ABSTRACT
Heparan sulfate proteoglycans (HSPGs) have been shown to regulate various developmental processes. However, the function of heparan sulfate (HS) during the development of mammalian stomach has not been characterized yet. Here, we investigate the role of epithelial HS in embryonic stomach by examining mice deficient in the glycosyltransferase gene Ext1. We show that HS exhibits a specific and dynamic expression pattern in mouse embryonic stomach. Depletion of the epithelial HS leads to stomach hypoplasia, with phenotypic differences in the gastric mucosa between the forestomach and hindstomach. In the posterior stomach, HS depletion disrupts glandular stomach patterning and cytodifferentiation via attenuation of Fgf signaling activity. Inhibition of Fgf signaling in vitro recapitulates the patterning defect. Ligand and carbohydrate engagement assay (LACE) reveals a diminished assembly of Fgf10 and Fgfr2b in the mutant. In the anterior stomach, loss of epithelial HS leads to stratification and differentiation defects of the multilayered squamous epithelium, along with reduced Hh and Bmp signaling activity. Our data demonstrate that epithelial HS plays multiple roles in regulating mammalian stomach morphogenesis in a regional-specific manner.
KEY WORDS: Ext1, Heparan sulfate, Glandular development, Fgf, Stratification, Hh-Bmp
Summary: Heparan sulfate is highly expressed in the embryonic mammalian stomach and is required for gastric development, including stratification of forestomach and specification of glandular stomach.
INTRODUCTION
The vertebrate stomach is a highly organized organ along the alimentary canal, and it is responsible for diverse biological functions, including digestion, immune defense and hormone secretion. The rodent stomach is divided into two major compartments along the proximal-distal axis. The most anterior part is the forestomach, which is absent in humans (McCracken and Wells, 2017). The forestomach mucosa comprises a multilayered keratinized squamous epithelium. The posterior region is the glandular stomach (hindstomach), which develops a columnar epithelium, and can be subdivided into a corpus and an antrum with regional-specific cell lineages. The corpus contains zymogenic chief cells, acid-secreting parietal cells, mucus-secreting cells and gastric endocrine cells, while the antrum region is devoid of chief cells and has few parietal cells (Karam et al., 1997; Kim and Shivdasani, 2016).
After the posterior foregut adopts its gastric identity, the stomach undergoes regionalization, specification and differentiation. A variety of regionally specific transcription factors (TFs) and intercellular signals including fibroblast growth factor (Fgf), sonic hedgehog (Shh), wingless-type MMTV integration site family (Wnt) and bone morphogenetic protein (Bmp) have been shown to control stomach development by cell autonomous and non-autonomous means (Kim et al., 2000; Ramalho-Santos et al., 2000; Jacobsen et al., 2002; Nyeng et al., 2007; Matsuyama et al., 2009; Rodriguez et al., 2010; McCracken et al., 2017). Such TFs are implicated in the generation of the boundary either between the stomach and other organs or between subdomains in the stomach, for example, Sox2 is predominately expressed in the forestomach, whereas Gata4 and Gata6 are uniquely expressed in the hindstomach (McCracken and Wells, 2017). Unlike other endoderm-derived organs, little is known about the mechanisms that confine the boundary of multiple TFs in the stomach. In addition, epithelial-mesenchymal interactions have been shown to be crucial for glandular stomach morphogenesis (Aubin et al., 2002; Kim et al., 2005a; Verzi et al., 2009). However, regulation of epithelial-mesenchymal communication and various signaling pathways has not been fully elucidated.
Distinct members of the Fgf family and Fgf receptors (Fgfrs) play essential roles in various biological events, such as stomach development, during embryogenesis and adult homeostasis in metazoans (Dorey and Amaya, 2010; Brewer et al., 2016). Association of Fgf and specific Fgfrs is highly dynamic during embryonic development, and their temporal and spatial epithelial-mesenchymal interaction is essential for endoderm patterning and specification (Allen and Rapraeger, 2003). One of the well-studied pairs in the gastrointestinal tract is Fgf10 and Fgfr2b. It has been reported that Fgf10 is expressed in the mesenchyme of the glandular stomach, cecum and rectum (Aubin et al., 2002; Spencer-Dene et al., 2006; Nyeng et al., 2007). In contrast, Fgfr2b, an isoform generated by alternative splicing of Fgfr2, is expressed in the epithelium of the lung bud, liver, stomach and intestine, and cooperates with Fgf10 to regulate organogenesis (Orr-Urtreger et al., 1993; Matsubara et al., 1996; Calmont et al., 2006; Cardoso, 2001; Herriges et al., 2015). Fgf10- and Ffgr2b-knockout mice show phenotypic similarities in the glandular mucosal defects (Spencer-Dene et al., 2006), implicating that Fgf10 acts as a major ligand for Fgfr2 during the glandular stomach development. Ectopic Fgf10 in mouse antral epithelium results in an abnormal gland formation with attenuated endocrine and parietal cell differentiation (Nyeng et al., 2007). It is possible that Fgf10 acts as a component of the epithelial-mesenchymal interaction network to regulate stomach development. However, little is known about how this intercellular signaling activity is modulated.
Heparan sulfate proteoglycans (HSPGs) are composed of a core protein and covalently attached to diverse heparan sulfate (HS) chains. HS chains are generated on the serine residues of the core protein via a complex process in the Golgi mediated by various enzymes, among which EXT1 and EXT2 are responsible for HS chain elongation (Bishop et al., 2007; Yan and Lin, 2009). HSPGs are enriched on the cell surface and in the extracellular matrix (ECM) depending on localization of the core protein. They are broadly implicated in the regulation of a variety of signaling pathways, including Fgf, Wnt and Bmp pathways, during development and diseases (Lin, 2004; Sarrazin et al., 2011; Iozzo and Schaefer, 2015; Poulain and Yost, 2015). Studies on the role of HSPGs in regulating signaling pathways have demonstrated that HS chains participate in the distribution of growth factors, chemokines and interleukins, as well as cellular responses to these molecules. Multiple mutants which are defective for HS exhibit abnormalities in early embryonic development (Lin et al., 2000; Stickens et al., 2005; Shimokawa et al., 2011; Kraushaar et al., 2012) and later organogenesis, including cardiovascular (Habuchi et al., 2007; Izvolsky et al., 2008; Pan et al., 2014; Zhang et al., 2015, 2014), respiratory (Fan et al., 2000; Ringvall et al., 2000; Izvolsky et al., 2003b) and nervous systems (Inatani et al., 2003; Grobe et al., 2005; Kalus et al., 2009; Pan et al., 2006; Cai et al., 2014; Tao and Zhang, 2016), indicating that HS plays indispensable roles in multiple stages of vertebrate development (specification, patterning and differentiation). Intriguingly, different tissues and organs exhibit dynamic changes in the composition of HS during embryonic development (Allen and Rapraeger, 2003; Ledin et al., 2004), adding another complexity to the regulatory mechanisms by which HS controls tissue-specific signaling events. Currently, the roles of HSPGs in mammalian gastric development are less well understood.
In this study, we examine the functions of epithelial HS on embryonic stomach development. To eliminate HS from the gastric epithelium, we selectively inactivate an essential glycosyltransferase gene for HS elongation, Ext1, during embryonic development. Depletion of HS leads to defects in glandular stomach regionalization and specification, as revealed by the impaired expression of posterior markers and a failure of gastric cell differentiation, which are associated with reduced Fgf signaling activity. We also show that epithelial HS enhances the binding of Fgf10 and Fgfr2b to the basement membrane and demonstrate the requirement of epithelial HS for the establishment of multilayered keratinized mucosa and Hh-Bmp signaling in the forestomach. Together, our data indicate that gastric epithelial HS plays multiple roles in stomach development.
RESULTS
Dynamic distribution of epithelial HS in the mouse embryonic stomach
After foregut regionalization, the gastric pseudostratified epithelium begins to differentiate from embryonic stage (E) 12.5 onwards. By E14.5, the morphological differences between forestomach and hindstomach epithelia are evident (Fig. 1A). The proximal stomach (forestomach) epithelium starts to stratify on E13.5, followed by keratinization at later stages, and finally develops into multilayered squamous mucosa that comprises a proliferative basal layer and suprabasal keratinized layers (Fukamachi et al., 1979; Thompson et al., 2018). In the distal stomach, glandular epithelium consists of columnar cells at E13.5, with few intra-epithelial vacuoles near the antral region. During development, the glandular epithelium undergoes morphogenesis and gives rise to gastric glands, which first appear at E15.5 and develop into the self-renewal glandular architecture in the adult (Fukamachi et al., 1979; Kim and Shivdasani, 2016).
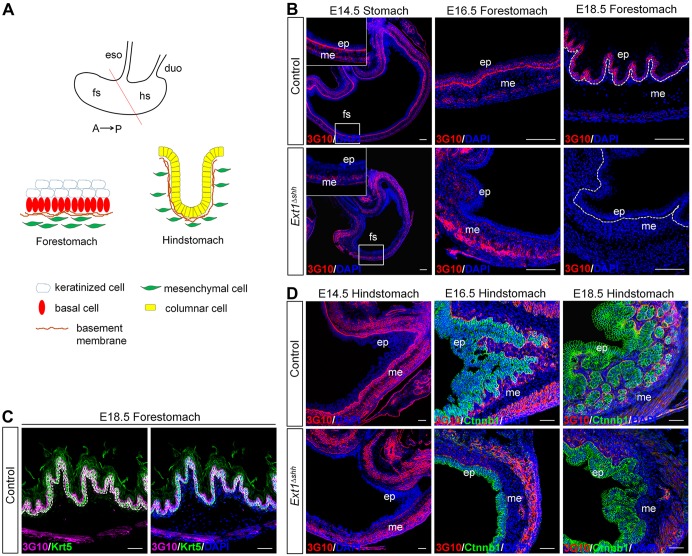
Fig. 1.
Dynamic expression of HS in gastric epithelium during embryonic development. (A) Schematic representation of the anatomy of stomach epithelium. The mouse embryonic stomach is subdivided into the anterior/non-glandular (forestomach) and the posterior/glandular (hindstomach) compartment, overlaid by multilayered keratinized squamous epithelia and complex glandular epithelia, respectively. A straight line distinguishes the forestomach and hindstomach. Distinct cell lineages are indicated. (B,C) HS is recognized by anti-3G10 antibody in control and mutant forestomach. An abundance of 3G10 signals are observed in the basement membrane and gastric epithelium in the forestomach. There is a switch of HS location from basement membrane to the cell surface from E14.5 to E18.5 (B, top panel). After epithelial deletion of Ext1, 3G10 staining is abolished both in the basement membrane and epithelium (B, bottom panel). At E18.5, expression of HS is highly enriched on the cell surface of Krt5-labeled basal cells (C). Magnified views of the boxed area are presented in the insets. Dashed lines separate the epithelium and mesenchyme. (D) HS is expressed in the basement membrane around forming glands in the hindstomach, which is consistent with glandular morphogenesis (top panel). HS is absent in the mutant (bottom panel). Ctnnb1 (green) staining denotes the epithelial cells. eso, esophagus; duo, duodenum; fs, forestomach; hs, hindstomach; ep, epithelium; me, mesenchyme. Scale bars: 100 μm (B), 50 μm (C and D).
