An international initiative takes conservation planning into the deep ocean to inform environmental management of deep-sea mining.
Abstract
Mineral exploitation has spread from land to shallow coastal waters and is now planned for the offshore, deep seabed. Large seafloor areas are being approved for exploration for seafloor mineral deposits, creating an urgent need for regional environmental management plans. Networks of areas where mining and mining impacts are prohibited are key elements of these plans. We adapt marine reserve design principles to the distinctive biophysical environment of mid-ocean ridges, offer a framework for design and evaluation of these networks to support conservation of benthic ecosystems on mid-ocean ridges, and introduce projected climate-induced changes in the deep sea to the evaluation of reserve design. We enumerate a suite of metrics to measure network performance against conservation targets and network design criteria promulgated by the Convention on Biological Diversity. We apply these metrics to network scenarios on the northern and equatorial Mid-Atlantic Ridge, where contractors are exploring for seafloor massive sulfide (SMS) deposits. A latitudinally distributed network of areas performs well at (i) capturing ecologically important areas and 30 to 50% of the spreading ridge areas, (ii) replicating representative areas, (iii) maintaining along-ridge population connectivity, and (iv) protecting areas potentially less affected by climate-related changes. Critically, the network design is adaptive, allowing for refinement based on new knowledge and the location of mining sites, provided that design principles and conservation targets are maintained. This framework can be applied along the global mid-ocean ridge system as a precautionary measure to protect biodiversity and ecosystem function from impacts of SMS mining.
INTRODUCTION
Mid-ocean ridges are located at divergent oceanic plate boundaries, where volcanism associated with seafloor spreading creates new oceanic crust. In these regions, seawater percolates through seafloor cracks and fissures to depths where it reacts with host rock at high temperature and pressure, stripping the rock of metals such as copper and zinc. The heated, chemically modified fluid is thermally buoyant and rises to exit the seafloor through hydrothermal vents, where metal sulfides precipitate and can accumulate as seafloor massive sulfides (SMS; also referred to as polymetallic sulfides). Where uplifted and exposed as ophiolite complexes on land, SMS deposits have long been exploited for their ores (1). They are now targeted for mining at the seabed (2). At slow seafloor spreading rates (<4 cm year−1), SMS deposits may accumulate over thousands of years and can be of sufficient size and ore quality to be of commercial interest (2, 3). Some large SMS deposits on the seabed are located at “active” hydrothermal vents, operationally defined as vents that emit diffuse and/or focused hydrothermal fluid and support symbiont-hosting invertebrate taxa that rely on uptake of inorganic compounds in the hydrothermal fluid to support microbial chemosynthesis (4). Large inactive, or “extinct” SMS accumulations on mid-ocean ridges are less studied than active vent systems. They generally lack biomass-rich assemblages of vent-endemic taxa but likely support highly diverse and complex benthic communities (5, 6). SMS deposits at inactive vents may be the preferred target for commercial mining based on environmental considerations (7), estimated size of the ore bodies (8–10), and the practicalities of avoiding equipment exposure to the high-temperature, acidic conditions at active vents (11).
The United Nations Convention on the Law of the Sea (UNCLOS) sets out the legal framework for seabed mining beyond the limits of national jurisdiction (referred to as “the Area”). The convention, along with the 1994 Implementing Agreement, established the International Seabed Authority (ISA) as the regulatory agency for deep-sea mining in the Area. The ISA is also charged with, among other things, ensuring effective protection of the marine environment from harmful effects arising from mining-related activities on the seabed (UNCLOS article 145). These responsibilities include the need to adopt and periodically review environmental rules, regulations, and procedures for the prevention, reduction, and control of pollution and other hazards to the marine environment, the protection and conservation of the natural resources of the Area, and the prevention of damage to the flora and fauna of the marine environment (UNCLOS article 145). Current regulatory efforts by the ISA focus on three mineral resources: SMS on mid-ocean ridges, polymetallic nodules on abyssal plains, and ferromanganese crusts on seamounts. Each occurs in different geological and ecological settings, with ecosystem processes that operate on different spatial and temporal scales (12) and with communities with varying degrees of resilience to mining activities (13). Environmental impacts from exploitation of SMS deposits are predicted to include loss of biological diversity resulting from direct habitat destruction and modification of vent fluid geochemistry, as well as degradation of surrounding benthic and pelagic environments through indirect impacts such as toxic and particle-rich sediment plumes, noise, vibration, and light created by the mining activity (4, 12, 14, 15). Any given SMS mine site on a mid-ocean ridge will encompass only a small area, with direct impacts covering up to a few square kilometers, but a series of small mines may be required to provide an overall profitable enterprise within a single mining contract area (3). Potential cumulative impacts of multiple or long-duration SMS mining events on regional scales are of concern. These impacts will result from direct and indirect effects and include disruption of population connectivity, loss of ecosystem functions and services, and the potential for regional and global extinctions (4).
To address potential impacts from deep-sea mining, the ISA is developing regional environmental management plans (EMPs) as a best practice (16). In 2012, the ISA approved its first EMP (17) for abyssal polymetallic nodule fields in the Clarion-Clipperton Zone (CCZ) in the central Pacific Ocean. The goals of the CCZ-EMP include facilitation of exploitation and cooperative research, monitoring of the environment, area-based management, application of an ecosystem-based approach to management, and broad stakeholder participation. Area-based planning to support management of the Area through EMPs should include, but should not be limited to, the design of networks of no-mining areas, consideration of vulnerable habitats at risk of serious harm outside of these conservation areas, and the identification of preservation and impact reference (18).
Operationally, the CCZ-EMP uses a network of no-mining areas (referred to by the ISA and, herein, as “Areas of Particular Environmental Interest” or APEIs) for preservation of unique and representative ecosystems and for protection of biodiversity and ecosystem structure and function (17). APEI networks contribute to a precautionary approach to environmental management of deep-sea mining by ensuring that representative benthic habitats and associated ecosystems are protected from serious harm on regional scales, particularly given uncertainties regarding the severity, frequency, and spatial extent of mining impacts (16). Establishment of these conservation areas does not preclude the need for additional regional environmental management actions that consider both benthic and pelagic ecosystems including, inter alia, environmental impact assessments, site-based conservation, transparent monitoring, and mitigation measures (18).
The CCZ-EMP adopts principles for area-based conservation used elsewhere (19) as elaborated by Wedding et al. (16, 20). These include “the principle that 30 to 50% of the total management area should be protected, that the network of protected areas should capture the full range of habitats and communities, and that each [APEI] should be large enough to maintain minimum viable population sizes for species potentially restricted to a subregion” (21). The APEI network design process for the CCZ polymetallic nodule beds used a regional benthic classification system where, in the absence of detailed data on the composition and distribution of benthic communities, surrogate measures and drivers of alpha and beta diversity, such as nodule abundance, particulate organic carbon (POC) flux to the seafloor, seamount distributions, bathymetry, and macrobenthic abundance, were assessed in the context of existing mining exploration claims. Biophysical surrogates of biodiversity have also been used to aid design of conservation networks [for example, in the Northeast Atlantic (22)] and have been tested at least once and proven to be effective (23). Through this surrogate approach, the CCZ was divided into nine representative subregions, each with a “no-mining” APEI of sufficient area (400 km × 400 km comprising a 200 km × 200 km core area surrounded by a 100-km-wide buffer zone) to support self-sustaining populations in each APEI core (20). To avoid overlap with existing exploration claim areas, the ISA positioned two of the APEIs from subregions within the core of the CCZ to the CCZ periphery (www.isa.org.jm/files/images/maps/CCZ-Sep2012-Official.jpg) (20). Together, the nine APEIs represent ~24% of the total CCZ management area. At the 22nd Session of the ISA in 2016, consideration was given to creation of two additional APEIs in the CCZ region, which would yield a total APEI coverage of ~29% of the CCZ management area.
The United Nations General Assembly (UNGA), in its resolution 68/70 adopted in 2013, encouraged the ISA to develop and approve EMPs for other seabed regions with potential to support deep-sea mining, in particular regions where exploration contracts had been granted. The UNGA reiterated this recommendation in subsequent annual resolutions on oceans and law of the sea (UNGA 69/245 and UNGA 70/235). The ISA followed with a call for EMPs “in particular where there are currently exploration contracts” (Council decisions ISBA/20/C/1 §9, ISBA/21/C/20 §10, and ISBA/22/C/28 §11). The ISA has yet to consider a regional EMP for any SMS deposits but has encouraged the scientific community to support the development of these EMPs. In response, an international initiative was begun in 2015 to advance a framework for the development of networks of APEIs on mid-ocean ridges using a portion of the Mid-Atlantic Ridge (MAR) as a case study. This region includes three SMS exploration contracts, covering a total area of 30,000 km2, granted by the ISA to France, the Russian Federation, and Poland (Fig. 1). This scientific initiative adopted an inclusive, expert-driven consultative process like that used for the CCZ APEI network design (20, 24). Two large international workshops were convened in June 2015 and November 2016 with deep-sea biologists, geospatial ecologists, lawyers, and mining contractors to discuss network designs. Supporting activities also fed into the workshops, including a comprehensive data report, a smaller working group that drafted design principles and assessed multiple network options, and outreach activities to obtain input from a larger scientific community. Through this process, a framework was developed for the design and assessment of various APEI network scenarios for the MAR. As reported below, this framework includes a conservation goal, specific conservation objectives and targets, and performance metrics.
Fig. 1. Study area and management context.
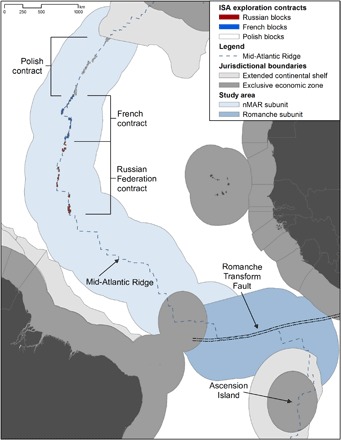
The case study area is centered on the ridge axis from the southern boundary of the Portuguese ECS claim to the northern boundary of the UK ECS claim at Ascension Island and extends 500 km to either side of the axis. Two management subunits are proposed here: nMAR and the RTF. Existing French, Polish, and Russian Federation exploration contracts for SMS are from the ISA database (www.isa.org.jm).
The CCZ-EMP served as a starting point for area-based planning for networks of no-mining areas on mid-ocean ridges. However, key features of ridge systems—including their quasi-linear nature, their along- and cross-axis bathymetric complexity, their complex and turbulent flow environments, and the patchy occurrence of hydrothermal vents and SMS on ridges—differ substantially from those of the abyssal plains of the CCZ and required de novo considerations for network design (25). A list of habitat indicators and biodiversity drivers on and around mid-ocean ridges was refined (table S1), and metrics for climate-change stressors based on model projections were introduced. In addition to the biodiversity variables of bathymetry and seamount distribution by Wedding et al. (20), this MAR case study included other variables for performance metrics, including biogeographic region, latitude, POC flux to the seafloor [replacing particulate organic nitrogen flux used by Wedding et al. (20)], slope, other habitat types (transform faults and hydrothermal vents), and future in situ environmental conditions (pH, temperature, dissolved O2 concentrations, and POC flux to the seafloor) derived from climate-change projections for the year 2100 (Table 1). Consideration was given to applying a more quantitative approach, including use of optimization tools such as MARXAN (26, 27), but given the limited available data on species distributions and alpha and beta diversity, a MARXAN or related approach would have conveyed a greater level of certainty with respect to the optimal placement of APEIs than is warranted. Furthermore, such an approach would indicate preferred placement of APEIs, which is counter to our intent to develop a framework and not to presuppose a specific solution before the ISA develops one.
