ABSTRACT
Retinoic acid (RA) is required for pancreas specification in Xenopus and other vertebrates. However, the gene network that is directly induced by RA signalling in this context remains to be defined. By RNA sequencing of in vitro-generated pancreatic explants, we identified the genes encoding the transcription factor Hnf1β and the Wnt-receptor Fzd4/Fzd4s as direct RA target genes. Functional analyses of Hnf1b and Fzd4/Fzd4s in programmed pancreatic explants and whole embryos revealed their requirement for pancreatic progenitor formation and differentiation. Thus, Hnf1β and Fzd4/Fzd4s appear to be involved in pre-patterning events of the embryonic endoderm that allow pancreas formation in Xenopus.
KEY WORDS: Pancreas, Retinoic acid, Hnf1β, Fzd4
Summary: The direct RA target genes Hnf1b and Fzd4 act as mediators of RA function in the pre-patterning of the dorsal endoderm during Xenopus gastrulation, resulting in pancreas specification in this context.
INTRODUCTION
The molecular mechanisms of vertebrate pancreas development appear to be largely conserved across species. Although the cascade of transcriptional regulatory events leading to the formation of the different specialised exo- and endocrine cells is understood in some detail, the preceding regionalisation of the endoderm that allows for the specification of a common pool of pancreatic precursor cells remains to be defined more precisely (reviewed in Pan and Wright, 2011; Shih et al., 2013). Lineage-tracing experiments making use of gastrula-stage Xenopus embryos revealed that cells within the dorsal endoderm contribute to the formation of the pancreatic organ (Chalmers and Slack, 2000). It was further demonstrated that, during this initial phase of organ specification, pancreatic precursor cells form under the influence of retinoic acid (RA) (Chen et al., 2004; Stafford et al., 2004). An essential role of the RA signalling pathway in the context of pancreas development had been discovered previously in the zebrafish (Stafford and Prince, 2002) and was later found to be equally relevant for mammalian pancreas specification (Martín et al., 2005). The concept of a RA gradient within the dorsal endoderm forming during early gastrula stages of Xenopus development is further supported by the expression characteristics of the key enzyme for RA biosynthesis, Raldh2 (Aldh1a2 – Xenbase), and of the major RA-degrading enzyme, Cyp26a1, during gastrulation. These two genes exhibit nonoverlapping expression patterns in the dorsal mesoderm, with Raldh2 being expressed immediately adjacent to the pre-pancreatic endoderm (Hollemann et al., 1998; Chen et al., 2001).
Tissue explants have been used to achieve pancreatic gene expression. Asashima and colleagues were the first to demonstrate RA-induced expression of pancreatic marker genes in dorsal lip explants from early gastrula-stage Xenopus embryos (Moriya et al., 2000a). The same group also used early embryonic ectodermal explants to promote the formation of pancreatic tissue by treatment with a combination of activin and RA (Moriya et al., 2000b). A similar result was obtained by programming the same type of explant with a cocktail including VegT, Noggin and RA (Chen et al., 2004; Borchers and Pieler, 2010). This same protocol, which was also utilised in the current study, aims to mimic early key regulatory events, given that RA signalling together with inhibition of BMP activity makes it possible to convert ventral endomesodermal explants from gastrula-stage Xenopus embryos to a pancreatic fate (Pan et al., 2007). Similarly, experimental protocols for the in vitro generation of β cells from embryonic stem cells (ESCs) (Kroon et al., 2008; Rezania et al., 2012; Schulz et al., 2012; Pagliuca et al., 2014) or induced pluripotent stem cells (iPSCs) (Zhang et al., 2009; Schiesser et al., 2014; Shaer et al., 2015) all include application of RA.
To the best of our knowledge, the RA-dependent gene network that drives pancreatic fate during gastrula stages of embryogenesis remains to be defined. The identification of such RA-dependent regulators of pancreas development could further improve protocols for the in vitro generation of pancreatic tissue. With the aim of identifying RA-regulated genes involved in pancreas specification, we made use of the Xenopus ectodermal explant system and RNA-sequencing (RNA-seq) analyses resulting in the identification of two direct, endodermally expressed RA target genes, namely Hnf1b and Fzd4. Functional studies performed in vitro and in vivo revealed their requirement for pancreas development.