To gain insights into the detailed profile of HS during embryonic stomach development, we examined the distribution of HS using anti-HS antibody (3G10), which recognizes neo-epitopes on the HS chains generated by heparinase III treatment. At the early stage of stomach differentiation (E14.5), HS was prominently expressed in the basement membrane of the gastric epithelium both in the forestomach and hindstomach (Fig. 1B,D, top left panel). In addition, HS was also present in the mesenchyme, shown by strong signals in smooth muscle cells. Intriguingly, during forestomach development, the distribution of HS in the basement membrane was gradually diminished while simultaneously enriched in the basal layer of the stratified epithelium (Fig. 1B, top, middle and right panel). At E18.5, co-staining of keratin-5 (Krt5) and 3G10 confirmed that a high level of HS was present on the basal cell surface; however, suprabasal cells were devoid of HS staining (Fig. 1C). In the hindstomach, HS was found in the basement membrane, particularly around the pit glands throughout the embryonic development (Fig. 1D, top panel). Our results demonstrate that HS exhibits temporal and spatial expression patterns in embryonic stomach, which coincides with morphogenesis processes, including epithelial invagination in the glandular stomach and stratification in the forestomach, indicating diverse roles of HS in the regulation of the stomach development.
Depletion of epithelial HS leads to gastric hypoplasia
To elucidate the role of HS in stomach development, we examined HS-deficient mice by inactivating the glycosyltransferase Ext1, which is essential for polymerization of HS chains in vitro (Busse and Kusche-Gullberg, 2003; Kim et al., 2003; Busse et al., 2007). Ext1−/− mutants exhibit disrupted gastrulation and early embryonic lethality (Lin et al., 2000), which precludes us investigating the function of Ext1 during the later organogenesis. To overcome this problem, we used Shh-Cre-EGFP induced recombination (Harfe et al., 2004) to conditionally delete Ext1 from endodermal epithelium. Cre is active throughout the gastrointestinal endoderm from E10.5 onwards (Ramalho-Santos et al., 2000; Spencer-Dene et al., 2006; Mao et al., 2010). Immunofluorescence staining of 3G10 was performed to evaluate the level of HS after Ext1 deletion. Ext1fl/fl; Shh-Cre (Ext1Δshh) mutant mice exhibited a complete lack of HS in the gastric epithelium from E14.5 to P0, but retained expression in the mesenchyme (Fig. 1B,D, bottom panel). These data demonstrate that Ext1 inactivation is sufficient to disrupt the synthesis of HS in vivo.
Next, we examined the morphology of the mutant embryonic stomach. Mice deficient in one allele of Ext1 appeared to be normal in terms of the gastrointestinal morphology, therefore Ext1fl/+; Shh-Cre mice were used as controls. In contrast, Ext1 mutant mice died shortly after birth due to respiratory defects (He et al., 2017), and exhibited several gastrointestinal anomalies, e.g. small stomach and abnormal intestines (Fig. 2A, Fig. S1A). In control stomach, the anterior and posterior compartments could be clearly recognized by the exterior structural features, including contrast folds in the forestomach and less transparent wall of the hindstomach. However, mutant stomach appeared to be even along the anterior-posterior axis, with a significantly reduced hindstomach (Fig. 2A, asterisks). By E18.5, a small fraction of mutant stomachs presented as rudimentary ‘buds’ along the gastrointestinal tract.
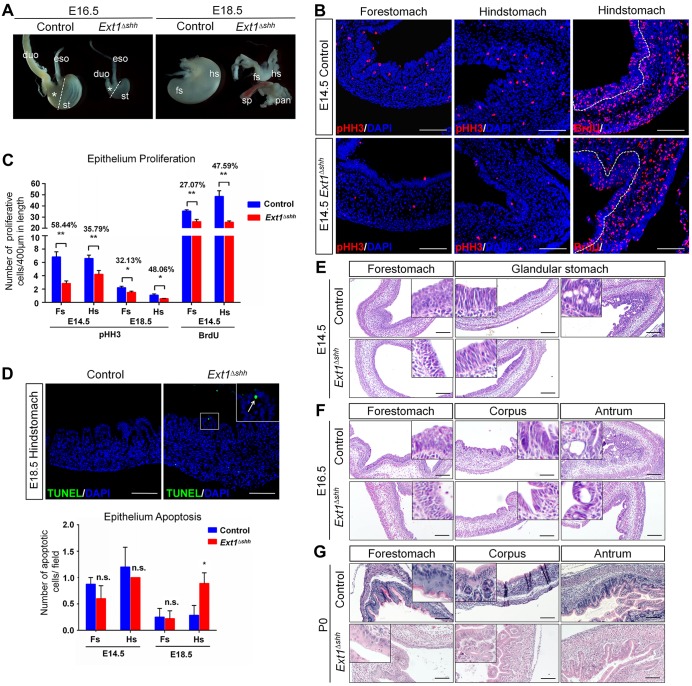
Fig. 2.
Depletion of epithelial HS causes stomach growth defects and impaired gastric morphogenesis. (A) Gross images of stomachs at indicated embryonic stages from control and Ext1Δshh mutant mice. Mutant embryos exhibit smaller stomachs and thinner intestines. Asterisks represent the glandular stomachs. Dashed lines separate the forestomach and hindstomach. Note that abnormal and rudimentary ‘buds’ are observed from some E18.5 mutant embryos. eso, esophagus; duo, duodenum; st, stomach; fs, forestomach; hs, hindstomach; sp, spleen; pan, pancreas. (B,C) Analysis of gastric epithelium proliferation by pHH3 and BrdU immunostaining. The mutant stomach shows a decreased proliferation in both the forestomach and hindstomach at E14.5. Quantification is achieved by counting the number of pHH3- and BrdU-positive cells relative to the length of the epithelium. The rates of reduction between control and mutant are shown. Nuclei are visualized with DAPI. Dashed lines outline the epithelium. n=5 per group. (D) Increased apoptosis at E18.5 is observed in mutant glandular stomach, demonstrated by TUNEL assay. The inset represents magnified image of the boxed area. The arrow indicates an apoptotic cell in the mutant epithelium. n=3 per group. (E-G) Hematoxylin and Eosin stained sections of mutant stomachs showing histological defects during gastric epithelium morphogenesis. *P<0.05, **P<0.01; n.s., no significant difference. Error bars indicate s.e.m. Scale bars: 100 μm.
The reduction in stomach size raised the possibility that inactivation of Ext1 causes a decrease in proliferation and/or an increase in apoptosis. To this end, we performed the following analyses. First, immunostaining of mitotic marker phospho-histone H3 (pHH3) revealed that mutant epithelium exhibited decreased proliferation capacity in the forestomach [reduced by 58.44% (P=0.0003) and 32.13% (P=0.0300) at E14.5 and E18.5, respectively], as well as in the hindstomach [reduced by 35.79% (P=0.0063) and 48.06% (P=0.0350) at E14.5 and E18.5, respectively] (Fig. 2B,C and Fig. S1B). Second, analysis of embryos exposed to BrdU for 2 h in utero and assayed for the efficiency of BrdU incorporation showed that the average number of BrdU-labeled cells in the mutant epithelium was decreased by 27.07% (P=0.0010) and 47.59% (P=0.0005) of those in control forestomach and hindstomach, respectively (Fig. 2B,C). Finally, we performed TUNEL assays to analyze apoptosis during stomach development. Apoptosis was rarely detected at early embryonic stages, and the level was comparable between control and mutant at E14.5. Intriguingly, the epithelial cell layer from E18.5 mutant stomach showed an elevated level of apoptosis (Fig. 2D). Together, these data indicate that both decreased proliferation and increased apoptosis might account for the gastric growth defects in the mutant embryos.
To gain deeper insights into the differences between control and mutant mice, we characterized stomach histology by Hematoxylin and Eosin staining (Fig. 2E-G). At E14.5, the forestomach epithelium appeared to be pseudostratified in the control, whereas it was pluricellular with cuboid shapes in the mutant. Strikingly, whereas in the hindstomach, the glandular epithelium of control mice comprised vacuolated, non-mucous epithelium, such epithelium was replaced by the stacking of naive columnar cells in the mutant (Fig. 2E). At E16.5 and later stages, histological differences between the control and the mutant epithelium were more evident. Control forestomach had already established multilayered squamous epithelium with longitudinal folding, whereas mutant epithelium showed less-stratified layers (Fig. 2F,G, left panel). Moreover, the proximal glandular mucosa of mutant embryos was covered by columnar epithelium without organized gland pits, which were normally displayed in the control corpus (Fig. 2F, middle and right panels). Sections of the P0 mutant stomach revealed that the atypical glandular mucosa contained simple branching tubular invaginations with rare glands, whereas there were complex glands containing many cell types in the control (Fig. 2G, middle and right panels). Therefore, we conclude that the disrupted forestomach development and gland formation lead to impaired stomach morphogenesis after Ext1 inactivation, indicating the importance of epithelial HS in gastric development.
Epithelial HS is necessary for patterning and specification of glandular stomach
The observed severe histological abnormalities inspired us to investigate the mechanisms for abnormal stomach morphogenesis in the Ext1 mutant mice. During embryonic development, the posterior foregut is specialized by instructive signals to define its gastric fate. Patterning of the stomach is then achieved and reinforced by regional-specific expression of various TFs. To characterize the nature of the gastric mucosa, we conducted immunohistochemistry analysis and RNA in situ hybridization (ISH) to examine markers of distinct gastric domains. Sox2 is not only expressed in the anterior stomach, but also has functional roles in patterning and specification of the forestomach (Rodriguez et al., 2010; McCracken et al., 2017; McCracken and Wells, 2017). Immunostaining of Sox2 at E14.5 revealed that its expression was extended to the entire stomach epithelium in the mutant embryo, compared with the restricted forestomach distribution in control (Fig. 3A). In wild-type stomach, the expression of Shh transcripts was strong in the forestomach epithelium, but weak in the presumptive corpus and antrum (Ramalho-Santos et al., 2000; Bitgood and McMahon, 1995). Consistent with misexpression of Sox2 in mutant, Shh mRNA was also detected at a high level in the glandular stomach (Fig. 3B). Caudal expansion of these anterior markers suggests that epithelial HS deficiency interferes with the defining of boundaries between anterior/posterior domains and causes stomach ‘anteriorization’.