Table 1. Network criteria, conservation targets, and metrics.
CBD network criteria (bold) including definitions quoted from CBD (29), metrics (italics), conservation targets, and metric equations used in this study, with relevant comments.
|
Network criteria Metrics |
Definitions and metric equations (normalized to 0 to 5 range) |
Conservation targets and comments |
| Important areas | “[Important Areas are] geographically or oceanographically discrete areas that provide important services to one or more species/populations of an ecosystem or to the ecosystem as a whole, compared to other surrounding areas…” |
|
| Major transform faults | APEI percent coverage/100% × 5. | The objective is to protect 100% of important areas. Scores are based on percent area conserved (for transition zones), percent by number of features conserved (for hybrid zones), and percent of length conserved (for transform faults). |
| Biogeographic transition zones | ||
| Genetic hybrid zones | ||
| Representativity | “Representativity is captured in a network when it consists of areas representing the different biogeographical subdivisions of the global oceans and regional seas that reasonably reflect the full range of ecosystems, including the biotic and habitat diversity of those marine ecosystems.” |
|
| Discrete habitat variables: Spreading ridge Active vents Inactive vents Fracture zones Seamounts |
APEI percent coverage/50% × 5, where any score greater than 5 was set to 5. |
The objective is to protect a representative amount (30 to 50%) of key habitat within the study region. Scores are based on percent area conserved (for spreading ridges), percent by number of features conserved (for active and inactive vents, and seamounts), and by percent of length conserved (for transform faults). |
| Note: Active hydrothermal vents and other vulnerable marine ecosystems are at risk of serious harm from SMS mining activities. We expect 100% of active hydrothermal vent ecosystems and other habitats at risk of serious harm to be protected through conservation measures, including, but not limited, to APEIs. | ||
| Continuous variables that describe the regional seascape: Slopes Depth Seafloor POC flux |
5 − (RMSE × 5) | The objective is to mimic the distribution of variables determined to be key drivers of biodiversity in proportion to their occurrence in the management subunit. Root mean square error (RMSE) was calculated as the difference between cumulative frequency distributions within the APEI scenario and the study region. All variables were classified into 10 to 15 bins to remove the effect of the number of bins on RMSE. |
| Connectivity | “Connectivity in the design of a network allows for linkages whereby protected sites benefit from larval and/or species exchanges, and functional linkages from other network sites. In a connected network individual sites benefit one another.” |
|
| Regional connectivity | 6 − (max distance between cores/75th percentile median dispersal distance), where any score greater than 5 was set to 5. |
The objective is to ensure that there is no major disruption to dispersal across the network of APEIs. The maximum distance between APEIs compared to median faunal dispersal distances is an indicator of the potential for disrupting dispersal within the entire management subunit. |
|
Network population persistence |
6 − mean gap ratio (that is, the mean distance between cores/mean core length), where any score greater than 5 was set to 5. |
The objective is to promote the viability of populations by self-seeding within APEIs and/or dispersal between APEIs. By minimizing the difference in length of APEI core areas versus distance between core areas, species that on average disperse beyond the APEI have a good chance of being able to disperse to adjacent APEIs. Minimizing this “gap ratio” should enhance persistence of species across the network, as well as within individual APEIs, and increase resilience across the network to localized disturbances. |
| Replication | “Replication of ecological features means that more than one site shall contain examples of a given feature in the given biogeographic area. The term “features” means “species, habitats and ecological processes” that naturally occur in the given biogeographic area.” |
|
| Replication | Number of APEIs where any score greater than 5 was set to 5. |
The objective is to have three to five replicate APEIs within a management unit, to decrease the likelihood of local catastrophes causing systemic biodiversity loss. |
| Viability and adequacy | “Adequate and viable sites indicate that all sites within a network should have size and protection sufficient to ensure the ecological viability and integrity of the feature(s) for which they were selected.” |
|
| Total area | (APEI percent coverage/50%) × 5, where any score greater than 5 was set to 5. |
The objective is to conserve an adequate portion (30 to 50%) of the management unit to ensure the viability of populations within it. Total area conserved is a proxy for overall adequacy of a network. The total area metric was calculated similarly to the habitat representativity metrics above. |
| Within APEI persistence | 5 × (APEI core length/200 km), where any score greater than 5 was set to 5. |
The objective is to ensure that APEIs are large enough to maintain minimum viable populations, and metapopulations, within a single APEI. The larger the APEI, the greater the probability self-recruitment within the APEI, and the lower the percentage of larval export from the APEI, which should enhance the persistence of populations, metapopulations, and communities within an APEI. 200 km was used as the minimum scale required to encompass two times the median dispersal distance of 75% of deep-sea fauna with known dispersal scales (53). |
|
Climate Change: Absolute similarity |
5 − (RMSE × 5) | The objective is to conserve areas where climate impacts would be minimized. The more close distributions of key climate variables (pH, temperature, dissolved O2 concentrations, and seafloor POC flux) in the future (that is, 2100) APEI cores mimic the current (that is, 2013) distribution in the management unit, the less impact is expected. RMSE was calculated as the difference between cumulative frequency distributions within the APEI scenario and the study region. All variables were classified into 10 to 15 bins to remove the effect of the number of bins on RMSE. |
|
Climate change: Relative local change |
(APEI percent coverage/50%) × 5, where any score greater than 5 was set to 5. |
The objective is to conserve 30 to 50% of the areas projected to be least affected by climate change. Least affected cells were defined as the 10% of cells with the lowest percent change between current (2013) and predicted (2100) values of the four key climate variables (pH, temperature, dissolved O2 concentrations, and POC flux to the seafloor). The percent of those cells falling in APEI cores for each scenario was calculated following the approach used for representativity metrics (continuously distributed variables). |
The development of the network of APEIs in the CCZ was based on scientific (ecological and biogeographic) principles and included both legal and socioeconomic considerations related to existing exploration contracts and commitments (20). Here, the linear nature of the mid-ocean ridge and the distribution of existing exploration contracts (Fig. 1) precluded the design of network of adequately sized and scientifically justifiable APEIs that avoided existing contracts. We thus use a solely science-based, ecological approach to adapt marine reserve design principles to the distinctive ridge setting. In doing so, we consider the APEI network design to be fungible, recognizing that mineral exploration will inform placement of networked APEIs that can meet conservation and exploitation objectives.
Article 4 of the Convention on Biological Diversity (CBD) states that the convention applies to Areas Beyond National Jurisdiction (ABNJ) “in the case of processes and activities, regardless of where their effects occur, carried out under its jurisdiction or control.” The CBD is also charged with “provision of scientific and, as appropriate, technical information and advice related to marine biological diversity” (28). In designing APEI network scenarios, we apply five network criteria identified by the CBD (29): important areas, representativity, connectivity, replication, and adequacy and viability. For each of these criteria, we propose conservation objectives for APEI networks (Box 1) that are used in an assessment of network performance. Our approach closely resembles that suggested in Annex III of the above CBD decision (albeit in a different order) and involved (i) delineation of a study area based on biogeographical considerations, (ii) identification of known ecologically or biologically important areas [analogous to Ecologically or Biologically Significant Areas (EBSAs) (29, 30)], (iii) iterative site selection, and (iv) consideration of ecological coherence (for example, ecological connectivity and viability), including viability under climate change. We then developed three network scenarios and assessed the performance of the scenarios. This approach allowed the development of scenarios that meet the current understanding of what an ecologically robust network of APEIs on a mid-ocean ridge would look like.
Box 1. Network criteria and conservation objectives for APEIs on a mid-ocean ridge based on CBD marine-protected area network criteria. Viability under climate change is newly integrated into the adequacy/viability criterion.
(1) Important areas
(a) Placement of APEIs within the network should capture areas considered to be ecologically and/or evolutionarily important based on best available science. APEIs should conserve 100% of identified important areas.
(2) Representativity
(a) APEI should conserve 30 to 50% of each habitat type (for example, the spreading ridge, seamounts, and transform faults) within each management unit.
(b) APEIs should be representative of the biophysical seascape (for example, depth, slope, and POC flux to the seafloor) within each management unit.
(3) Connectivity
(a) The APEI network should minimize the average and maximum distances between core areas to the greatest extent possible to conserve all dispersal scales and to ensure exchange across the entire network.
(4) Replication
(a) APEIs should be replicated within biogeographic provinces (where the size of the management unit permits) to capture along-axis variation in faunal composition and protect against localized catastrophes.
(5) Adequacy/viability
(a) The APEI network should protect 30 to 50% of the total management unit.
(b) Each APEI unit within the network should include a core area of sufficient length and width to maintain viable populations and ecosystem function.
(c) Each APEI unit within the network should include an appropriately sized buffer zone to protect core areas from indirect mining effects.
(d) Viability under climate change
(i) Projected biophysical conditions (temperature, pH, dissolved O2 concentrations, and POC flux to the seafloor) in APEIs should include the range of current conditions across the study area.
(ii) APEIs should include at least 30% of the area projected to be least affected by reasonable climate change scenarios (based on predicted changes in temperature, pH, dissolved O2 concentrations, and POC flux to the seafloor).
Although we focus our study on the northern and equatorial MAR, the general principles, design criteria, and evaluation approach should be applicable to mid-ocean ridge systems (and potentially other deep-sea settings) worldwide. Our intent was to develop a framework for the design and assessment of networks of no-mining areas based on internationally agreed conservation network criteria to inform the sustainable use of SMS mineral resources. While we consider networks of APEIs to be necessary elements of sustainable use of these resources, we emphasize that they are not sufficient on their own; additional environmental management tools will be needed to protect and preserve the marine environment. For mid-ocean ridges and exploitation of SMS deposits, one such additional tool may be site-based closures to protect all active hydrothermal vent ecosystems, which have been identified as vulnerable and at risk of serious harm (7, 12). Vulnerable marine ecosystems, including cold-water corals and sponges outside of APEIs, will also need protection. Non–area-based tools might include, for example, management of the frequency and timing of mining activities in a region or monitoring of environmental thresholds for turbidity and toxicity.
Building on the conservation goal reported by Wedding et al. (20) for the CCZ, the conservation goal for the design of an APEI network on the MAR is to contribute to “the protection of the natural diversity, ecosystem structure, function, connectivity, and resilience of deep-sea communities in the context of seabed mining in the region.”