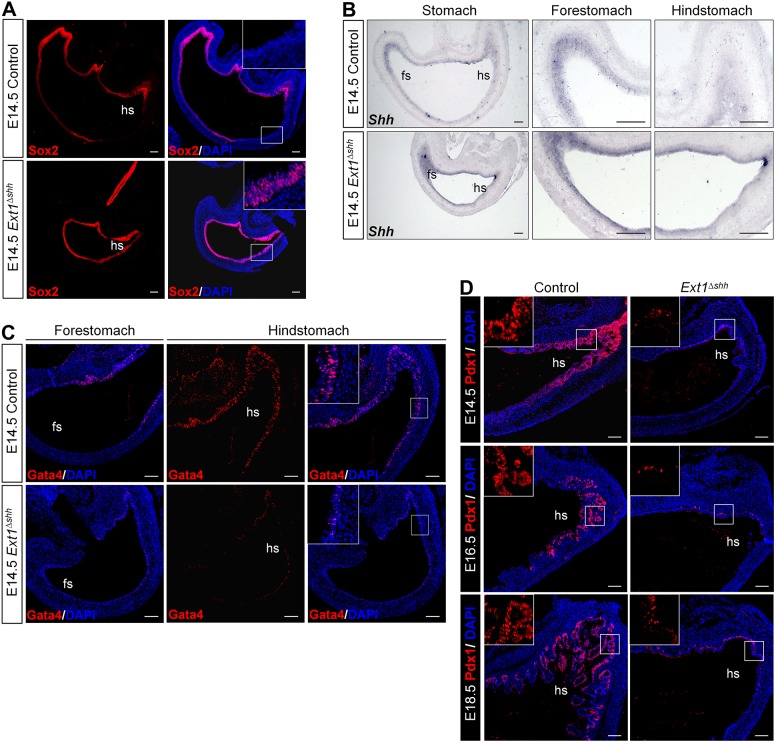
Fig. 3.
Epithelial HS is required for A-P patterning and glandular specification during the development of stomach. (A) Immunofluorescence staining of Sox2 in E14.5 stomach. HS deletion results in strong expression of Sox2 in the glandular stomach epithelium. (B) In situ hybridization (ISH) using Shh riboprobe on E14.5 paraffin sections. Expression of Shh is spread throughout the entire gastric epithelium in the mutant. Images at high magnification are shown on the right. (C) Immunofluorescence staining with antibody against Gata4. Nuclear stained Gata4 is decreased in mutant glandular stomach, compared with the strong and wide Gata4 pattern in the control. (D) Immunofluorescence staining with antibody against Pdx1 reveals a dramatic reduction in the pre-antral region of the mutant stomach during embryonic development. Insets represent magnified images of the boxed area with no DAPI stain. hs, hindstomach; fs, forestomach. Scale bars: 100 μm (A,B), 50 μm (C,D).
Regionalization of the glandular stomach is also achieved by localized expression of TFs. The zinc-finger transcription factor Gata4 is required for the glandular epithelial specification and to be involved in attenuating Shh expression in the distal stomach (Jacobsen et al., 2002, 2005). We evaluated the expression of Gata4 after Ext1 inactivation. At E14.5, this regional TF was detected exclusively in the glandular mucosa in control mice. However, Gata4 nuclear signals were remarkably decreased in the mutant mice (Fig. 3C). Expression of the homeobox gene Pdx1 is normally limited to the antral-pyloric segment, serving as a reliable marker of this region. We performed immunohistochemistry for Pdx1 at different embryonic stages and found that Pdx1 was almost absent in the mutant stomach during development (Fig. 3D), further supporting the patterning defects in the glandular stomach. Together, the absence of glandular patterning markers and overexpression of the forestomach patterning markers suggest that epithelial HS plays an important role in the specification of glandular stomach.
Epithelial HS is required for proper gastric mucosal differentiation and is dispensable for mesenchyme development
As described above, glandular specification was disrupted in the mutant stomach. To determine whether the differentiation of gastric epithelium was impaired, we examined the differentiation of mutant glandular stomach at late developmental stages (E18.5 and P0). Strikingly, analysis by immunohistochemistry staining and ISH revealed the defective differentiation of gastric cells, including chief cells (pepsinogen I), parietal cells (Atp4b) and mucus cells (PAS), which was consistent with the lack of Gata4 in the glandular stomach (Fig. 4A and Fig. 3C), indicating that epithelial HS was required for cytodifferentiation of the glandular epithelium. However, endocrine cells were still present in the mutant mucosa, as shown by the pan-endocrine cell marker (Fig. 4A, arrows).
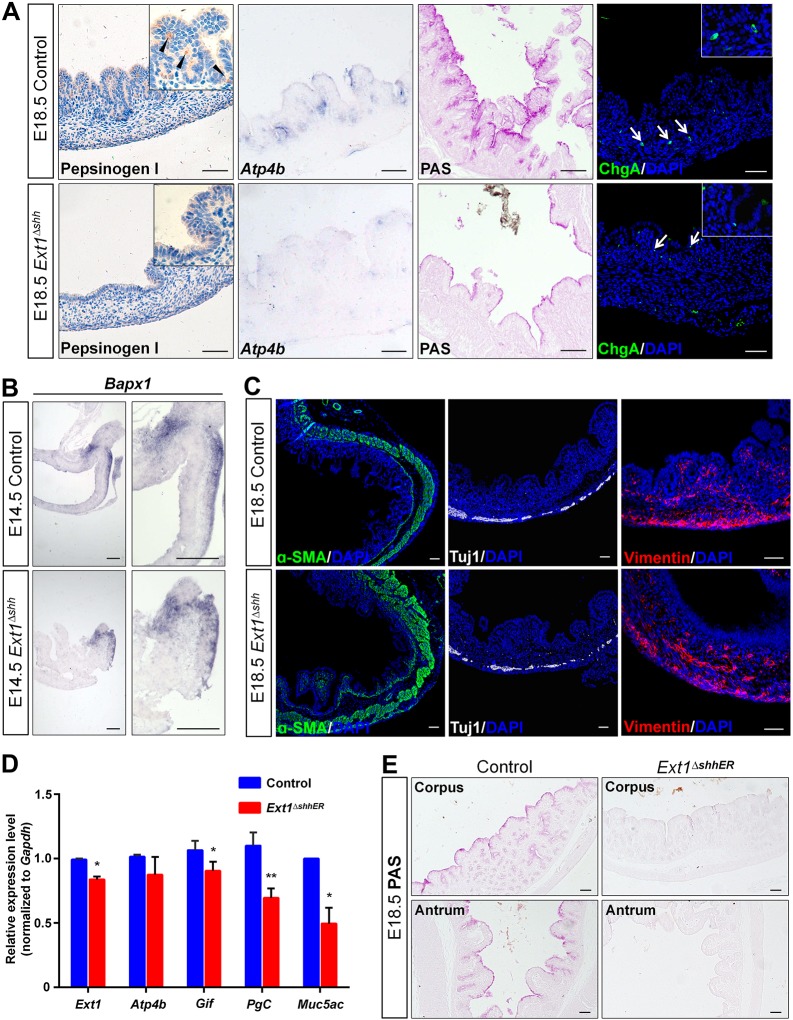
Fig. 4.
Epithelial HS is indispensable for differentiation of epithelial cell lineages in the glandular stomach. (A) Differentiation of the glandular epithelium at E18.5. Immunohistochemistry and ISH analysis indicate loss of chief cells (pepsinogen I), parietal cells (Atp4b) and fewer mucus cell (PAS) in the corpus after depletion of HS, whereas ChgA-positive endocrine cells are present. Arrowheads denote chief cells, and arrows indicate endocrine cells. Magnified views are presented in the insets. (B) ISH on paraffin sections using Bapx1 riboprobe. Expression of Bapx1 in the distal gastric mesenchyme is comparable between the control and mutant. (C) Differentiation of the gastric mesenchyme is examined by immunostaining of α-SMA for smooth muscle cells, Tuj1 for enteric neurons and vimentin for mesenchyme cells. Major mesenchymal cell types emerge normally at E18.5 in HS-deficient mice. (D) Ext1 and gastric-specific markers examined by qPCR in Ext1 inducible knockout mice. Several cell lineages are impaired, suggesting that HS is essential for the differentiation of glandular lineages. *P<0.05, **P<0.01. Error bars indicate s.e.m. (n=3). (E) PAS staining confirms the reduction in mucus production. Scale bars: 50 μm (A,C,E), 100 μm (B).
Epithelial-mesenchymal interaction plays a critical role in stomach development. To understand whether the underlying mesenchyme contributed to the epithelial defects in mutant stomach, we studied mesenchyme development in mutant embryos. Bapx1 (also known as Nkx3-2), a well-known regulator of gastric epithelial specification (Verzi et al., 2009), was analyzed on paraffin sections from the control and mutant embryos. Bapx1 expression was not altered in the posterior mesenchyme of the mutants (Fig. 4B). We further characterized the differentiation of cells in gastric mesenchyme by immunostaining with α-SMA for smooth muscle cells, Tuj1 for enteric nerve cells and vimentin for mesenchyme cells (Fig. 4C, Fig. S2A). These cell lineages were not altered in the mutant. To confirm the identity of the gastric epithelium, we examined the typical markers of intestinal epithelium by staining for villin and AB-PAS (Fig. S2B,C). We found that both markers were absent in the atypical mucosa of the mutant stomach, indicating that there was no intestinal metaplasia after HS depletion. Our data led us to the conclusion that the epithelium and the mesenchyme of the mutant stomach maintained the gastric identity with normal mesenchyme development.
Stomach differentiation markers such as Atp4b and PgC start to appear at late embryonic stages. Thus their absence in the mutant fetus might be due to developmental delay. To determine whether Ext1 deletion leads to defective cell differentiation per se, an inducible knockout mouse model was constructed by using Shh-CreERT2, in which the activity of Cre recombinase expressed in Shh-producing cells was induced via tamoxifen administration, and the differentiation markers were assessed following injection of tamoxifen at E16.5, when the cell fate of the glandular epithelium had been determined. We examined the deletion of HS by 3G10 immunostaining. Our results showed that the epithelial HS was strongly localized in the forestomach epithelium and basement membrane of the hindstomach, including corpus and antrum, as previously reported (Fig. 1B,D and Fig. S2D). However, its expression was reduced in knockout mice (Fig. S2D). The expression of some glandular cell markers such as Gif, Pgc and Muc5ac were significantly decreased (Fig. 4D). PAS staining confirmed the reduction of mucus secretion in the mutant, with no alteration of Gata4 expression (Fig. 4E, Fig. S2E), indicating that epithelial HS is involved in the differentiation of some gastric lineages, including chief cells and mucus cells.