RESULTS
Study area and biogeographic approach
To inform governance of deep-sea mining on the seafloor in the Area, the UNGA and ISA call for regional EMPs in areas that contain exploration contracts. We focus on ABNJ on the northern MAR with existing exploration contracts, and an extension to the south that illustrates how regional management units may be defined by biogeography. The study area is centered on the axis of the MAR and extends latitudinally from the southern boundary of the Portuguese extended continental shelf (ECS) claim at 32.84°N to the northern boundary of the UK ECS claim for Ascension Island at 02.43°S, exclusive of the Brazilian Exclusive Economic Zone (EEZ) (Fig. 1). The study area extends 500 km on either side of the axis of the MAR (unless restricted by national jurisdictions) to include the range of representative benthic habitats that might be affected by deep-sea mining of SMS or other seabed resources and provide a zone of sufficient size for population connectivity through larval dispersal.
To ground the study of ecological principles underpinning ecosystem-based management (31, 32), we apply a biogeographic approach using the most recent classification scheme for ocean floor biogeography (33). The primary management feature is the spreading axis of the MAR, which, for most of its length in the study area, is encompassed by the lower bathyal (800 to 3500 m) and abyssal (3501 to 6500 m) North Atlantic biogeographic provinces by Watling et al. (33). There is an isolated hadal (>6500 m) biogeographic unit [HD9 by Watling et al. (33)] and a bathyal (North Atlantic/South Atlantic) biogeographic transition zone at the southern margin of the study area. The study area was thus partitioned into two subunits: (i) the northern MAR (nMAR) subunit, north of the Brazilian EEZ, and (ii) the Romanche Transform Fault (RTF) subunit, south of the Brazilian EEZ (Fig. 1).
Identification of important areas
APEI network design should incorporate features of ecological importance. For the MAR, these features include (i) major transform faults that serve as conduits for deep-water circulation between west and east basins of the Atlantic (34, 35) and support a diverse set of habitats and fauna (36); (ii) transition zones between biogeographic units (so-called “biogeographic crossroads” or “suture zones”), where there is high species richness, beta diversity (37), and hybridization that may foster evolution (38); and (iii) recognized genetic hybrid zones [for example, Won et al. (39)]. As noted above, all active hydrothermal vent ecosystems on the mid-ocean ridge are vulnerable and at risk of serious harm and thus deserve protection (7, 12); some of these ecosystems will fall within APEI units, while the others will need to be protected through other area-based conservation measures.
Placement of APEIs in the MAR region was designed to capture the following important ecological features (Fig. 2A):
Fig. 2. Biogeographic context, important areas, and APEI scenarios.
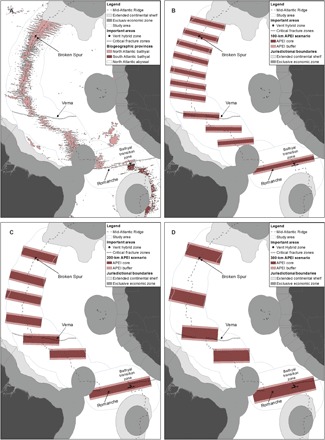
APEI scenarios were anchored by important areas identified by expert opinion before scenario development began. Important areas include (A) critical transform faults (that is, Vema and Romanche), biogeographic transition zones (that is, the bathyal transition zone in the region of the RTF), and genetic hybrid zones (that is, Broken Spur). Three APEI network scenarios were developed for the nMAR subunit, with core lengths along the ridge axis of (B) 100 km, (C) 200 km, and (D) 300 km; each APEI also has a 50-km buffer on the northern and southern sides of the core zone.
nMAR subunit:
(1) The Vema Transform Fault, a major water-mass transport pathway between the deep western and eastern Atlantic Basins (34) and an area with presumed reducing habitats as suggested by the record of the indicator species Abyssogena southwardae (Krylova et al., 2010).
(2) The hybrid zone at Broken Spur (39, 40). While multiple mussel hybrids are known along the MAR (the symbiont-bearing mussels Bathymodiolus azoricus and Bathymodiolus puteoserpensis), Broken Spur has the greatest proportion of hybrid individuals in a stabilized population with indications of local adaptation (41, 42); this region also corresponds to a biogeographic sub-boundary between northern “bathyal” and southern “abyssal” vent faunas (43).
RTF subunit:
(1) The bathyal biogeographic transition zone between the North Atlantic and South Atlantic units (33).
(2) The RTF, which includes a hadal biogeographic unit (33). The Romanche is a major transport pathway between the western and eastern Atlantic basins for dense water masses originating in polar regions (34, 35, 44). The proposed RTF subunit also overlaps substantively with the EBSA known as the “Atlantic Equatorial Fracture Zone and High Productivity System” (45).
Iterative site selection: Orientation, size, and spacing of APEI units
The cross-axis bathymetric profile of the MAR incudes a central axial valley with ridge flanks, canyons, seamounts, flat sedimented areas, and abyssal hills extending laterally from the axis. To capture cross-axis habitat heterogeneity, APEIs are recommended as rectangular bands with their length following the strike of the ridge axis and their width oriented perpendicular to the ridge axis. The cross-axis orientation of a banded-APEI approach also captures the special characteristics of transform faults, which represent extremes in depth and other environmental variables, including hydrographic regimes that support diverse deep-sea habitats and thus merit protection.
Latitudinal variation in POC flux to the seafloor (46, 47), a primary determinant of biodiversity and ecosystem structure and function in the deep sea (22, 48–51), indicates that a network of APEIs should be distributed along the entire length of the ridge axis in the study area to capture this and other latitudinal variations in biophysical characteristics. Such a network of APEIs provides replication that protects against catastrophic loss of habitat in any locality and increases demographic stability by promoting inter-APEI connectivity.
Core length along the ridge axis
APEIs consist of core and buffer areas, where mining should not occur. Each core should be large enough to maintain a minimum viable population size for a large percentage of deep-sea invertebrates through self-replenishment (20). The 75th percentile median dispersal distance for deep-sea benthic invertebrates is used to define the distance from the core-area center point required to capture ecological dispersal within the APEI. This distance is calculated from both genetic, reflecting evolutionary time scales (52), and larval dispersal models, reflecting contemporary time scales (53–55). These calculated distances were 103 km for vent invertebrates and 74 km for nonvent deep-sea invertebrates (52). A nominal 100-km dispersal distance thus captures estimated dispersal distances for vent and nonvent deep-sea invertebrate datasets, within the 75th percentile allowance. This 100-km dispersal distance matches the dispersal distance used in the APEI network design for the CCZ (20), but was derived using a new synthesis of dispersal distances for deep-sea (rather than shallow-water) organisms (52). As in the case of the CCZ-EMP, the length (and width) of the core conservation area is at least two times the median faunal dispersal distance (20, 56). This indicates that the minimum APEI core length along the ridge axis should be 200 km. Large-scale genetic connectivity over evolutionary time (57) is possibly the result of temporally discontinuous short-distance (for example, <26 km) dispersal mediated by stepping stone habitats (55).These short dispersal distances that occur discontinuously at contemporary time scales should also be contained well within the minimum core length of 200 km.
Core width across the ridge axis
The mid-ocean ridge has complex, cross-axis physical characteristics (including depth gradients and hydrographic regimes) that drive ecosystem processes, and there is evidence for differentiation in the faunal composition of the eastern and western flanks of the MAR (58, 59). Near-bed currents on the flanks of the ridge axis can be channeled in canyons and faults, resulting in a topographically forced flow toward the ridge crest (60, 61). Because large, buried SMS deposits are expected on the flanks of the ridge crest (3, 62–65), flow toward the ridge crest enhances the potential for mining plumes from flank SMS deposits to affect habitats closer to the crest. Where species’ distributions extend across ridge flanks, protecting cross-ridge swaths will be important for internal connectivity within an APEI. To capture representative habitats that vary with depth (from upper bathyal to abyssal) and other biophysical characteristics along the flanks (33), we extend the width of the core area to 500 km on either side of the ridge axis. Such an approach protects the bathyal-abyssal biogeographic transition areas on the ridge flanks and the ridge axis, helps meet the conservation target of 30 to 50% of each habitat type in the management unit, and accommodates future exploitation of buried SMS deposits and of other minerals on ridge flanks.
Buffer zones
SMS mining is expected to produce plumes of particulates at the seabed during mining activities and plumes at some height above the seabed during discharge of water and fine particles from the shipboard dewatering plant (12). While details of SMS mining plume structure and dispersion are not constrained well at present, SMS plumes are expected to affect a smaller region than those created by polymetallic nodule mining, where dispersion distances may extend to 100 km (66). Test mining of deep-sea sulfides was undertaken in 2017 off Japan, but the results of the associated environmental monitoring program have not yet been made publicly available. Given that passive particles suspended in the water at 1000 m on the MAR travel on average more than 2 km/day (based on Argo float data and models), we assume that plume dispersal may be on the order of tens of kilometers. Until more data are available on plume dispersal and toxicity, we use a buffer zone of 50 km on the northern and southern borders of the APEI cores. We assume an absence of exploitable mineral resources beyond 500 km on the western and eastern flanks of the ridge axis and thus do not indicate buffer zones on these borders of the core area.
Spacing
All conservation networks involve trade-offs between (i) promoting larval connectivity between closed areas (improved by smaller spacing between closures); (ii) providing spillover of larvae (or emigrants) from closed areas to unprotected areas, thus enhancing productivity/recovery outside protected zones (improved by creating many small closures); and (iii) maintenance of self-sustaining populations within APEI cores (improved by increasing the size of individual closures). We adopt a common design guideline for conservation networks, namely, to minimize the difference between the maximum dispersal distance protected by the core area and the distance between core areas (67). Using this approach, species with larval dispersal distances greater than the length of the core areas should be able to disperse to adjacent APEIs, while those with dispersal distances less than the core length are likely to maintain populations (including metapopulations) within a single APEI core. Consideration also needs to be given to the maximum distance between adjacent core areas. Large gaps between core areas can result in core areas effectively acting as separate units rather than as a network. To address this issue, we minimize the maximum distance between adjacent core areas to ensure network functionality. Spacing between APEIs is also necessarily affected by the overall percentage of the management unit to be protected (in this case, 30 to 50%).
nMAR management subunit APEI network design
On the basis of the size and spacing requirements outlined above, network scenarios of APEIs with 100-, 200-, and 300-km core lengths along the ridge axis (oriented approximately north-south), with 1000 km width (centered on the ridge axis), and spaced at distances as near as possible to the length of the APEI core were placed in the nMAR subunit (Fig. 2, B to D). These APEI network scenarios were “anchored” by two important areas identified on the nMAR: the Broken Spur hybrid zone and the Vema transform fault. While our premise is that the 200-km core length scenario is a minimum core length, the 100- and 300-km core length scenarios allow us to understand what ecological performance might be lost (or gained) by changing the core length of an APEI.