HS promotes Fgf signaling activity in gastric epithelium
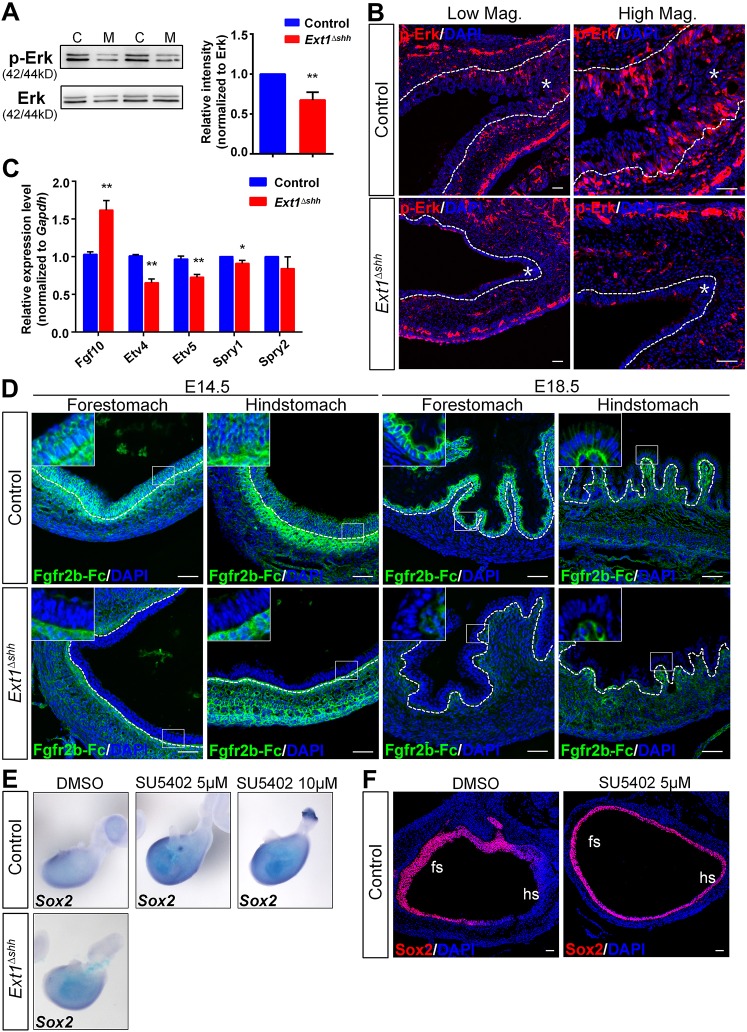
Stomach development is dependent on diverse signaling pathways; however, the mechanisms by which these pathways are regulated and integrated are enigmatic. The Fgf signaling pathway has been implicated in many aspects of foregut patterning and organogenesis (Wells and Melton, 2000; Zorn and Wells, 2009; Kim and Shivdasani, 2016; McCracken and Wells, 2017). It is also reported to be crucial for glandular stomach development (Spencer-Dene et al., 2006; Nyeng et al., 2007). Therefore, we decided to characterize the effects of Ext1 inactivation on Fgf signaling. Consistent with previous studies, ISH of Fgf10 probes confirmed the localization of Fgf10 in the mesenchyme of the posterior stomach (Fig. S3A). Moreover, one of the FGF receptor isoforms, Fgfr2b, was at comparable levels in both the control and mutant stomach (Fig. S3B). The fact that glandular defects displayed in Ext1 mutants phenocopied the abnormalities in Fgf10−/− and Fgfr2b−/− mutants (Spencer-Dene et al., 2006), raised the possibility that Fgf signaling activity was downregulated in HS-deficient stomach. To investigate the activity of Fgf signaling, we examined the phosphorylation status of Fgf signaling effector Erk1/2 and its downstream targets. Western blotting revealed the reduction in the level of phospho-Erk1/2 (p-Erk), which indicated the decreased activity of Erk signaling (Fig. 5A). Immunostaining with anti-p-Erk1/2 antibody showed a localized distribution of p-Erk in the epithelium of control glandular stomach, in concert with Fgf10 expression pattern (Fig. 5B, Fig. S3A). By contrast, the epithelium of the mutant stomach exhibited diminished p-Erk staining and a reduced number of p-Erk-positive cells in the gastric epithelium (Fig. S3C). Furthermore, ISH and/or qPCR analyses revealed the decreased expression of the Fgf targets Etv4, Etv5 and Sprys in the mutant stomach, regardless of elevated expression of Fgf10 mRNA after HS deletion (Fig. 5C, Fig. S3D). Altogether these results support the conclusion that epithelial HS plays a crucial role in promoting Fgf signaling activity in embryonic gastric epithelium.
Fig. 5.
Epithelial HS modulates Fgf signaling activity in embryonic stomach. (A) Representative image of western blot of p-Erk shows a reduction in its protein level. Graph shows the quantification of band intensity from three independent western blots. C, control; M, mutant (n=3). (B) p-Erk signals are absent in mutant glandular epithelium. Asterisks indicate epithelium. (C) Analysis of Fgf signaling targets. qPCR shows decreased expression of Etv4, Etv5 and Spry1 in mutant stomach in spite of elevated Fgf10 mRNA level (n=5 per group). (D) LACE staining of Fgf10 and Fgfr2b in forestomach and hindstomach. Strong signals near the basement membrane, as well as in the epithelial cells both in the forestomach and hindstomach are detected in the control, which is rarely observed in the mutant. Magnified views of the boxed areas are shown in the insets. (E,F) Fgfr inhibitor (SU5402) treatment leads to enhanced Sox2 expression in a concentration-dependent manner shown by ISH and immunostaining. Organ culture experiments were performed independently three times (n=6 under each control condition; n=2 for mutant). fs, forestomach; hs, hindstomach. The dashed lines outline the gastric epithelium. **P<0.05 and *P<0.01. Error bars indicate s.e.m. Scale bars: 50 μm.
To elucidate the molecular mechanisms underlying the effects of epithelial HS on Fgf signaling in the stomach, we carried out ligand and carbohydrate engagement (LACE) assay to examine whether ablation of epithelial HS affected the assembly of Fgf-Fgfr complex in gastric mucosa. E14.5 stomach sections of both control forestomach and hindstomach showed a strong binding of Fgf10–Fgfr2b-Fc to the gastric mucosal layer, in the epithelium and near the basement membrane (Fig. 5D, top panel). Such a binding pattern was in agreement with 3G10 staining (Fig. 1B,D). As expected, signals were scarcely detected in the epithelium of mutant stomach, similar to results in control sections without Fgf10 incubation, demonstrating that the binding was specific and mediated by both Fgf and HS (Fig. 5D, bottom panel and data not shown). Decreased Fgf10 and Fgfr2b binding in the mutant epithelium was more evident at E18.5, when no signals were observed in the epithelium of the mutant stomach (Fig. 5D). Our data indicate that epithelial HS enhances the binding affinity of Fgf and Fgfrs to the gastric epithelium.
Previous studies have shown that exogenous Fgf10 upregulates Nkx2.1 and downregulates Sox2 in esophagus explants (Rodriguez et al., 2010), and Fgf10 transgenic mice have reduced Sox2 transcripts in the stomach (Nyeng et al., 2007). We speculated that the expanded distribution of Sox2 in Ext1 mutant stomach was caused by decreased Fgf signaling. To evaluate this possibility, we manipulated the Fgf signaling in the stomach explants in vitro, and examined its influence on Sox2 expression. Following treatment with the Fgfr inhibitor SU5402 for 72 h, wild-type stomachs exhibited increased expression of Sox2 mRNA in a concentration-dependent manner (Fig. 5E). Notably, mutant stomach explants displayed elevated Sox2 expression, which was consistent with what we observed in vivo (Fig. 3A). Immunostaining of paraffin sections also confirmed the abnormal Sox2 expression in the hindstomach with a thinner epithelial layer after inhibition of Fgf signaling (Fig. 5F). Accordingly, we demonstrate that ectopic expression of Sox2 in glandular epithelium is associated with decreased Fgf signaling activity after HS depletion.
Epithelial HS is necessary for epithelium stratification and is required for Hh and Bmp signaling activities
Since we demonstrated the spatial and temporal distribution of HS in the forestomach mucosa during embryonic development (Fig. 1B,C), we asked whether such dynamic expression had functional roles in forestomach development. Analysis of the composition of the forestomach epithelium showed that control epithelium contained a continuous p63 (TP63)- and Krt5-positive cell layer (Fig. 6A, top panel). In contrast, HS ablation not only dramatically reduced the number of p63- and Krt5-positive basal cells, but also led to their scattered distribution (Fig. 6A, bottom panel). Many studies have demonstrated that p63, an important player in keratinocyte proliferation and differentiation, is essential for establishing and sustaining most squamous epithelia (Koster et al., 2004; Romano et al., 2012; Yang et al., 1999; Daniely et al., 2004; Wang et al., 2011). We found that the remaining basal cells in the mutant epithelium retained proliferative capacity as revealed by co-staining of Krt5 and BrdU (Fig. 6B, left panel). Intriguingly, while wild-type epithelium cells in the upper layers at E18.5 were Sox2 negative, those cells in the mutant were positive for Sox2 (Fig. 6B, right panel). Notably, cells in the suprabasal layer, which are derived from basal cells, lost the proliferative capacity in the control; likewise, these abnormal Sox2-positive cells also lack BrdU signals in the mutant. We also examined the differentiation of keratinized cells, and found that mutant epithelium was negative for the keratinization marker involucrin (Fig. 6C), consistent with the decreased squamous layers. Our results highlight the important role of HS in the forestomach stratification and differentiation.
Fig. 6.
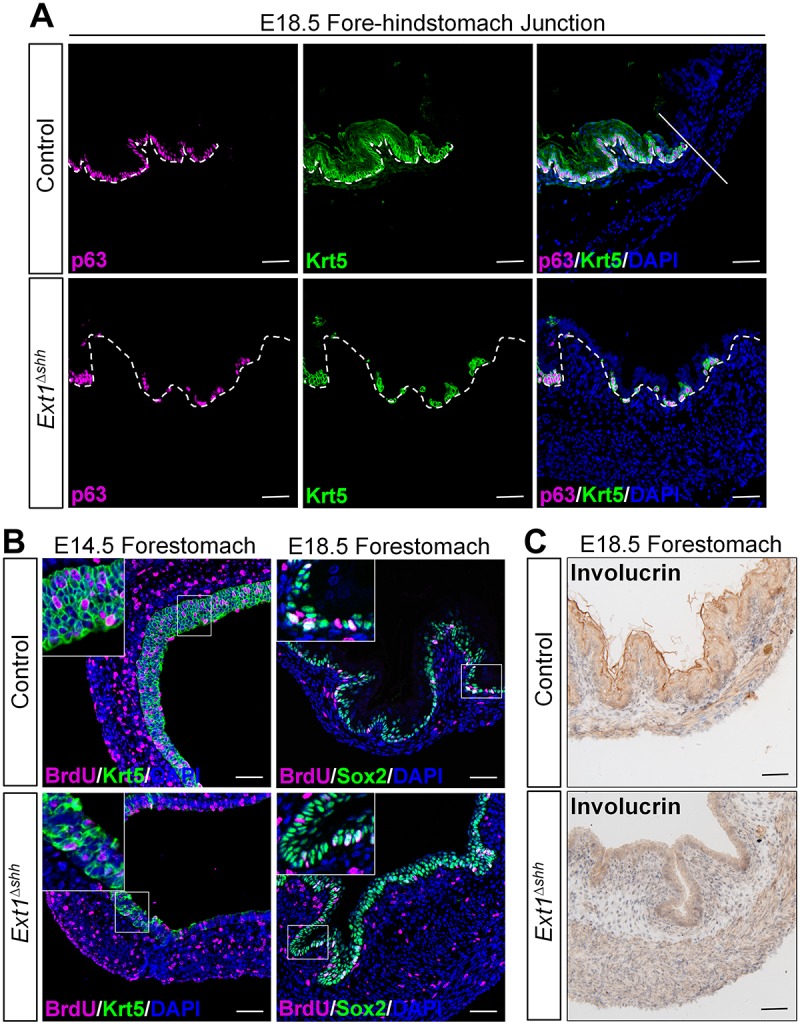
Epithelial HS regulates forestomach stratification and differentiation. (A) Immunostaining for basal cell markers on forestomach sections. The number of p63+ and Krt5+ basal cells declined and their distribution is sporadic rather than continuous in the mutant epithelium. The straight line separates the forestomach and glandular stomach. White dashed lines outline the epithelium. (B) Analysis of the proliferation in forestomach epithelium. Co-staining for BrdU and Krt5 indicates that remaining basal cells in mutant have proliferation capacity. However, those abnormal Sox2-stained cells in the supralayer are negative for BrdU staining. Magnified views of the boxed area are presented in the insets. (C) Immunohistochemistry analysis of keratinization marker involucrin reveals that the formation of squamous layers is disrupted in the mutant. Scale bars: 50 μm.
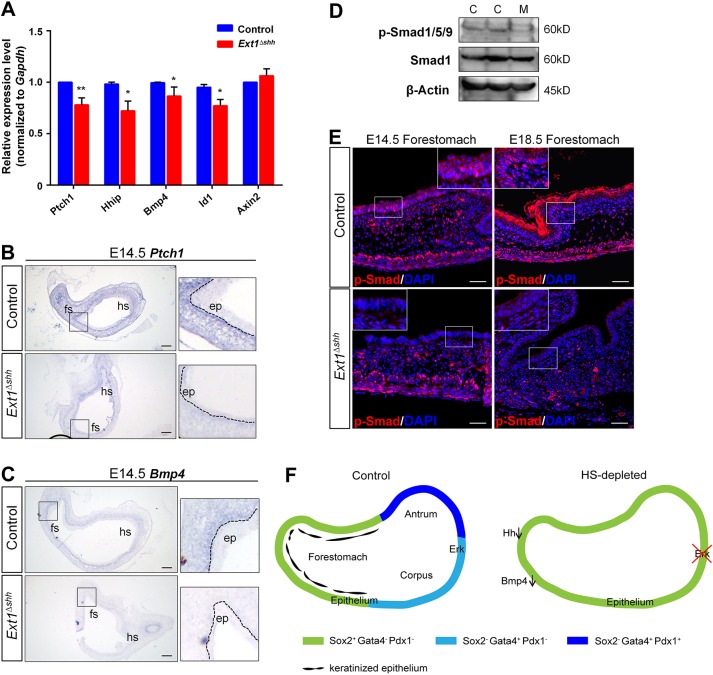
To address the possible involvement of other HS-dependent intercellular pathways in the forestomach phenotypes, we examined the expression of Hh, Bmp, Wnt targets by qPCR. The Hh targets Ptch1 and Hhip were significantly decreased in the mutant stomach, whereas the canonical Wnt target Axin2 was unaffected (Fig. 7A). Ptch1 is expressed in the gastric mesenchyme. We used ISH to detect Ptch1 expression in the stomach, and our results showed a significant difference in its signal strength between the mutant and control (Fig. 7B). Bmp4 is a well-established target of the Hh signaling pathway in many embryonic tissues, especially in the intestine (Shyer et al., 2015). The interaction between Bmp4 and Hh signaling pathways in the embryonic stomach has been implicated previously (Bitgood and McMahon, 1995). We found that Bmp4 and its target Id1 were both reduced in the Ext1 mutant (Fig. 7A,C). Notably, the expression pattern of Bmp4 was consistent with that of Ptch1, supporting the potential interaction between Hh and Bmp signaling in the embryonic stomach. In addition, the phosphorylation level of Smad1/5/9, an intracellular indicator of Bmp signaling activation, and its nuclear distribution were remarkably decreased during stomach development (Fig. 7D,E).
Fig. 7.
Epithelial HS depletion attenuates Hh-Bmp signaling activity in the stomach. (A) Expression of Hh, Bmp and Wnt signaling targets. Both Hh targets (Ptch1, Hhip1, Bmp4) and Bmp target (Id1) are downregulated after HS ablation at E14.5. **P<0.05, *P<0.01. Error bars indicate s.e.m. (n=4 per group). (B) ISH for Ptch1 transcripts. Mutant displays decreased Ptch1 signal strength in the mesenchyme, compared with the control. (C) ISH for Bmp4 confirms its decreased expression in mutant mesenchyme. (D,E) Phosphorylation of Smad1/5/9 is reduced in mutant revealed by western blotting and immunostaining. C, control; M, mutant (n=3). White dashed lines outline the epithelium. Magnified views of the boxed areas are presented in the insets (B,C,E). Scale bars: 100 μm (B,C), 50 μm (E). (F) Model for the functions of epithelial HS on stomach patterning and specification. In wild-type glandular stomach, epithelial HS promotes Fgf-Erk signaling activation in the overlying epithelium to regulate proper patterning and glandular cytodifferention. In the forestomach, epithelial HS is required for epithelial stratification and modulation of Hh-Bmp signaling. In HS-deficient mice, gastric epithelium is disorganized in both forestomach and hindstomach.
DISCUSSION
The vertebrate stomach is derived from posterior foregut followed by a series of specification and regionalization events, which are orchestrated by signaling activities mediated by various morphogens, including Shh, Wnt, Fgf10, Bmp and activin (Kim et al., 2000; Ramalho-Santos et al., 2000; Kim et al., 2005a,b; Wang et al., 2006; Shin et al., 2006; Spencer-Dene et al., 2006; McCracken et al., 2017; Rodriguez et al., 2010), as well as the interactions between the epithelium and the underlying mesenchyme (Kim et al., 2005a; Koike and Yasugi, 1999; Aubin et al., 2002). Our data presented here demonstrate that gastric epithelial HS also plays essential roles in gastric development. We provide evidence that epithelial HS regulates stomach morphogenesis by modulating the activities of multiple signaling pathways.
HS exhibits dynamic distribution during embryonic stomach development
HSPGs are distributed on the cell surface and in the ECM, including the basement membrane. Intriguingly, different tissues and organs exhibit dynamic changes in the composition of HSPGs during embryonic development (Allen and Rapraeger, 2003; Ledin et al., 2004), indicative of highly diversified demands on HSPGs in developmental processes. Here, for the first time, we have characterized HS distribution during stomach development. HS was found to be persistently expressed in the basement membrane, with high expression level surrounding the forming glands. Ext1 inactivation ablated the epithelial HS without disrupting the integrity of the laminin network (data not shown), suggesting that HS from the gastric epithelium is distributed in the basement membrane, but is not required for its stabilization and maintenance. Notably, we found a switch in HS distribution in forestomach from the basement membrane to the cell surface. This spatial-temporal expression pattern might be indicative of a transient change in HSPG composition in the context of different developmental stages. Elucidating the molecular mechanisms underlying the regulation of HSPG expression in a spatial-temporal manner would shed more light on our understanding of alteration of HSPG biosynthesis related to human diseases.
Epithelium HS regulates embryonic stomach development
Our data demonstrate the multiple roles of HS during stomach development. In the forestomach, HS inactivation leads to defective squamous epithelium formation. In the hindstomach, HS inactivation at early embryonic stages disrupts the patterning and specification of glandular epithelium, including the differentiation of parietal cells, chief cells and mucus-producing cells. The failure of cell lineage specification is probably not due to developmental delay. First, by E18.5, although mutant epithelium lacks the cells expressing chief cell marker (pepsinogen I) and parietal cell marker (Atp4b), the expression of enteroendocrine cell marker (chromograninA) is evident in mutant (Fig. 4A), suggesting that the glandular development is not simply delayed. Second, we confirm the differentiation defect by using an inducible knockout mouse model in which we selectively deleted Ext1 after the fate of gastric epithelium has been determined. It is known that Gata4 expression is exclusively restricted in the glandular region and has functions during glandular development; therefore, it is widely used as a glandular epithelium marker (Jacobsen et al., 2002; McCracken et al., 2017). Here, we show that Gata4 patterns normally in Ext1ΔshhER gastric epithelium at late fetal stages, indicating that the glandular patterning is not altered. However, similar to expression in Ext1Δshh mice, expression of some glandular cell lineage markers is decreased compared with levels in the control (Fig. 4D). Together, these data suggest that HS-deficient epithelial cells have an intrinsic deficiency in their ability to differentiate.
Epithelial HS is required for Fgf signaling in glandular stomach
HS is well known to regulate Fgf signaling in multiple biological processes (Rapraeger et al., 1991; Nurcombe et al., 1993; Shimokawa et al., 2011; Qu et al., 2012; Venero Galanternik et al., 2015), through multiple means: by regulating ligand diffusion; by acting as a co-receptor; and by controlling ligand concentrations (Bishop et al., 2007; Matsuo and Kimura-Yoshida, 2013; Balasubramanian and Zhang, 2016). Both gain-of-function and loss-of-function studies in the mouse and chicken have shown that Fgf signaling has regional effects on stomach morphogenesis (Spencer-Dene et al., 2006; Shin et al., 2006; Nyeng et al., 2007). However, little is known about the regulation of Fgf signaling transduction and its downstream effectors during gastric epithelium differentiation. Here, we demonstrate that epithelial HS in the glandular stomach has a key role in regulating Fgf signaling activity. Consistent with the important roles of HS in the activation of other signaling pathways (Lin and Perrimon, 2000; Häcker et al., 2005; Bishop et al., 2007; Iozzo and Schaefer, 2015), we show that epithelial HS facilitates the binding of Fgf10 and Fgfr2b in the basement membrane, and regulates Fgf-Erk signaling in the glandular epithelium. Although intensive studies have demonstrated the instructive roles of various mesenchymal factors during glandular stomach development, the communication network between mesenchyme and epithelium has not been clarified. Our data highlight the importance of mesenchymal derived Fgf during organogenesis and show that HS potentially serves as an important regulator in an epithelial-mesenchymal interplay.