RTF management subunit
Assuming that APEI core lengths should be 200 km or more and the identification of the RTF as an important area, the areal extent of the RTF subunit does not allow for a replicated network of APEIs. We proposed a single APEI centered on the RTF. The width of the RTF APEI is extended to protect the full extent of the transform offset and the hadal biogeographic unit between the ridge axes. In addition, the APEI extends 500 km to either side of the adjacent northern and southern ridge axes, as for the nMAR APEIs.
APEI network performance assessment: nMAR management subunit
The guidelines for size and spacing of APEIs described above are based on scientific theory but do not guarantee that such a network would meet the network criteria set out by the CBD, that is, that the network would be ecologically coherent (68, 69). We assessed ecological coherence of APEI network scenarios with core lengths of 100, 200, and 300 km by evaluating performance against conservation targets for 17 metrics developed to quantify the five CBD network criteria (Fig. 3, bottom). The representativity criterion is subdivided into metrics for discrete habitats and for continuous biological or physical oceanographic variables that describe the regional seascape. We also reported summary scores for each scenario for each network criterion (Fig. 3, top).
Fig. 3. APEI network performance assessment (nMAR management subunit).
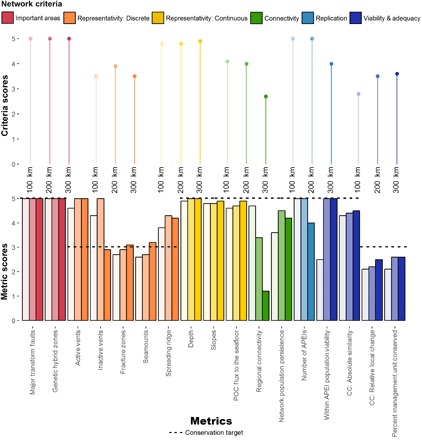
Bottom: Scores for 17 metrics derived to capture performance (5 being the best) of scenarios against the five CBD network criteria (see legend for color code; light shading, 100-km scenario; medium shading, 200-km scenario; dark shading, 300-km model). Table 1 defines the metrics and metric equations. Table S2 shows the raw values and commentary. Dotted line, conservation targets for each score; CC, climate change. Top: Summary scores for each network criterion (calculated by taking the average scenario score of the metrics for a criterion). Scenario core lengths are provided on the x axis.
All scenarios met the target for important areas in this management subunit and did well at representing current biophysical seascape conditions (Representativity: Continuous). Each scenario also outperformed the other scenarios in at least one criterion (Fig. 3, top). The 100-km scenario performed better in the connectivity and replication criteria. The 200-km core scenario outperformed in representing key discrete habitat types and replication. The 300-km scenario did slightly better in achieving targets to represent the regional biophysical seascape and in mitigating effects from projected changes under climatic conditions (Fig. 3). While the 200-km scenario performed well across all criteria, the 100-km scenario underperformed in adequacy and viability, and the 300-km scenario underperformed in connectivity. As noted in the Introduction, the 100-km scenario also does not meet our critical design requirement for a ≥200-km core length.
APEI network performance assessment: RTF management subunit
For the RTF management subunit, all scenarios protect the hadal biogeographic province. Two of the important areas identified by experts are in the RTF management subunit, namely, the RTF itself and the biogeographic transition zone between the North Atlantic and South Atlantic bathyal biogeographic provinces. Only the 300-km scenario completely protected both the RTF and the biogeographic transition zone within a single APEI. The 200-km scenario performed well, protecting the RTF and greater than 70% of the biogeographic transition zone, but the 100-km scenario was unable to adequately conserve either the RTF or the biogeographic transition zone. Other network criteria were not evaluated, as there was only one APEI and, thus, consideration of metrics for network criteria was inappropriate.
DISCUSSION
From the assessment above, it is evident that there are trade-offs in scenario performance across network criteria. While all scenarios performed well in certain criteria, each criterion must be met to support an ecologically coherent network. The poor performance of the 100-km scenario in the viability and adequacy criterion and the 300-km scenario in the connectivity criterion raise questions about the ecological coherence of those network scenarios. Furthermore, the 100-km scenario failed to meet the basic target to conserve the 75th percentile median dispersal distance for deep-sea benthic invertebrates within core areas and was unable to fully conserve the important areas in the RTF management unit. Given the need to place buffers around core areas, smaller APEIs are a less-efficient mechanism with which to meet conservation targets. Therefore, we recommend the use of a 200-km core length for APEIs but recognize that the size of an APEI is contingent on the characteristics of the management unit (for example, the need to use an APEI with a 300-km core length to fully conserve important areas in the RTF subunit).
The nMAR network scenarios described here do not take into account locations of existing exploration contracts. Exploration contracts influenced decisions by the ISA regarding the placement of APEIs in the CCZ, leading to a network of APEIs that are not necessarily representative of the local and regional biodiversity (16, 20). Exploration contracts on the MAR continue to be granted, with the most recent contract awarded in 2017. Before applying for exploitation contracts, contractors will have to relinquish 75% of the area under the exploration contract. Future exploration and exploitation contracts may also need to consider what other management measures with overlapping objectives have been introduced by other intergovernmental organizations with mandates to regulate human activities (for example, fisheries). Thus, the legal and geographic landscape in which networks of APEIs are being developed continues to (and is designed to) change. Given this situation, the size and spacing of core areas is flexible, and the network development process can be adaptive to accommodate mineral extraction (16), as long as the overall regional conservation goal and design targets are not compromised. Critically, lengths of APEIs along the ridge axis can be varied to fit between existing exploration or exploitation contracts, provided that these conditions are met.
More important than the precise dimensions of each APEI is the distribution of those APEIs along the ridge axis; size and spacing of APEIs along the ridge must deliver a network of areas that maintain population connectivity. Connectivity is not merely a function of the mean and maximum distance between APEIs but also of the size of individual APEIs and the percent of habitat protected (67). Thus, any network design should ensure that (i) habitat conservation targets are met, (ii) the average length of a core area is at least 200 km, (iii) the distance between APEIs is as close as possible to the core lengths of adjacent APEIs, and (iv) the maximum distance between adjacent APEIs is minimized. Maintaining average core lengths of 200 km should promote self-sustaining populations within APEIs. Limiting what we refer to as the “gap ratio” (the ratio of the APEI core length to the distance to adjacent APEIs) will help ensure connectivity between APEIs. Given the highly linear nature of mid-ocean ridge systems, the maximum distance between APEIs is a critical factor in determining whether the design will act as a network or whether it will simply be multiple isolated conservation areas with concomitant losses in resilience. This becomes more critical as the average size of APEIs decreases, resulting in more larval export from the no-mining area.
The conservation targets, network criteria, and performance assessment framework applied here can provide the scientific basis for the design of banded APEIs on mid-ocean ridges across the globe, facilitating broad applications of a precautionary approach for the protection of biodiversity and ecosystem function in the context of SMS mining. This process can be readily adapted for design of APEI networks on the other mid-ocean ridges where there are, or may be, mining interests. These include the spreading ridges in the Indian Ocean, where the ISA has already awarded SMS exploration leases to India, Germany, Korea, and China, and the southern and more northern extensions of the MAR.
Our APEI design process also considered, for the first time in the deep sea, mitigation of projected climate-induced changes. Projected climate-driven changes in pH, temperature, dissolved O2 concentrations, and POC flux to the seafloor will occur throughout the water column and at the sea floor (70). These environmental shifts could alter connectivity regimes (71), induce species range shifts, change latitudinal or depth distributions of species, alter food webs, weaken carbonate skeletons, and ultimately alter biodiversity and ecosystem functions (72). In the context of area-based planning in the deep sea, conservation areas should incorporate existing syntheses and future projections of warming, deoxygenation, acidification, and POC flux to the seafloor into the evaluation of habitat vulnerability and resilience (73). We used projected changes in these variables to capture current biogeochemical habitat conditions (and their associated biota) within APEI networks in the future (specifically in the year 2100). Climate-induced changes in ecosystem structure and function are critical to include in the design of APEI networks to ensure that the goals of the protected area networks are sustainable as deep-sea ecosystems are altered by climate change.
Although change in seafloor conditions appears inevitable, it is unknown exactly how much change might be physiologically stressful. POC flux is a proxy for food supply, with effects on species diversity, trophic interactions, and other ecosystem attributes (51), and POC flux to the seabed is projected to decrease in some parts of the management area by as much as 10 to 25%. Projected increases in temperature (0.1° to 0.2°C) and reductions in O2 seem modest (74) but could raise metabolic energy demands of resident species, and when combined with decreased POC flux to the seafloor, even small increases might be detrimental (75). Impacts of climate change are not restricted to metazoan life. Microbial and microbial-metazoan systems in the deep sea are also expected to be influenced by climate-induced changes in temperature, O2 concentration, POC flux, and pH, with the potential for consequences that modify or disrupt ecosystem structure and function (76). Climate-induced stressors will not act alone; changes in environmental conditions will co-occur (77) and may interact in unpredictable ways (78), highlighting the need for a precautionary approach. Uncertainty in climate projections and their ecological impacts should not preclude, considering climate issues in ongoing spatial planning for APEIs. The analysis undertaken here represents a first attempt to assess how APEI scenarios will reflect or resist change in key environmental variables under future climate change and demonstrated the relatively poor performance of the 100-km core length APEI network scenario in these metrics (Fig. 3, bottom). We strongly encourage future studies to expand on the climate change–related metrics developed here and test their ecological relevance [for example, (74)].
Our current knowledge of deep-sea ecosystems is sparse and spatially biased (79). The development of validated models of potential habitat suitability (80) and other methods to predict the distribution of deep-sea habitats in unsurveyed areas (81) can be an important next step in refining network design. Higher resolution and more comprehensive data sets of habitat and species’ distributions, important ecological drivers, population genetic structure, connectivity at ecological and evolutionary time scales, oceanographic currents, and higher resolution of earth system models to describe future change and ecological response are needed. In the near term, it is critically important to validate plume dispersal models to inform adaptive management of the size of buffer zones around APEIs to better understand the impacts on the deep pelagic and interlinkages between benthic and pelagic systems in the deep sea (82). The designation and valuation of ecosystem services for high-sea and deep-sea ecosystems are just beginning (83–86) and will also be important for refining APEI network design in the future. With sufficient data, it should be possible to map the supply and demand of ecosystem services to guide area-based planning (87–89). Network criterion 1 (Box 1) should then be revised so that those areas providing multiple or highly valued ecosystem services would receive priority for protection from activities that may deteriorate these services. Because of the prohibitive costs of sampling in deep and distant locations under extreme environmental conditions, meeting the data needs for these management approaches will require engagement with mining contractors, who must collect high-quality baseline environmental data as part of their exploration contract, as well as the scientific research community.
The ultimate design and timing of implementation of regional APEI networks on mid-ocean ridges remain to be resolved. Regional EMPs, including area-based tools, are within the aegis of the ISA. Placement of APEI networks on the ridge axis before awarding exploration contracts is, at face value, an optimal precautionary approach for protection of the marine environment. However, given that few commercially viable mine sites are thought to exist even over many thousands of kilometers of ridge axis (3), such a strategy reduces the likelihood of discovering a commercially viable mine along the ridge axis or identifying important biodiversity areas. Furthermore, large extents of ridge axis in the Atlantic and Indian oceans are already under exploration contracts, potentially compromising the ability to design networks to meet the conservation goal, objectives, and design targets, if these contracted areas must be excluded from APEI network design.