In addition, our study reveals that Fgf signaling in the hindstomach is required for the restriction of Sox2 and expression of Gata4 in the hindstomach, indicating that there might be a counterbalance between regional-specific TFs in the embryonic stomach. A previous study has shown that Fgf10 prevents Sox2 expression in epithelial cells of the airway in part by activating epithelial β-catenin signaling (Volckaert et al., 2013). Further study is needed to address whether a similar signaling axis modulates Sox2 expression in the stomach.
In our recent study, we examined the role of epithelial HS in lung branching morphogenesis and demonstrated a surprisingly expanded distribution of Fgf10 expression and increased Fgf signaling activity in lung buds (He et al., 2017). The data shown here are very different from what we observed in the lung buds, raising an interesting question of the specific activities of HS in regulation of the Fgf signaling pathway. Studies have shown that the epithelium-associated O-sulfated HS is required for binding of Fgf to the epithelium (Izvolsky et al., 2003a,b). However, N-sulfation of HS is shown to be essential for activating Fgf-Shp2-Erk during lacrimal gland development (Pan et al., 2008). Given the lack of knowledge of the details of HS modification and components of HSPGs in the stomach, we speculate that the divergent effects of HS on Fgf signaling in lung and stomach development is probably due to context-dependent differences in the modification pattern and subcellular distribution of HS in various developmental events. Further studies are needed to define the related mechanisms.
Epithelial HS is associated with activities of multiple signaling pathways during stomach development
HS plays essential roles in vertebrate development by regulating multiple signaling pathways. Previous studies in Drosophila have demonstrated that HS chains regulate the distribution of distinct growth factors, such as Wg, Hh and Dpp, which are crucial for patterning the wing disc (Lin, 2004; Yan and Lin, 2009). During vertebrate development, HSPGs are also implicated in various signaling events to regulate cell proliferation, specification and migration. The zebrafish Glypican4 (knypek) mutant exhibits severe cardiac defects, which are attributed to elevated Bmp and canonical Wnt signaling in the presumptive cardiac mesoderm (Strate et al., 2015). In mouse embryonic lungs, ablation of epithelial HS decreases the biological activity of Shh in the underlying mesenchyme and causes lung dysplasia through Shh-Fgf signaling (He et al., 2017). Therefore, we sought to determine whether lack of epithelial HS affects Hh, Bmp and Wnt signaling pathways during stomach morphogenesis. Intriguingly, mutant embryos display a moderate but significant reduction in Hh and Bmp signaling at E14.5. Given that forestomach morphology is largely normal in the Fgfr2b mutant (Spencer-Dene et al., 2006), the stratification and differentiation defect in the Ext1 mutant might be caused by disruption of other signaling pathways. For example, Shh-Bmp signaling has been demonstrated to be important in squamous epithelial stratification during ureter development (Bohnenpoll et al., 2017). As a result of the pleotropic effects of HS on morphogen signaling pathways, it is highly likely that the complex stomach phenotypes of the Ext1 mutant may be caused by a combination of defects in multiple signaling pathways.
On the basis of these data, we conclude that epithelial HS has multiple functions on the development of the embryonic stomach. In the hindstomach, epithelial HS regulates glandular stomach patterning and gastric cytodifferentiation by promoting Fgf-Erk signaling, which is required for restriction of Sox2 expression. In the forestomach, HS is required for squamous epithelium stratification and Hh-Bmp signaling activation (Fig. 7F). Our data may help to enhance our comprehension of the functional roles of HSPGs in tissue morphogenesis and provide some clues for identifying molecular mechanisms for stomach diseases.
MATERIALS AND METHODS
Mice
Ext1flox mice (stock no. 011699-UCD) from Mutant Mouse Resource Research Centers (MMRRC) were previously described (Zhang et al., 2015). Shh-Cre (stock no.005622) and Shh-CreERT2 (stock no.005623) mice were obtained from Jackson Laboratory (Harfe et al., 2004). Ext1fl/fl; Shh-Cre (Ext1Δshh) mice were generated by crossing Ext1fl/+; Shh-Cre males with Ext1fl/fl females. Ext1fl/fl; Shh-CreERT2 (Ext1ΔshhER) mice were generated in a similar way and submitted to tamoxifen (75 mg kg−1, Sigma, T5648) administration on E16.5 and E17.5. The morning of a vaginal plug present was regarded as embryonic day (E) 0.5, and mice were euthanized at indicated developmental stages. Mice were handled in accordance with institutional guidelines, and the animal protocols were approved by the Ethical Review Committee of the Institute of Zoology, Chinese Academy of Sciences, China.
Immunohistochemistry and histology
Stomachs from embryos were fixed in 4% paraformaldehyde overnight at 4°C. Tissues were dehydrated through a series of ethanol, immersed in xylene and then embedded in paraffin. For immunostaining, paraffin sections were cut at 5 μm. The sections were deparaffinized and rehydrated. Antigen retrieval was performed in sodium citrate buffer (pH 6.0), and endogenous peroxidase enzyme activity was blocked with 3% H2O2 for 15 min. After blocking with 10% goat serum, slides were incubated with primary antibodies overnight at 4°C. On the next day, following PBS washes, slides were incubated with Cy3- and Alexa Fluor 488-conjugated secondary antibodies (1:400, Jackson ImmunoResearch) at room temperature for 1 h. For p63, p-Erk, p-Smad, Sox2, Pdx1 and pepsinogen I staining, slides were incubated with biotin-conjugated anti-mouse, anti-goat or anti-rabbit IgG (1:200, Vector Labs). The ABC immunoperoxidase system (Vector Labs) was used, and then signals were developed by TSA amplification system (PerkinElmer) or DAB Peroxidase Substrate Kit (Vector Labs) for immunofluorescence or immunohistochemistry, respectively. Apoptosis was detected using a DeadEnd Fluorometric TUNEL Kit (Promega). All slides were examined and photographed using a Zeiss LSM 780 laser-scanning confocal microscope under the same laser exposure between control and mutant sections.
Primary antibodies against the following proteins were used: 3G10 (1:100, AMSBIO, 370260-s); Ctnnb1 (1:400, Cell Signaling Technology, 8814); Krt5 (1:100, Covance, PRB-160P); pHH3 (1:200, Cell Signaling Technology, 9701); BrdU (1:200, Millipore, mab3510); Sox2 (1:500, Abcam, ab97959); Sox2 (1:50, Santa Cruz, sc-17320); Gata4 (1:100, Santa Cruz, sc-25310); Pdx1 (1:300, Abcam, ab47267); Pepsinogen I (1:80, Bio-Rad, 7240-1009); Chromogranin A (1:200, Santa Cruz, sc13090); Villin (1:500, Millipore, mab1671); α-SMA (1:200, Zsbio, ZM0003); Tuj1 (1:500, Sigma, t2200); Vimentin (1:200, Santa Cruz, sc7558); p-Erk (1:200, Cell Signaling Technology, 4370); p63 (1:200, Abcam, ab735); Involucrin (1:100, rabbit, Abcam, ab53112); p-Smad1/5/9 (1:200, Cell Signaling Technology, 13820).
For BrdU incorporation, pregnant mice were injected intraperitoneally with BrdU (100 mg kg−1 body weight) and euthanized 2 h later for analysis. Samples were processed as described above. Paraffin sections were treated with 2 N HCl at 37°C for 30 min and washed in PBS before blocking. Then, the standard immunostaining protocol was applied for the following steps.
RNA in situ hybridization (ISH)
DIG-labeled riboprobes were generated from murine cDNA clone using the DIG RNA Labeling Kit (Roche) followed by RNA purification. Sense riboprobes transcribed by T3 RNA polymerase were used as a negative control. ISH for paraffin sections was performed as previously described with some modifications (Kim et al., 2011). Briefly, 10 μm paraffin sections were cut, deparaffinized and rehydrated. Samples were permeabilized with 20 μg ml−1 proteinase K (Promega) at 37°C for 10 min, then washed with PBS and fixed in 4% paraformaldehyde for 10 min. Samples were hybridized with RNA riboprobes overnight at 65°C. After hybridization, slides were washed twice for 30 min each with 50% formamide/2× SSC, 2× SSC, 0.2× SSC and MA buffer (100 mM maleic acid, 150 mM NaCl, pH 7.5) at 65°C. Then, they were washed with PBST (PBS with 0.1% Tween 20) and blocked for 2 h in blocking buffer (Roche) at 4°C. Tissues were incubated with AP-conjugated anti-DIG antibody (1:600, Roche, 11093274910) at 4°C overnight. Next day, slides were washed with PBS and NTMT buffer (0.1 M NaCl, 0.1 M Tris-HCl, pH 9.5, 50 mM MgCl2, 0.5% Tween 20), then stained with BM Purple (Roche). Primers for RNA riboprobes are listed in Table S1. Whole-mount in situ hybridization on isolated stomach tissues was conducted using an optimized protocol (Piette et al., 2008).
Western blotting
Stomachs were dissected from embryos in ice-cold PBS and homogenized in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing proteinase and phosphatase inhibitor cocktails (Roche). Tissue lysates were reduced in the loading buffer. Approximately 100 μg of total proteins for each sample were subjected to 10% SDS-PAGE gel, transferred to PVDF membranes and blocked for 1 h at room temperature. Primary antibodies were incubated in 5% nonfat dried milk in TBST overnight at 4°C: antibodies against Erk (1:1000, Cell Signaling Technology, 4695); p-Erk (1:1000, Cell Signaling Technology, 4370); p-Smad1/5/9 (1:1000, Cell Signaling Technology, 13820); Smad1 (1:1000, Santa Cruz, sc-81378); and β-actin (1:2000, CWBIO, CW0096M). After washing with TBST, membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 1 h. Chemiluminescent signals were developed by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA) and images were captured by ImageQuant LAS 4000 (GE Healthcare Life Sciences). Band densities were determined using ImageJ (NIH, Bethesda, MD, USA). Images of western blots are representative of at least three independent experiments.
RNA isolation and qPCR
Total RNA was extracted in Trizol (Invitrogen) according to the product manuals. 1 μg RNA was reverse transcribed to cDNA using GoScript Reverse Transcription System (Promega). qPCR was carried out using GoTaq qPCR Master Mix (Promega) and mRNA expression was determined by CFX-96 Detection System (Bio-Rad). Expression levels were normalized to Gapdh. Each sample was run in triplicate and at least three biological replicates were performed. Primer sequences are listed in Table S2.