We encourage the ISA and civil society to consider incentives for regional-scale environmental baseline surveys to identify commercially viable mine sites and important biodiversity areas. Our knowledge of deep-sea ecosystems is scant and, without investment in regionally intensive baseline data collection, will likely remain so for decades. Partnerships involving the ISA, contractors, and the scientific community in the environmental planning process, including baseline surveys, are critical if we are to ensure that mining activities can proceed with due regard to the environment. For now, we recommend that the best approach is for regional EMPs, including APEI networks based on a representative approach such as the one described here, to be implemented as soon as possible. The ISA recently released a preliminary strategy for the development of these plans especially for areas where there are current contracts for exploration (90), with supporting activities proposed through 2020.
MATERIALS AND METHODS
Data
To ensure repeatability, only published data were used. Biogeographic units of interest were the abyssal, bathyal, and hadal regions extracted from (33). Depth and slope were derived from the General Bathymetric Chart of the Oceans 2014 Grid (v. 20150318; www.gebco.net). The spreading ridge feature was extracted from GRID-Arendal’s Global Seafloor Geomorphic Features data set (91). Locations of known and inferred active and inactive hydrothermal vents sites were taken from the InterRidge Vent Database (92). Seamounts were clipped from the Global Seamount Database (93). Transform faults were obtained from the Global Seafloor Fabric and Magnetic Lineation Data Base (94). Data for contemporary (2013) pH, temperature, dissolved O2 concentrations, and POC flux to the seafloor were those used by Sweetman et al. (70), as were the projected (2100) variables generated from Coupled Model Intercomparison Project Phase 5 (CMIP5) (95, 96).
All geospatial analyses were carried out in ArcGIS 10.4.1, and all data layers were clipped to the case study area using a custom projection (Mollweide, with the central meridian set to −36.00°) that allowed for the best compromise between exact area calculations and exact distance calculations.
Derived variables
Distance, total area, and area by habitat coverage
Pairwise distances between APEI core areas in each scenario were calculated by running the “Near” tool using geodesic distances between nearest edges of cores. The area of the management unit conserved in each scenario was calculated by summing the core areas of the APEIs and dividing by the area of the management subunit to describe the percent area conserved. To analyze the degree to which targets for areal coverage of specific habitat types (that is, area of spreading ridges and biogeographic units, number of active and inactive hydrothermal vents and seamounts, and length of transform faults) were achieved, habitats falling within the core areas of each scenario were computed using the “Identify” tool; area of the habitat within the cores was divided by the total area of the management subunit to get the percent conserved by each network scenario.
Geomorphologic, oceanographic, and climate change variables
Distributions of depth and slope (geomorphological features) within APEI core areas were compared to their distributions within the entire management subunit for each scenario. Core and management subunit areas were converted to 1-km resolution rasters to ensure that the succeeding calculations in ArcMap were not performed at a coarser resolution. Variables were then binned by depth (100-m bins) or slope (1° bins) before extracting values. The “Zonal Histogram” tool was used to generate frequency histograms for each variable within APEI cores and for the management units. The same process was used to calculate histograms for the current (2013) and future (2100) distributions of four oceanographic variables at the seafloor, each binned into 20 equal-interval variables: acidity (pH), temperature (°C), O2 (ml liter−1), and POC flux to the seafloor (mg of C m−2 day−1). Percent change between current and future conditions for pH, temperature, dissolved O2 concentrations, and POC flux to the seafloor was calculated for each grid cell in the study area.
Performance assessment of APEI network scenarios: nMAR management subunit
Eighteen quantifiable metrics were developed to gauge network performance against the conservation targets identified in Box 1 (Table 1). The three APEI scenarios with core lengths of 100, 200, or 300 km were evaluated to assess how size and spacing of APEIs influence the degree to which the conservation targets were met. Each scenario was scored on the basis of how well it achieved specific conservation goals for individual metrics and each criterion. Equations and conservation targets for all metrics are included in Table 1. For ease of interpretation and to allow summarizing within a criterion, all scores were normalized to a range of 0 to 5, with 5 being the best score.
The metrics used in each criterion were linked by their properties and objectives. Hence, we opted to include a summary metric for each criterion to improve ease of interpretation of the results. The criteria scores were calculated by taking the average of the scores across the metrics included in that criterion. Because of differences in what the criteria measure, and in accordance with current consensus on multicriteria analytical methods, no effort was made to average across all criteria.
Supplementary Material
Acknowledgments
We thank F. Cardigos, G. Carreira, D. Freestone, M. Jungwiwattanaporn, M. King, P. Lourinho, C. Mann, H. Marques da Silva, G. Menezes, F. Porteiro, R. Serrao Santos, I. Shepherd, M. Silva, R. Tinch, S. van den Hove, and V. Zykov for the contributions to the development of the framework described in this paper. We also thank A. Sweetman for supplying the climate change models used in the performance assessment. We extend our appreciation to G. Le Gurun, S. Mulsow, and M. Lodge of the International Seabed Authority for the contributions to the SEMPIA process. Funding: The SEMPIA Workshops were supported by funding from the European Union Seventh Framework Programme (FP7/2007-2013) under the Managing Impacts of Deep-Sea Resource Exploitation (MIDAS) Project (grant agreement no. 603418), Direção Regional dos Assuntos do Mar, Governo Regional dos Açores, the Deep-Sea Conservation Coalition, the Pew Charitable Trusts, the Deep Sea Conservation Coalition, the Kaplan Fund, and Oceans 5. Work on the manuscript was supported through funding received by C.L.V.D., D.C.D., and P.N.H. from the Pew Charitable Trusts and, for these authors and D.J., from the Global Ocean Biodiversity Initiative through a grant from the International Climate Initiative (IKI). The Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety supports IKI on the basis of a decision adopted by the German Bundestag. D.C.D. was also supported, in part, by the NF-UBC Nereus Program. T.M. was funded by Programa Investigador Fundação para a Ciência e Tecnologia (FCT) (IF/01194/2013/CP1199/CT0002 and UID/MAR/04292/2013). A.G. was supported, in part, by Russian Science Foundation grant 14-50-00095. A.C. is supported by Program Investigador (IF/00029/2014/CP1230/CT0002) from FCT. This study had also the support of FCT through the strategic project UID/MAR/04292/2013 granted to the Marine and Environmental Sciences Center (MARE). J.A.A.P. was supported by the National Council for Scientific and Technological Development–CNPq (process 310504/2016-3). M.C.R. was supported by CORAL–Sustainable Ocean Exploitation: Tools and Sensors (CIIMAR-NORTE 2020-ERDF). L.L. was supported, in part, by the JM Kaplan Foundation and was a co-lead of Deep-Ocean Stewardship Initiative and Deep Ocean Observing Strategy. Author contributions: C.L.V.D., T.M., A.C., D.C.D., and P.W. conceived the project. D.C.D., T.M., and P.N.H. undertook the Geographic Information System (GIS) mapping. D.C.D. extracted the feature data for performance assessment. R.J.E., C.R.S., L.A.L., D.C.D., and C.L.V.D. undertook the performance assessment. All authors contributed to iterative discussions, writing, and editing to the manuscript. D.D. and C.L.V.D. led those discussions and the writing and editing. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All geospatial information used in this analysis is publicly available. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SEMPIA Workshop Participants
Odd Aksel Bergstad1, Christopher Barrio2, Inês Barros3, Meri Bilan3, David Billett4, Sabine Christiansen5, Jesse Cleary6, Henko de Stigter7, Matthew Gianni8, Anthony Grehan9, Aline Jaeckel10, Daniel O. B. Jones11, Tina Kutti3, Laura Lallier12, Kirsty McQuaid13, Lénaïck Menot14, Anna Metaxas15, Tina N. Molodtsova16, Francesc Montserrat17, John Mouat18, Gordon Paterson19, Christopher K. Pham3, Jozée Sarrazin14, Andrew Sweetman20, Gerald H. Taranto3, Torsten Thiele21, Phillip Turner6, Alexander Turra17, Frederic Vandeperre3, Hiroyuki Yamamoto22, Sébastien Ybert14
1Institute of Marine Research, Norway. 2Seascape Consultants, UK. 3Institute of Marine Research (IMAR), Portugal. 4Deep Seas Environmental Solutions Ltd, UK. 5Institute for Advanced Sustainability Studies, Germany. 6Duke University, USA. 7NIOZ-Royal Netherlands Institute for Sea Research, Netherlands. 8Deep Sea Conservation Coalition, Netherlands. 9National University of Ireland, Galway, Ireland. 10Macquarie University, Sydney, Australia. 11National Oceanography Centre, UK. 12University of Ghent, Belgium. 13Plymouth University, UK. 14IFREMER, France. 15Dalhousie University, Canada. 16P.P. Shirshov Institute of Oceanology, Russia. 17Oceanographic Institute, University of São Paulo (IO-USP), Brazil. 18OSPAR Commission, UK. 19Natural History Museum, UK. 20The Lyell Centre for Earth and Marine Science and Technology, Heriot-Watt University, UK. 21Global Ocean Trust, UK. 22Japan Agency for Marine Earth Science and Technology, Japan.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/7/eaar4313/DC1
Table S1. Surrogate parameters related to biodiversity and deep-sea ecosystem structure and function and examples.
Table S2. Raw values and performance metric scores.
Table S3. Climate change metric results.
REFERENCES AND NOTES
- 1.Cann J. R., Availability of sulphide ores in the ocean crust. J. Geol. Soc. London 137, 381–384 (1980). [Google Scholar]
- 2.Hannington M., Jamieson J., Monecke T., Petersen S., Beaulieu S., The abundance of seafloor massive sulfide deposits. Geology 39, 1155–1158 (2011). [Google Scholar]
- 3.Petersen S., Krätschell A., Augustin N., Jamieson J., Hein J. R., Hannington M. D., News from the seabed – Geological characteristics and resource potential of deep-sea mineral resources. Mar. Policy 70, 175–187 (2016). [Google Scholar]
- 4.Van Dover C. L., Impacts of anthropogenic disturbances at deep-sea hydrothermal vent ecosystems: A review. Mar. Environ. Res. 102, 59–72 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Erickson K. L., Macko S. A., VanDover C. L., Evidence for a chemoautotrophically based food web at inactive hydrothermal vents (Manus Basin). Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 1577–1585 (2009). [Google Scholar]
- 6.Collins P. C., Kennedy R., VanDover C. L., A biological survey method applied to seafloor massive sulphides (SMS) with contagiously distributed hydrothermal-vent fauna. Mar. Ecol. Prog. Ser. 452, 89–107 (2012). [Google Scholar]
- 7.Van Dover C. L., Arnaud-Haond S., Gianni M., Helmreich S., Huber J. A., Jaeckel A. L., Metaxas A., Pendleton L. H., Petersen S., Ramirez-Llodra E., Steinberg P. E., Tunnicliffe V., Yamamoto H., Scientific rationale and international obligations for protection of active hydrothermal vent ecosystems from deep-sea mining. Mar. Policy 90, 20–28 (2018). [Google Scholar]
- 8.Hannington M., Monecke T., Global exploration models for polymetallic sulphides in the Area: An assessment of lease block selection under the draft regulations on prospecting and exploration for polymetallic sulphides. Mar. Georesour. Geotechnol. 27, 132–159 (2009). [Google Scholar]
- 9.Petersen S., Hannington M., Krätschell A., Technology developments in the exploration and evaluation of deep-sea mineral resources. Ann. Mines Responsab. Environ. 85, 14–18 (2017). [Google Scholar]
- 10.Fouquet Y., Minéralisations hydrothermales: Les enjeux scientifiques de leur exploration. Ann. Mines Responsab. Environ. 85, 9–13 (2017). [Google Scholar]
- 11.Scott S. D., Deep ocean mining. Geosci. Canada 28, 87–96 (2001). [Google Scholar]
- 12.Levin L. A., Mengerink K., Gjerde K. M., Rowden A. A., Lee Van Dover C., Clark M. R., Ramirez-Llodra E., Currie B., Smith C. R., Sato K. N., Gallo N., Sweetman A. K., Lily H., Armstrong C. W., Brider J., Defining “serious harm” to the marine environment in the context of deep-seabed mining. Mar. Policy 74, 245–259 (2016). [Google Scholar]
- 13.Gollner S., Kaiser S., Menzel L., Jones D. O. B., Brown A., Mestre N. C., van Oevelen D., Menot L., Colaço A., Canals M., Cuvelier D., Durden J. M., Gebruk A., Egho G. A., Haeckel M., Marcon Y., Mevenkamp L., Morato T., Pham C. K., Purser A., Sanchez-Vidal A., Vanreusel A., Vink A., Martinez Arbizu P., Resilience of benthic deep-sea fauna to mining activities. Mar. Environ. Res. 129, 76–101 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Van Dover C. L., Mining seafloor massive sulphides and biodiversity: What is at risk?. ICES J. Mar. Sci. 68, 341–348 (2011). [Google Scholar]
- 15.Boschen R. E., Rowden A. A., Clark M. R., Gardner J. P. A., Mining of deep-sea seafloor massive sulfides: A review of the deposits, their benthic communities, impacts from mining, regulatory frameworks and management strategies. Ocean Coast. Manag. 84, 54–67 (2013). [Google Scholar]
- 16.Wedding L. M., Reiter S. M., Smith C. R., Gjerde K. M., Kittinger J. N., Friedlander A. M., Gaines S. D., Clark M. R., Thurnherr A. M., Hardy S. M., Crowder L. B., Managing mining of the deep seabed. Science 349, 144–145 (2015). [DOI] [PubMed] [Google Scholar]
- 17.International Seabed Authority, Environmental Management Plan for the Clarion-Clipperton Zone. ISBA/17/LTC/7 (2011); www.isa.org.jm/sites/default/files/files/documents/isba-17ltc-7_0.pdf.
- 18.Therivel R., Wilson E., Heaney D., Thompson S., Strategic Environmental Assessment (Routledge, 2013). [Google Scholar]
- 19.Foster N. L., Foggo A., Howell K. L., Using species-area relationships to inform baseline conservation targets for the deep North East Atlantic. PLOS ONE 8, e58941 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wedding L. M., Friedlander A. M., Kittinger J. N., Watling L., Gaines S. D., Bennett M., Hardy S. M., Smith C. R., From principles to practice: A spatial approach to systematic conservation planning in the deep sea. Proc. Biol. Sci. 280, 20131684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodge M., Johnson D., Le Gurun G., Wengler M., Weaver P., Gunn V., Seabed mining: International Seabed Authority environmental management plan for the Clarion–Clipperton Zone. A partnership approach. Mar. Policy. 49, 66–72 (2014). [Google Scholar]
- 22.Howell K. L., A benthic classification system to aid in the implementation of marine protected area networks in the deep/high seas of the NE Atlantic. Biol. Conserv. 143, 1041–1056 (2010). [Google Scholar]
- 23.Sutcliffe P. R., Klein C. J., Pitcher C. R., Possingham H. P., The effectiveness of marine reserve systems constructed using different surrogates of biodiversity. Conserv. Biol. 29, 657–667 (2015). [DOI] [PubMed] [Google Scholar]
- 24.C. R. Smith, S. Gaines, A. Friedlander, C. Morgan, A. Thurnherr, S. Mincks, L. Watling, A. Rogers, M. Clark, A. Baco-Taylor, A. Bernardino, F. De Leo, P. Dutrieux, A. Rieser, J. Kittinger, J. Padilla-Gamino, R. Prescott, P. Srsen, Preservation reference areas for nodule mining in the Clarion-Clipperton Zone: Rationale and recommendations to the international seabed authority, in Design Marine Protected Areas for Seamounts and the Abyssal Nodule Province in Pacific High Seas (University of Hawaii, 2008). [Google Scholar]
- 25.Van Dover C. L., Smith C. R., Ardron J., Dunn D., Gjerde K., Levin L., Smith S. The Dinard Workshop Contributors , Designating networks of chemosynthetic ecosystem reserves in the deep sea. Mar. Policy 36, 378–381 (2012). [Google Scholar]
- 26.I. Ball, H. P. Possingham, M. E. Watt, Marxan and relatives: Software for spatial conservation prioritisation, in Spatial Conservation Prioritisation: Quantitative Methods and Computational Tools (Oxford Univ. Press, 2009), pp. 185–195. [Google Scholar]
- 27.Watts M. E., Ball I. R., Stewart R. S., Klein C. J., Wilson K., Steinback C., Lourival R., Kircher L., Possingham H. P., Marxan with Zones: Software for optimal conservation based land- and sea-use zoning. Environ Model Softw. 24, 1513–1521 (2009). [Google Scholar]
- 28.Convention on Biological Diversity, VIII/24. Protected areas, in Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity at its Eighth Meeting (Convention Biological Diversity, 2006); www.cbd.int/doc/decisions/cop-08/cop-08-dec-24-en.pdf.
- 29.Convention on Biological Diversity, IX/20. Marine and coastal biodiversity, in Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity at its Ninth Meeting (Convention Biological Diversity, 2008); www.cbd.int/decision/cop/default.shtml?id=11663.
- 30.Dunn D. C., Ardron J., Bax N., Bernal P., Cleary J., Cresswell I., Donnelly B., Dunstan P., Gjerde K., Johnson D., Kaschner K., Lascelles B., Rice J., von Nordheim H., Wood L., Halpin P. N., The Convention on Biological Diversity’s Ecologically or Biologically Significant Areas: Origins, development, and current status. Mar. Policy 49, 137–145 (2014). [Google Scholar]
- 31.Lourie S. A., Vincent A. C. J., Using biogeography to help set priorities in marine conservation. Conserv. Biol. 18, 1004–1020 (2004). [Google Scholar]
- 32.Brooks T. M., Mittermeier R. A., da Fonseca G. A. B., Gerlach J., Hoffmann M., Lamoreux J. F., Mittermeier C. G., Pilgrim J. D., Rodrigues A. S. L., Global biodiversity conservation priorities. Science 313, 58–61 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Watling L., Guinotte J., Clark M. R., Smith C. R., A proposed biogeography of the deep ocean floor. Prog. Oceanogr. 111, 91–112 (2013). [Google Scholar]
- 34.E. G. Morozov, A. N. Demidov, R. Y. Tarakanov, W. Zenk, Abyssal Channels in the Atlantic Ocean: Water Structure and Flows (Springer Science & Business Media, 2010). [Google Scholar]
- 35.Mercier H., Speer K. G., Transport of bottom water in the Romanche Fracture Zone and the Chain Fracture Zone. J. Phys. Oceanogr. 28, 779–790 (1998). [Google Scholar]
- 36.Gebruk A. V., Krylova E. M., Megafauna of the Charlie–Gibbs Fracture Zone (northern Mid-Atlantic Ridge) based on video observations. J. Mar. Biol. Assoc. U. K. 93, 1143–1150 (2013). [Google Scholar]
- 37.Spector S., Biogeographic crossroads as priority areas for biodiversity conservation. Conserv. Biol. 16, 1480–1487 (2002). [Google Scholar]
- 38.Dibattista J. D., Rocha L. A., Hobbs J. P. A., He S., Priest M. A., Sinclair-Taylor T. H., Bowen B. W., Berumen M. L., When biogeographical provinces collide: Hybridization of reef fishes at the crossroads of marine biogeographical provinces in the Arabian Sea. J. Biogeogr. 42, 1601–1614 (2015). [Google Scholar]
- 39.Won Y., Hallam S. J., O’Mullan G. D., Vrijenhoek R. C., Cytonuclear disequilibrium in a hybrid zone involving deep-sea hydrothermal vent mussels of the genus Bathymodiolus. Mol. Ecol. 12, 3185–3190 (2003). [DOI] [PubMed] [Google Scholar]
- 40.O’Mullan G. D., Maas P. A. Y., Lutz R. A., Vrijenhoek R. C., A hybrid zone between hydrothermal vent mussels (Bivalvia: Mytilidae) from the Mid-Atlantic Ridge. Mol. Ecol. 10, 2819–2831 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Breusing C., Vrijenhoek R. C., Reusch T. B. H., Widespread introgression in deep-sea hydrothermal vent mussels. BMC Evol. Biol. 17, 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzpatrick B. M., Ryan M. E., Johnson J. R., Corush J., Carter E. T., Hybridization and the species problem in conservation. Curr. Zool. 61, 206–216 (2015). [Google Scholar]
- 43.A. V. Gebruk, A. N. Mironov, Biogeography of Atlantic hydrothermal vents, in Ecosystems of Atlantic Hydrothermal Vents, M. Vinogradov, A. Vereshchaka, Eds. (Nauka, 2006), pp. 119–162. [Google Scholar]
- 44.German C. R., Ramirez-Llodra E., Baker M. C., Tyler P. A. ChEss Scientific Steering Committee , Deep-water chemosynthetic ecosystem research during the census of marine life decade and beyond: A proposed deep-ocean road map. PLOS ONE 6, e23259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Convention on Biological Diversity, XI/17. Marine and coastal biodiversity: Ecologically or biologically significant marine areas, in Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity at its Eleventh Meeting (Convention Biological Diversity, 2012); www.cbd.int/doc/decisions/cop-11/cop-11-dec-17-en.pdf.