Ligand and carbohydrate engagement (LACE) assays
LACE assay was performed as previously described (Allen and Rapraeger, 2003; Tao and Zhang, 2016). Briefly, paraffin sections were cut at the thickness of 10 μm. After deparaffinization and rehydration, slides were immersed in 0.5 mg ml−1 NaBH4 at room temperature for 10 min to block autofluorescence and then placed in 0.1 M glycine for 30 min. After washing with PBS three times, slides were blocked with 2% BSA in TBS for 1 h at room temperature. For Fgf-Fgfr2 binding assay, sections were incubated with 20 nM recombinant human FGF10 (R&D Systems, #345-FG) and 20 nM human recombinant Fgfr2α (IIIb)/Fc chimera (R&D Systems, #663-FR) in DMEM with 10% FBS (Gibco) overnight at 4°C. Alexa Fluor 488-conjugated anti-human Fc antibody (1:400, Jackson ImmunoResearch, 109-547-008) was applied to determine the binding of Fgfr. Sections were counterstained with DAPI to localize cell nuclei. This experiment was replicated three times.
In vitro organ culture
Stomach tissues were isolated from both control and mutant embryos at E11.5 and cultured on filters (Nucleopore Track-Etch Membrane, 0.8 µm; Whatman, Clifton, NJ, USA) in DMEM/F12 containing 1% penicillin-streptomycin and 100 µM non-essential amino acids (Invitrogen). Control organs were treated with or without Fgfr inhibitor SU5402 (SelleckChem) for 72 h. After culture, organs were harvested and fixed in 4% paraformaldehyde. Whole-mount ISH and immunofluorescence staining were performed to determine Sox2 expression. Images were acquired on a ZEISS stereotypical microscope. Organ culture experiments were performed independently three times (n=6 of each concentration; n=2 of mutant).
Statistical analysis
Values are presented as means±s.e.m. For comparative analysis, unpaired Student's t-test and graphs were performed using GraphPad Prism 6 software. P<0.05 was considered statistically significant; P<0.01 highly significant.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: M.H., H.H., X.L.; Methodology: M.H., H.H.; Investigation: M.H., H.H.; Writing - original draft: M.H.; Writing - review & editing: H.H., T.B., X.L.; Supervision: X.L.; Funding acquisition: X.L.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31730044 and 31571507) to X.L. and National Institutes of Health (R01GM115995 and R01HL136722) to X.L. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.210781.supplemental
References
- Allen B. L. and Rapraeger A. C. (2003). Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly. J. Cell Biol. 163, 637-648. 10.1083/jcb.200307053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J., Dery U., Lemieux M., Chailler P. and Jeannotte L. (2002). Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development 129, 4075-4087. [DOI] [PubMed] [Google Scholar]
- Balasubramanian R. and Zhang X. (2016). Mechanisms of FGF gradient formation during embryogenesis. Semin. Cell Dev. Biol. 53, 94-100. 10.1016/j.semcdb.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. R., Schuksz M. and Esko J. D. (2007). Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030-1037. 10.1038/nature05817 [DOI] [PubMed] [Google Scholar]
- Bitgood M. J. and McMahon A. P. (1995). Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172, 126-138. 10.1006/dbio.1995.0010 [DOI] [PubMed] [Google Scholar]
- Bohnenpoll T., Wittern A. B., Mamo T. M., Weiss A.-C., Rudat C., Kleppa M.-J., Schuster-Gossler K., Wojahn I., Lüdtke T. H.-W., Trowe M.-O. et al. (2017). A SHH-FOXF1-BMP4 signaling axis regulating growth and differentiation of epithelial and mesenchymal tissues in ureter development. PLoS Genet. 13, e1006951 10.1371/journal.pgen.1006951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. R., Mazot P. and Soriano P. (2016). Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 30, 751-771. 10.1101/gad.277137.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse M. and Kusche-Gullberg M. (2003). In vitro polymerization of heparan sulfate backbone by the EXT proteins. J. Biol. Chem. 278, 41333-41337. 10.1074/jbc.M308314200 [DOI] [PubMed] [Google Scholar]
- Busse M., Feta A., Presto J., Wilén M., Grønning M., Kjellén L. and Kusche-Gullberg M. (2007). Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J. Biol. Chem. 282, 32802-32810. 10.1074/jbc.M703560200 [DOI] [PubMed] [Google Scholar]
- Cai Z., Grobe K. and Zhang X. (2014). Role of heparan sulfate proteoglycans in optic disc and stalk morphogenesis. Dev. Dyn. 243, 1310-1316. 10.1002/dvdy.24142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmont A., Wandzioch E., Tremblay K. D., Minowada G., Kaestner K. H., Martin G. R. and Zaret K. S. (2006). An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev. Cell 11, 339-348. 10.1016/j.devcel.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Cardoso W. V. (2001). Molecular regulation of lung development. Annu. Rev. Physiol. 63, 471-494. 10.1146/annurev.physiol.63.1.471 [DOI] [PubMed] [Google Scholar]
- Daniely Y., Liao G., Dixon D., Linnoila R. I., Lori A., Randell S. H., Oren M. and Jetten A. M. (2004). Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 287, C171-C181. 10.1152/ajpcell.00226.2003 [DOI] [PubMed] [Google Scholar]
- Dorey K. and Amaya E. (2010). FGF signalling: diverse roles during early vertebrate embryogenesis. Development 137, 3731-3742. 10.1242/dev.037689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G. P., Xiao L., Cheng L., Wang X. H., Sun B. and Hu G. X. (2000). Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 467, 7-11. 10.1016/S0014-5793(00)01111-X [DOI] [PubMed] [Google Scholar]
- Fukamachi H., Mizuno T. and Takayama S. (1979). Epithelial-mesenchymal interactions in differentiation of stomach epithelium in fetal mice. Anat. Embryol. 157, 151-160. 10.1007/BF00305155 [DOI] [PubMed] [Google Scholar]
- Grobe K., Inatani M., Pallerla S. R., Castagnola J., Yamaguchi Y. and Esko J. D. (2005). Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development 132, 3777-3786. 10.1242/dev.01935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habuchi H., Nagai N., Sugaya N., Atsumi F., Stevens R. L. and Kimata K. (2007). Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J. Biol. Chem. 282, 15578-15588. 10.1074/jbc.M607434200 [DOI] [PubMed] [Google Scholar]
- Häcker U., Nybakken K. and Perrimon N. (2005). Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol. 6, 530-541. 10.1038/nrm1681 [DOI] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P. and Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528. 10.1016/j.cell.2004.07.024 [DOI] [PubMed] [Google Scholar]
- He H., Huang M., Sun S., Wu Y. and Lin X. (2017). Epithelial heparan sulfate regulates Sonic Hedgehog signaling in lung development. PLoS Genet. 13, e1006992 10.1371/journal.pgen.1006992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges J. C., Verheyden J. M., Zhang Z., Sui P. F., Zhang Y., Anderson M. J., Swing D. A., Zhang Y., Lewandoski M. and Sun X. (2015). FGF-regulated ETV transcription factors control FGF-SHH feedback loop in lung branching. Dev. Cell 35, 322-332. 10.1016/j.devcel.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M., Irie F., Plump A. S., Tessier-Lavigne M. and Yamaguchi Y. (2003). Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science 302, 1044-1046. 10.1126/science.1090497 [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. and Schaefer L. (2015). Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11-55. 10.1016/j.matbio.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izvolsky K. I., Shoykhet D., Yang Y., Yu Q., Nugent M. A. and Cardoso W. V. (2003a). Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev. Biol. 258, 185-200. 10.1016/S0012-1606(03)00114-3 [DOI] [PubMed] [Google Scholar]
- Izvolsky K. I., Zhong L., Wei L., Yu Q., Nugent M. A. and Cardoso W. V. (2003b). Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L838-L846. 10.1152/ajplung.00081.2003 [DOI] [PubMed] [Google Scholar]
- Izvolsky K. I., Lu J. N., Martin G., Albrecht K. H. and Cardoso W. V. (2008). Systemic inactivation of Hs6st1 in mice is associated with late postnatal mortality without major defects in organogenesis (vol 46, pg 8, 2008). Genesis 46, 574-574. 10.1002/dvg.20455 [DOI] [PubMed] [Google Scholar]
- Jacobsen C. M., Narita N., Bielinska M., Syder A. J., Gordon J. I. and Wilson D. B. (2002). Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev. Biol. 241, 34-46. 10.1006/dbio.2001.0424 [DOI] [PubMed] [Google Scholar]
- Jacobsen C. M., Mannisto S., Porter-Tinge S., Genova E., Parviainen H., Heikinheimo M., Adameyko I. I., Tevosian S. G. and Wilson D. B. (2005). GATA-4:FOG interactions regulate gastric epithelial development in the mouse. Dev. Dyn. 234, 355-362. 10.1002/dvdy.20552 [DOI] [PubMed] [Google Scholar]
- Kalus I., Salmen B., Viebahn C., von Figura K., Schmitz D., D'Hooge R. and Dierks T. (2009). Differential involvement of the extracellular 6-O-endosulfatases Sulf1 and Sulf2 in brain development and neuronal and behavioural plasticity. J. Cell. Mol. Med. 13, 4505-4521. 10.1111/j.1582-4934.2008.00558.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam S. M., Li Q. T. and Gordon J. I. (1997). Gastric epithelial morphogenesis in normal and transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 272, G1209-G1220. 10.1152/ajpgi.1997.272.5.G1209 [DOI] [PubMed] [Google Scholar]
- Kim T.-H. and Shivdasani R. A. (2016). Stomach development, stem cells and disease. Development 143, 554-565. 10.1242/dev.124891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Hebrok M., Li E., Oh S. P., Schrewe H., Harmon E. B., Lee J. S. and Melton D. A. (2000). Activin receptor patterning of foregut organogenesis. Genes Dev. 14, 1866-1871. [PMC free article] [PubMed] [Google Scholar]
- Kim B.-T., Kitagawa H., Tanaka J., Tamura J.-I. and Sugahara K. (2003). In vitro heparan sulfate polymerization - crucial roles of core protein moieties of primer substrates in addition to the EXT1-EXT2 interaction. J. Biol. Chem. 278, 41618-41623. 10.1074/jbc.M304831200 [DOI] [PubMed] [Google Scholar]
- Kim B.-M., Buchner G., Miletich I., Sharpe P. T. and Shivdasani R. A. (2005a). The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev. Cell 8, 611-622. 10.1016/j.devcel.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Huang Z. and Mo R. (2005b). Gli3 null mice display glandular overgrowth of the developing stomach. Dev. Dyn. 234, 984-991. 10.1002/dvdy.20542 [DOI] [PubMed] [Google Scholar]
- Kim T.-H., Kim B.-M., Mao J., Rowan S. and Shivdasani R. A. (2011). Endodermal Hedgehog signals modulate Notch pathway activity in the developing digestive tract mesenchyme. Development 138, 3225-3233. 10.1242/dev.066233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T. and Yasugi S. (1999). In vitro analysis of mesenchymal influences on the differentiation of stomach epithelial cells of the chicken embryo. Differentiation 65, 13-25. 10.1046/j.1432-0436.1999.6510013.x [DOI] [PubMed] [Google Scholar]
- Koster M. I., Kim S., Mills A. A., DeMayo F. J. and Roop D. R. (2004). p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 18, 126-131. 10.1101/gad.1165104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraushaar D. C., Rai S., Condac E., Nairn A., Zhang S., Yamaguchi Y., Moremen K., Dalton S. and Wang L. (2012). Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J. Biol. Chem. 287, 22691-22700. 10.1074/jbc.M112.368241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledin J., Staatz W., Li J.-P., Götte M., Selleck S., Kjellén L. and Spillmann D. (2004). Heparan sulfate structure in mice with genetically modified heparan sulfate production. J. Biol. Chem. 279, 42732-42741. 10.1074/jbc.M405382200 [DOI] [PubMed] [Google Scholar]
- Lin X. (2004). Functions of heparan sulfate proteoglycans in cell signaling during development. Development 131, 6009-6021. 10.1242/dev.01522 [DOI] [PubMed] [Google Scholar]
- Lin X. and Perrimon N. (2000). Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biol. 19, 303-307. 10.1016/S0945-053X(00)00073-1 [DOI] [PubMed] [Google Scholar]
- Lin X., Wei G., Shi Z., Dryer L., Esko J. D., Wells D. E. and Matzuk M. M. (2000). Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev. Biol. 224, 299-311. 10.1006/dbio.2000.9798 [DOI] [PubMed] [Google Scholar]
- Mao J., Kim B. M., Rajurkar M., Shivdasani R. A. and McMahon A. P. (2010). Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137, 1721-1729. 10.1242/dev.044586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara Y., Ichinose M., Tatematsu M., Ichinose M., Oka M., Yahagi N., Kurokawa K., Kageyama T., Miki K. and Fukamachi H. (1996). Stage-specific elevated expression of the genes for hepatocyte growth factor, keratinocyte growth factor, and their receptors during the morphogenesis and differentiation of rat stomach mucosa. Biochem. Biophys. Res. Commun. 222, 669-677. 10.1006/bbrc.1996.0802 [DOI] [PubMed] [Google Scholar]
- Matsuo I. and Kimura-Yoshida C. (2013). Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Curr. Opin. Genet. Dev. 23, 399-407. 10.1016/j.gde.2013.02.004 [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Aizawa S. and Shimono A. (2009). Sfrp controls apicobasal polarity and oriented cell division in developing Gut epithelium. PLoS Genet. 5, e1000427 10.1371/journal.pgen.1000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken K. W. and Wells J. M. (2017). Mechanisms of embryonic stomach development. Semin. Cell Dev. Biol. 66, 36-42. 10.1016/j.semcdb.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken K. W., Aihara E., Martin B., Crawford C. M., Broda T., Treguier J., Zhang X., Shannon J. M., Montrose M. H. and Wells J. M. (2017). Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature 541, 182-187. 10.1038/nature21021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe V., Ford M. D., Wildschut J. A. and Bartlett P. F. (1993). Developmental regulation of neural response to Fgf-1 and Fgf-2 by Heparan-sulfate proteoglycan. Science 260, 103-106. 10.1126/science.7682010 [DOI] [PubMed] [Google Scholar]
- Nyeng P., Norgaard G. A., Kobberup S. and Jensen J. (2007). FGF10 signaling controls stomach morphogenesis. Dev. Biol. 303, 295-310. 10.1016/j.ydbio.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A., Bedford M. T., Burakova T., Arman E., Zimmer Y., Yayon A., Givol D. and Lonai P. (1993). Developmental localization of the splicing alternatives of Fibroblast Growth-Factor Receptor-2 (Fgfr2). Dev. Biol. 158, 475-486. 10.1006/dbio.1993.1205 [DOI] [PubMed] [Google Scholar]
- Pan Y., Woodbury A., Esko J. D., Grobe K. and Zhang X. (2006). Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133, 4933-4944. 10.1242/dev.02679 [DOI] [PubMed] [Google Scholar]
- Pan Y., Carbe C., Powers A., Zhang E. E., Esko J. D., Grobe K., Feng G.-S. and Zhang X. (2008). Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development 135, 301-310. 10.1242/dev.014829 [DOI] [PubMed] [Google Scholar]
- Pan Y., Carbe C., Kupich S., Pickhinke U., Ohlig S., Frye M., Seelige R., Pallerla S. R., Moon A. M., Lawrence R. et al. (2014). Heparan sulfate expression in the neural crest is essential for mouse cardiogenesis. Matrix Biol. 35, 253-265. 10.1016/j.matbio.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette D., Hendrickx M., Willems E., Kemp C. R. and Leyns L. (2008). An optimized procedure for whole-mount in situ hybridization on mouse embryos and embryoid bodies. Nat. Protoc. 3, 1194-1201. 10.1038/nprot.2008.103 [DOI] [PubMed] [Google Scholar]
- Poulain F. E. and Yost H. J. (2015). Heparan sulfate proteoglycans: a sugar code for vertebrate development? Development 142, 3456-3467. 10.1242/dev.098178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Pan Y., Carbe C., Powers A., Grobe K. and Zhang X. (2012). Glycosaminoglycan-dependent restriction of FGF diffusion is necessary for lacrimal gland development. Development 139, 2730-2739. 10.1242/dev.079236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M., Melton D. A. and McMahon A. P. (2000). Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127, 2763-2772. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A. and Olwin B. B. (1991). Requirement of Heparan-sulfate for Bfgf-mediated fibroblast growth and myoblast differentiation. Science 252, 1705-1708. 10.1126/science.1646484 [DOI] [PubMed] [Google Scholar]
- Ringvall M., Ledin J., Holmborn K., van Kuppevelt T., Ellin F., Eriksson I., Olofsson A.-M., Kjellén L. and Forsberg E. (2000). Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J. Biol. Chem. 275, 25926-25930. 10.1074/jbc.C000359200 [DOI] [PubMed] [Google Scholar]
- Rodriguez P., Da Silva S., Oxburgh L., Wang F., Hogan B. L. M. and Que J. W. (2010). BMP signaling in the development of the mouse esophagus and forestomach. Development 137, 4171-4176. 10.1242/dev.056077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano R.-A., Smalley K., Magraw C., Serna V. A., Kurita T., Raghavan S. and Sinha S. (2012). DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 139, 772-782. 10.1242/dev.071191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S., Lamanna W. C. and Esko J. D. (2011). Heparan sulfate proteoglycans. Cold Spring Harbor Perspect. Biol. 3, a004952 10.1101/cshperspect.a004952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa K., Kimura-Yoshida C., Nagai N., Mukai K., Matsubara K., Watanabe H., Matsuda Y., Mochida K. and Matsuo I. (2011). Cell surface Heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell 21, 257-272. 10.1016/j.devcel.2011.06.027 [DOI] [PubMed] [Google Scholar]
- Shin M., Noji S., Neubüser A. and Yasugi S. (2006). FGF10 is required for cell proliferation and gland formation in the stomach epithelium of the chicken embryo. Dev. Biol. 294, 11-23. 10.1016/j.ydbio.2005.12.019 [DOI] [PubMed] [Google Scholar]
- Shyer A. E., Huycke T. R., Lee C. H., Mahadevan L. and Tabin C. J. (2015). Bending gradients: how the intestinal stem cell gets its home. Cell 161, 569-580. 10.1016/j.cell.2015.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Dene B., Sala F. G., Bellusci S., Gschmeissner S., Stamp G. and Dickson C. (2006). Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology 130, 1233-1244. 10.1053/j.gastro.2006.02.018 [DOI] [PubMed] [Google Scholar]
- Stickens D., Zak B. M., Rougier N., Esko J. D. and Werb Z. (2005). Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132, 5055-5068. 10.1242/dev.02088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strate I., Tessadori F. and Bakkers J. (2015). Glypican4 promotes cardiac specification and differentiation by attenuating canonical Wnt and Bmp signaling. Development 142, 1767-1776. 10.1242/dev.113894 [DOI] [PubMed] [Google Scholar]
- Tao C. Q. and Zhang X. (2016). Retinal proteoglycans act as cellular receptors for basement membrane assembly to control astrocyte migration and angiogenesis. Cell Rep. 17, 1832-1844. 10.1016/j.celrep.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. A., DeLaForest A. and Battle M. A. (2018). Patterning the gastrointestinal epithelium to confer regional-specific functions. Dev. Biol. 435, 97-108. 10.1016/j.ydbio.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venero Galanternik M. V., Kramer K. L. and Piotrowski T. (2015). Heparan sulfate proteoglycans regulate Fgf signaling and cell polarity during collective cell migration. Cell Rep. 10, 414-428. 10.1016/j.celrep.2014.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi M. P., Stanfel M. N., Moses K. A., Kim B.-M., Zhang Y., Schwartz R. J., Shivdasani R. A. and Zimmer W. E. (2009). Role of the homeodomain transcription factor Bapx1 in mouse distal stomach development. Gastroenterology 136, 1701-1710. 10.1053/j.gastro.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., Campbell A., Dill E., Li C. G., Minoo P. and De Langhe S. (2013). Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 140, 3731-3742. 10.1242/dev.096560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. X., Dollé P., Cardoso W. V. and Niederreither K. (2006). Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 297, 433-445. 10.1016/j.ydbio.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Wang X., Ouyang H., Yamamoto Y., Kumar P. A., Wei T. S., Dagher R., Vincent M., Lu X., Bellizzi A. M., Ho K. Y. et al. (2011). Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell 145, 1023-1035. 10.1016/j.cell.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. M. and Melton D. A. (2000). Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development 127, 1563-1572. [DOI] [PubMed] [Google Scholar]
- Yan D. and Lin X. H. (2009). Shaping morphogen gradients by proteoglycans. Cold Spring Harbor Perspect. Biol. 1, a002493 10.1101/cshperspect.a002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Schweitzer R., Sun D., Kaghad M., Walker N., Bronson R. T., Tabin C., Sharpe A., Caput D., Crum C. et al. (1999). p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714-718. 10.1038/19539 [DOI] [PubMed] [Google Scholar]
- Zhang B., Xiao W., Qiu H., Zhang F., Moniz H. A., Jaworski A., Condac E., Gutierrez-Sanchez G., Heiss C., Clugston R. D. et al. (2014). Heparan sulfate deficiency disrupts developmental angiogenesis and causes congenital diaphragmatic hernia. J. Clin. Investig. 124, 209-221. 10.1172/JCI71090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Cao P., Yang Z., Wang Z., Wu J.-L., Chen Y. and Pan Y. (2015). Heparan sulfate biosynthesis enzyme, Ext1, contributes to outflow tract development of mouse heart via modulation of FGF signaling. PLoS ONE 10, e0136518 10.1371/journal.pone.0136518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn A. M. and Wells J. M. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221-251. 10.1146/annurev.cellbio.042308.113344 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.