- 46.Lutz M. J., Caldeira K., Dunbar R. B., Behrenfeld M. J., Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean. J. Geophys. Res. 112, C10011 (2007). [Google Scholar]
- 47.Henson S. A., Sanders R., Madsen E., Global patterns in efficiency of particulate organic carbon export and transfer to the deep ocean. Global Biogeochem. Cycles 26, GB1028 (2012). [Google Scholar]
- 48.Wei C.-L., Rowe G. T., Escobar-Briones E., Boetius A., Soltwedel T., Caley M. J., Soliman Y., Huettmann F., Qu F., Yu Z., Pitcher C. R., Haedrich R. L., Wicksten M. K., Rex M. A., Baguley J. G., Sharma J., Danovaro R., MacDonald I. R., Nunnally C. C., Deming J. W., Montagna P., Lévesque M., Weslawski J. M., Wlodarska-Kowalczuk M., Ingole B. S., Bett B. J., Billett D. S., Yool A., Bluhm B. A., Iken K., Narayanaswamy B. E., Global patterns and predictions of seafloor biomass using random forests. PLOS ONE 5, e15323 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woolley S. N. C., Tittensor D. P., Dunstan P. K., Guillera-Arroita G., Lahoz-Monfort J. J., Wintle B. A., Worm B., O’Hara T. D., Deep-sea diversity patterns are shaped by energy availability. Nature 533, 393–396 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Sibuet M., Lambert C. E., Chesselet R., Laubier L., Density of the major size groups of benthic fauna and trophic input in deep basins of the Atlantic Ocean. J. Mar. Res. 47, 851–867 (1989). [Google Scholar]
- 51.Smith C. R., De Leo F. C., Bernardino A. F., Sweetman A. K., Arbizu P. M., Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 23, 518–528 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Baco A. R., Etter R. J., Ribeiro P. A., von der Heyden S., Beerli P., Kinlan B. P., A synthesis of genetic connectivity in deep-sea fauna and implications for marine reserve design. Mol. Ecol. 25, 3276–3298 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Ross R. E., Nimmo-Smith W. A. M., Howell K. L., Increasing the depth of current understanding: Sensitivity testing of deep-sea larval dispersal models for ecologists. PLOS ONE 11, e0161220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hilário A., Metaxas A., Gaudron S. M., Estimating dispersal distance in the deep sea: Challenges and applications to marine reserves. Front. Mar. Sci. 2, 1–14 (2015). [Google Scholar]
- 55.Breusing C., Biastoch A., Drews A., Metaxas A., Jollivet D., Vrijenhoek R. C., Bayer T., Melzner F., Sayavedra L., Petersen J. M., Dubilier N., Schilhabel M. B., Rosenstiel P., Reusch T. B., Biophysical and population genetic models predict the presence of “phantom” ptepping stones connecting Mid-Atlantic Ridge vent ecosystems. Curr. Biol. 26, 2257–2267 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Gaines S. D., White C., Carr M. H., Palumbi S. R., Designing marine reserve networks for both conservation and fisheries management. Proc. Natl. Acad. Sci. U.S.A. 107, 18286–18293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowe W. H., Allendorf F. W., What can genetics tell us about population connectivity?. Mol. Ecol. 19, 3038–3051 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Alt C. H. S., Rogacheva A., Boorman B., Alan Hughes J., Billett D. S. M., Gooday A. J., Jones D. O. B., Trawled megafaunal invertebrate assemblages from bathyal depth of the Mid-Atlantic Ridge (48°–54°N). Deep Sea Res. Part II Top. Stud. Oceanogr. 98, 326–340 (2013). [Google Scholar]
- 59.Priede I. G., Aksel Bergstad O., Miller P. I., Vecchione M., Gebruk A., Falkenhaug T., Billett D. S. M., Craig J., Dale A. C., Shields M. A., Tilstone G. H., Sutton T. T., Gooday A. J., Inall M. E., Jones D. O. B., Martinez-Vicente V., Menezes G. M., Niedzielski T., Sigurðsson Þ., Rothe N., Rogacheva A., Alt C. H. S., Brand T., Abell R., Brierley A. S., Cousins N. J., Crockard D., Rus Hoelzel A., Høines Å., Letessier T. B., Read J. F., Shimmield T., Cox M. J., Galbraith J. K., Gordon J. D. M., Horton T., Neat F., Lorance P., Does presence of a mid-ocean ridge enhance biomass and biodiversity?. PLOS ONE 8, e61550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St. Laurent L. C., Toole J. M., Schmitt R. W., Buoyancy forcing by turbulence above rough topography in the abyssal Brazil Basin. J. Phys. Oceanogr. 31, 3476–3495 (2001). [Google Scholar]
- 61.Thurnherr A. M., St. Laurent L. C., Speer K. G., Toole J. M., Ledwell J. R., Mixing associated with sills in a canyon on the midocean ridge flank. J. Phys. Oceanogr. 35, 1370–1381 (2005). [Google Scholar]
- 62.G. Cherkashov, Seafloor massive sulfide deposits: Distribution and prospecting, in Deep-Sea Mining, R. Sharma, Ed. (Springer International Publishing, 2017), pp. 143–164. [Google Scholar]
- 63.German C. R., Petersen S., Hannington M. D., Hydrothermal exploration of mid-ocean ridges: Where might the largest sulfide deposits be forming?. Chem. Geol. 420, 114–126 (2016). [Google Scholar]
- 64.Andersen C., Theissen-Krah S., Hannington M., Rüpke L., Petersen S., Faulting and off-axis submarine massive sulfide accumulation at slow spreading mid-ocean ridges: A numerical modeling perspective. Geochem. Geophys. Geosyst. 18, 2305–2320 (2017). [Google Scholar]
- 65.Kelley D. S., Karson J. A., Blackman D. K., Früh-Green G. L., Butterfield D. A., Lilley M. D., Olson E. J., Schrenk M. O., Roe K. K., Lebon G. T., Rivizzigno P. AT3-60 Shipboard Party , An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature 412, 145–149 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Oebius H. U., Becker H. J., Rolinski S., Jankowski J. A., Parametrization and evaluation of marine environmental impacts produced by deep-sea manganese nodule mining. Deep Sea Res. Part II Top. Stud. Oceanogr. 48, 3453–3467 (2001). [Google Scholar]
- 67.Moffitt E. A., Wilson White J., Botsford L. W., The utility and limitations of size and spacing guidelines for designing marine protected area (MPA) networks. Biol. Conserv. 144, 306–318 (2011). [Google Scholar]
- 68.Johnson D., Ardron J., Billett D., Hooper T., Mullier T., Chaniotis P., Ponge B., Corcoran E., When is a marine protected area network ecologically coherent? A case study from the North-east Atlantic. Aquat. Conserv. 24, 44–58 (2014). [Google Scholar]
- 69.Ardron J. A., Three initial OSPAR tests of ecological coherence: Heuristics in a data-limited situation. ICES J. Mar. Sci. 65, 1527–1533 (2010). [Google Scholar]
- 70.Sweetman A. K., Thurber A. R., Smith C. R., Levin L. A., Mora C., Wei C.-L., Gooday A. J., Jones D. O. B., Rex M., Yasuhara M., Ingels J., Ruhl H. A., Frieder C. A., Danovaro R., Würzberg L., Baco A., Grupe B. M., Pasulka A., Meyer K. S., Dunlop K. M., Henry L.-A., Murray Roberts J., Major impacts of climate change on deep-sea benthic ecosystems. Elem. Sci. Anth. 5, 4 (2017). [Google Scholar]
- 71.Fox A. D., Henry L.-A., Corne D. W., Roberts J. M., Sensitivity of marine protected area network connectivity to atmospheric variability. R. Soc. Open Sci. 3, 160494 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levin L. A., Le Bris N., The deep ocean under climate change. Science 350, 766–768 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Johnson D., Ferreira M. A., Kenchington E., Climate change is likely to severely limit the effectiveness of deep-sea ABMTs in the North Atlantic. Mar. Policy 87, 111–122 (2017). [Google Scholar]
- 74.Sperling E. A., Frieder C. A., Levin L. A., Biodiversity response to natural gradients of multiple stressors on continental margins. Proc. Biol. Sci. 283, 20160637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomsen J., Casties I., Pansch C., Körtzinger A., Melzner F., Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: Laboratory and field experiments. Glob. Chang. Biol. 19, 1017–1027 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Danovaro R., Corinaldesi C., Dell’Anno A., Rastelli E., Potential impact of global climate change on benthic deep-sea microbes. FEMS Microbiol. Lett. 364, fnx214 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Gruber N., Warming up, turning sour, losing breath: Ocean biogeochemistry under global change. Philos. Trans. A Math. Phys. Eng. Sci. 369, 1980–1996 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Breitburg D. L., Salisbury J., Bernhard J. M., Cai W.-J., Dupont S., Doney S. C., Kroeker K. J., Levin L. A., Long W. C., Milke L. M., Miller S. H., Phelan B., Passow U., Seibel B. A., Todgham A. E., Tarrant A. M., And on top of all that… Coping with ocean acidification in the midst of many stressors. Oceanography 28, 48–61 (2015). [Google Scholar]
- 79.Webb T. J., Vanden Berghe E., O’Dor R., Biodiversity’s big wet secret: The global distribution of marine biological records reveals chronic under-exploration of the deep pelagic ocean. PLOS ONE 5, e10223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ross R. E., Howell K. L., Use of predictive habitat modelling to assess the distribution and extent of the current protection of “listed” deep-sea habitats. Divers. Distrib. 19, 433–445 (2013). [Google Scholar]
- 81.Almada G. V. d. M. B., Bernardino A. F., Conservation of deep-sea ecosystems within offshore oil fields on the Brazilian margin, SW Atlantic. Biol. Conserv. 206, 92–101 (2017). [Google Scholar]
- 82.Ban N. C., Maxwell S. M., Dunn D. C., Hobday A. J., Bax N. J., Ardron J., Gjerde K. M., Game E. T., Devillers R., Kaplan D. M., Dunstan P. K., Halpin P. N., Presseya R. L., Better integration of sectoral planning and management approaches for the interlinked ecology of the open oceans. Mar. Policy 49, 127–136 (2014). [Google Scholar]
- 83.Armstrong C. W., Foley N. S., Tinch R., van den Hove S., Services from the deep: Steps towards valuation of deep sea goods and services. Ecosyst. Serv. 2, 2–13 (2012). [Google Scholar]
- 84.Thurber A. R., Sweetman A. K., Narayanaswamy B. E., Jones D. O. B., Ingels J., Hansman R. L., Ecosystem function and services provided by the deep sea. Biogeosciences 11, 3941–3963 (2014). [Google Scholar]
- 85.Weiss M. C., Sousa F. L., Mrnjavac N., Neukirchen S., Roettger M., Nelson-Sathi S., Martin W. F., The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 16116 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Martin S. L., Ballance L. T., Groves T., An ecosystem services perspective for the oceanic Eastern Tropical Pacific: Commercial fisheries, carbon storage, recreational fishing, and biodiversity. Front. Mar. Sci. 3, 50 (2016). [Google Scholar]
- 87.Naidoo R., Balmford A., Costanza R., Fisher B., Green R. E., Lehner B., Malcolm T. R., Ricketts T. H., Global mapping of ecosystem services and conservation priorities. Proc. Natl. Acad. Sci. U.S.A. 105, 9495–9500 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burkhard B., Kroll F., Nedkov S., Müller F., Mapping ecosystem service supply, demand and budgets. Ecol. Indic. 21, 17–29 (2012). [Google Scholar]
- 89.Le J. T., Levin L. A., Carson R. T., Incorporating ecosystem services into environmental management of deep-seabed mining. Deep Sea Res. Part II Top. Stud. Oceanogr. 137, 486–503 (2016). [Google Scholar]
- 90.International Seabed Authority, “Preliminary strategy for the development of regional environmental management plans for the Area. ISBA/24/C/3” (2018).
- 91.Harris P. T., Macmillan-Lawler M., Rupp J., Baker E. K., Geomorphology of the oceans. Mar. Geol. 352, 4–24 (2014). [Google Scholar]
- 92.Beaulieu S. E., Baker E. T., German C. R., Maffei A., An authoritative global database for active submarine hydrothermal vent fields. Geochem. Geophys. Geosyst. 14, 4892–4905 (2013). [Google Scholar]
- 93.Kim S.-S., Wessel P., New global seamount census from altimetry-derived gravity data. Geophys. J. Int. 186, 615–631 (2011). [Google Scholar]
- 94.Wessel P., Matthews K. J., Dietmar Müller R., Mazzoni A., Whittaker J. M., Myhill R., Chandler M. T., Semiautomatic fracture zone tracking. Geochem. Geophys. Geosyst. 16, 2462–2472 (2015). [Google Scholar]
- 95.Taylor K. E., Stouffer R. J., Meehl G. A., An overview of CMIP5 and the experiment design. Bull. Am. Meteorol. Soc. 93, 485–498 (2012). [Google Scholar]
- 96.Mora C., Wei C.-L., Rollo A., Amaro T., Baco A. R., Billett D., Bopp L., Chen Q., Collier M., Danovaro R., Gooday A. J., Grupe B. M., Halloran P. R., Ingels J., Jones D. O. B., Levin L. A., Nakano H., Norling K., Ramirez-Llodra E., Rex M., Ruhl H. A., Smith C. R., Sweetman A. K., Thurber A. R., Tjiputra J. F., Usseglio P., Watling L., Wu T., Yasuhara M., Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st Century. PLOS Biol. 11, e1001682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson T. J., Nichol S. L., Syms C., Przeslawski R., Harris P. T., Deep-sea bio-physical variables as surrogates for biological assemblages, an example from the Lord Howe Rise. Deep Sea Res. Part II Top. Stud. Oceanogr. 58, 979–991 (2011). [Google Scholar]
- 98.Bongiorni L., Ravara A., Parretti P., Santos R. S., Rodrigues C. F., Amaro T., Cunha M. R., Organic matter composition and macrofaunal diversity in sediments of the Condor Seamount (Azores, NE Atlantic). Deep Sea Res. Part II Top. Stud. Oceanogr. 98, 75–86 (2013). [Google Scholar]
- 99.Brault S., Stuart C. T., Wagstaff M. C., McClain C. R., Allen J. A., Rex M. A., Contrasting patterns of α- and β-diversity in deep-sea bivalves of the eastern and western North Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 92, 157–164 (2013). [Google Scholar]
- 100.Brown A., Thatje S., Explaining bathymetric diversity patterns in marine benthic invertebrates and demersal fishes: Physiological contributions to adaptation of life at depth. Biol. Rev. 89, 406–426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho W., Shank T. M., Incongruent patterns of genetic connectivity among four ophiuroid species with differing coral host specificity on North Atlantic seamounts. Mar. Ecol. 31, 121–143 (2010). [Google Scholar]
- 102.Dunn D. C., Halpin P. N., Rugosity-based regional modeling of hard-bottom habitat. Mar. Ecol. Prog. Ser. 377, 1–11 (2009). [Google Scholar]
- 103.Durden J. M., Bett B. J., Jones D. O. B., Huvenne V. A. I., Ruhl H. A., Abyssal hills – Hidden source of increased habitat heterogeneity, benthic megafaunal biomass and diversity in the deep sea. Prog. Oceanogr. 137, 209–218 (2015). [Google Scholar]
- 104.Etter R., Grassle J., Patterns of species diversity in the deep sea as a function of sediment particle size diversity. Nature 360, 576–578 (1992). [Google Scholar]
- 105.van der Heijden K., Petersen J. M., Dubilier N., Borowski C., Genetic connectivity between north and south Mid-Atlantic Ridge chemosynthetic bivalves and their symbionts. PLOS ONE 7, e39994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henry L.-A., Vad J., Findlay H. S., Murillo J., Milligan R., Roberts J. M., Environmental variability and biodiversity of megabenthos on the Hebrides Terrace Seamount (Northeast Atlantic). Sci. Rep. 4, 5589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.V. E. Kostylev, C. G. Hannah, Process-driven characterization and mapping of seabed habitats, in Mapping the Seafloor for Habitat Characterization: Geological Association of Canada, Special Paper 47 (2007), pp. 171–184. [Google Scholar]
- 108.Kvile K., Taranto G. H., Pitcher T. J., Morato T., A global assessment of seamount ecosystems knowledge using an ecosystem evaluation framework. Biol. Conserv. 173, 108–120 (2014). [Google Scholar]
- 109.Lacey N. C., Rowden A. A., Clark M. R., Kilgallen N. M., Linley T., Mayor D. J., Jamieson A. J., Community structure and diversity of scavenging amphipods from bathyal to hadal depths in three South Pacific Trenches. Deep Res. Part I Oceanogr. Res. Pap. 111, 121–137 (2016). [Google Scholar]
- 110.Leduc D., Rowden A. A., Keith Probert P., Pilditch C. A., Nodder S. D., Vanreuseld A., Duineveld G. C. A., Witbaard R., Further evidence for the effect of particle-size diversity on deep-sea benthic biodiversity. Deep Res. Part I Oceanogr. Res. Pap. 63, 164–169 (2012). [Google Scholar]
- 111.McClain C. R., Rex M. A., Toward a conceptual understanding of β-diversity in the deep-sea benthos. Annu. Rev. Ecol. Evol. Syst. 623–642 (2015). [Google Scholar]
- 112.Menezes G. M., Sigler M. F., Silva H. M., Pinho M. R.. Structure and zonation of demersal fish assemblages off the Azores Archipelago (mid-Atlantic). Mar. Ecol. Prog. Ser. 324, 241–260 (2006). [Google Scholar]
- 113.Mohn C., Rengstorf A., White M., Duineveld G., Mienis F., Soetaert K., Grehan A., Linking benthic hydrodynamics and cold-water coral occurrences : A high-resolution model study at three cold-water coral provinces in the NE Atlantic. Prog. Oceanogr. 122, 92–104 (2014). [Google Scholar]
- 114.Morris K., Tyler P. A., Murton B., Rogers A. D., Lower bathyal and abyssal distribution of coral in the axial volcanic ridge of the Mid-Atlantic Ridge at 45°N. Deep Res. Part I Oceanogr. Res. Pap. 62, 32–39 (2012). [Google Scholar]
- 115.Roland Pitcher C., Lawton P., Ellis N., Smith S. J., Incze L. S., Wei C. L., Greenlaw M. E., Wolff N. H., Sameoto J. A., Snelgrove P. V., Cadotte M., Exploring the role of environmental variables in shaping patterns of seabed biodiversity composition in regional-scale ecosystems. J. Appl. Ecol. 49, 670–679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rengstorf M. A., Grehan A., Yesson C., Brown C., Towards high-resolution habitat suitability modeling of vulnerable marine ecosystems in the deep-sea: Resolving terrain attribute dependencies. Mar. Geod. 35, 343–361 (2012). [Google Scholar]
- 117.Rengstorf M. A., Mohn C., Brown C., Wisz S. M., Grehan J. A., Predicting the distribution of deep-sea vulnerable marine ecosystems using high-resolution data: Considerations and novel approaches. Deep Sea Res. Part I Oceanogr. Res. Pap. 93, 72–82 (2014). [Google Scholar]
- 118.Rex M. A., Stuart C. T., Coyne G., Latitudinal gradients of species richness in the deep-sea benthos of the North Atlantic. Proc. Natl. Acad. Sci. U.S.A. 97, 4082–4085 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sarrazin J., Legendre P., de Busserolles F., Fabri M.-C., Guilini K., Ivanenko V. N., Morineaux M., Vanreusel A., Sarradin P.-M., Biodiversity patterns, environmental drivers and indicator species on a high-temperature hydrothermal edifice, Mid-Atlantic Ridge. Deep Res. Part II Top. Stud. Oceanogr. 121, 177–192 (2015). [Google Scholar]
- 120.Stuart C. T., Brault S., Rowe G. T., Wei C.-L., Wagstaff M., McClain C. R., Rex M. A., Nestedness and species replacement along bathymetric gradients in the deep sea reflect productivity: A test with polychaete assemblages in the oligotrophic north-west Gulf of Mexico. J. Biogeogr. 44, 548–555 (2017). [Google Scholar]
- 121.Sutton T. T., Letessier T. B., Bardarson B., Midwater fishes collected in the vicinity of the Sub-Polar Front, Mid-North Atlantic Ocean, during ECOMAR pelagic sampling. Deep Res. Part II Top. Stud. Oceanogr. 98, 292–300 (2013). [Google Scholar]
- 122.C. L. Van Dover, The Ecology of Deep-Sea Hydrothermal Vents (Princeton Univ. Press, 2000). [Google Scholar]
- 123.Vetter E. W., Smith C. R., De Leo F. C., Hawaiian hotspots: Enhanced megafaunal abundance and diversity in submarine canyons on the oceanic islands of Hawaii. Mar. Ecol. 31, 183–199 (2010). [Google Scholar]
- 124.Wagstaff M. C., Howell K. L., Bett B. J., Billett D. S. M., Brault S., Stuart C. T., Rex M. A., β-Diversity of deep-sea holothurians and asteroids along a bathymetric gradient (NE Atlantic). Mar. Ecol. Prog. Ser. 508, 177–185 (2014). [Google Scholar]
- 125.Woodall L. C., Robinson L. F., Rogers A. D., Narayanaswamy B. E., Paterson G. L. J., Deep-sea litter: A comparison of seamounts, banks and a ridge in the Atlantic and Indian Oceans reveals both environmental and anthropogenic factors impact accumulation and composition. Front. Mar. Sci. 2, 3 (2015). [Google Scholar]
- 126.Yasuhara M., Danovaro R., Temperature impacts on deep-sea biodiversity. Biol. Rev. 91, 275–287 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Zeppilli D., Bongiorni L., Cattaneo A., Danovaro R., Santos R. S., Meiofauna assemblages of the Condor Seamount (North-East Atlantic Ocean) and adjacent deep-sea sediments. Deep Sea Res. Part II Top. Stud. Oceanogr. 98, 87–100 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/7/eaar4313/DC1
Table S1. Surrogate parameters related to biodiversity and deep-sea ecosystem structure and function and examples.
Table S2. Raw values and performance metric scores.
Table S3. Climate change metric results.