RESULTS
Programmed pancreatic explants recapitulate the in vivo temporal pattern of pancreatic gene expression
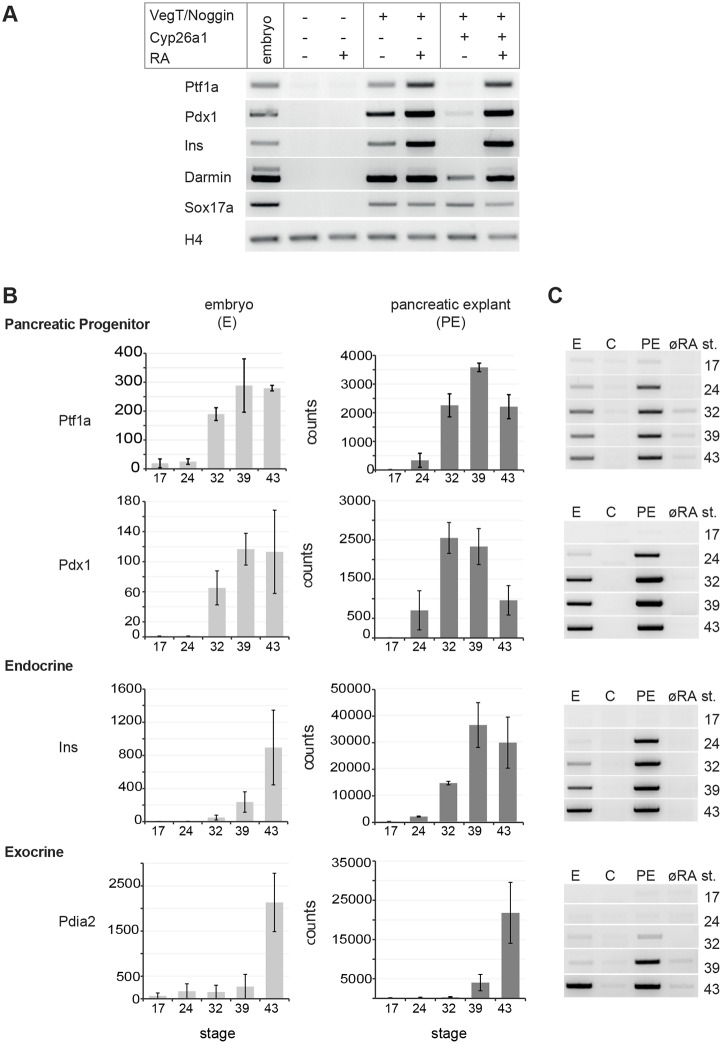
We previously demonstrated that programming of ectodermal explants from Xenopus blastulae with a cocktail containing Noggin, VegT and RA is sufficient to drive pancreatic gene expression (Chen et al., 2004). Furthermore, we also found that Noggin by itself induces significant expression levels of the RA-generating enzyme Raldh2 in this system (Pan et al., 2007). Thus, even in the absence of exogenously added RA, a basal level of pancreatic marker gene expression is induced by a combination of VegT and Noggin alone, which appears to be further increased by addition of excess RA (Fig. 1A). To create a control situation that is devoid of RA, we co-injected the RA-degrading enzyme Cyp26a1 together with VegT and Noggin, resulting in the almost complete loss of pancreatic gene expression, which was rescued upon addition of excess exogenous RA (Fig. 1A). We used whole-mount in situ hybridisation (WMISH) to estimate the proportion of pancreatic tissue that would form in such programmed explants at different concentrations of RA (Fig. S1A,B). Insulin expression was detected in singular, scattered cells, whereas Ptf1a and Pdx1 expression occurred in coherent groups of cells. Whereas an increase in RA concentration from 5 to 15 µM enhanced pancreatic gene expression to some extent, a further advance to 30 µM had no significant additional effect (Fig. S1C). Under all conditions, a significant portion of the cells in the explants was not positive for pancreatic marker gene transcription, probably because they had adopted a mesodermal or ectodermal fate (see below).
Fig. 1.
Comparative analysis of the temporal profile of pancreatic marker gene expression in vivo and in programmed explants. (A) Ectodermal explants isolated from stage 8/9 embryos and injected as indicated were treated with 5 µM RA and cultivated until the equivalent of stage 30. Detection of marker gene expression for the genes indicated was done by RT-PCR. (B) and (C) Transcript quantification using Nanostring analysis (B) and RT-PCR (C) of whole embryos (five embryos per condition) and explants (∼50 explants per condition) from two biological replicates with untreated and treated embryos grown to the equivalent of the developmental stages indicated. Average values are given as mean and error bars as s.e.m. C, unprogrammed explants; E, embryo; Ins, Insulin; øRA, programmed explants with blocked endogenous RA signalling (VegT/Noggin/Cyp26a1); PE, pancreatic explants (VegT/Noggin/RA).
We next examined the temporal pattern of pancreatic progenitor and differentiation marker gene transcription in programmed explants. Representative transcripts were analysed at different time points by RT-PCR as well as by Nanostring analysis in explants and whole embryos (Fig. 1B,C, Table S1). Absolute transcript levels for all pancreatic progenitor and differentiation markers increased more than 10-fold in pancreatic explants compared with whole embryos at maximum expression levels. We also observed a simultaneous onset of transcription for the progenitor markers Ptf1a and Pdx1 at stage 24 in RA-treated explants, but not in Noggin/VegT/Cyp26a1-programmed or control explants. Insulin expression occurred at stage 24 in explants, but, similar to Ptf1a and Pdx1, was somewhat delayed in embryos. The exocrine differentiation marker Pdia2, as well as the endocrine marker glucagon (RT-PCR data, not shown), first increased at the equivalent of stage 39 in pancreatic explants, which was slightly earlier than in stage 43 whole embryos, whereas amylase, as an additional exocrine marker gene, was induced at stage 43 in both explants and embryos (Table S1). Finally, Tm4sf3 (Tspan8 – Xenbase), a gene specific for the ventral pancreatic Anlage, was also detected in stage 43 RA-treated explants and control embryos. Induction of lung differentiation, as an example of another endodermal organ, reflected by the expression of surfactant protein, was observed in late-stage embryos but never in programmed explants (Table S1). As expected, early transient expression of the general endodermal marker Sox17 was observed in both pancreatic explants and whole embryos at early stages, but not in a RA-dependent manner. Conversely, early explant expression of Darmin, a different general endodermal marker, was strictly dependent on RA signalling and occurred at a level similar to that in the embryo. Significant levels of RA-independent gene transcription of mesodermal and (neuro-) ectodermal genes were detected in the explants (Table S1).
Taken together, these data revealed the partial but efficient conversion of the pluripotent ectodermal explants to a pancreatic fate, in principle following the temporal profile of pancreas organogenesis in the embryo.
Hnf1b and Fzd4 are early endodermal RA-responsive genes
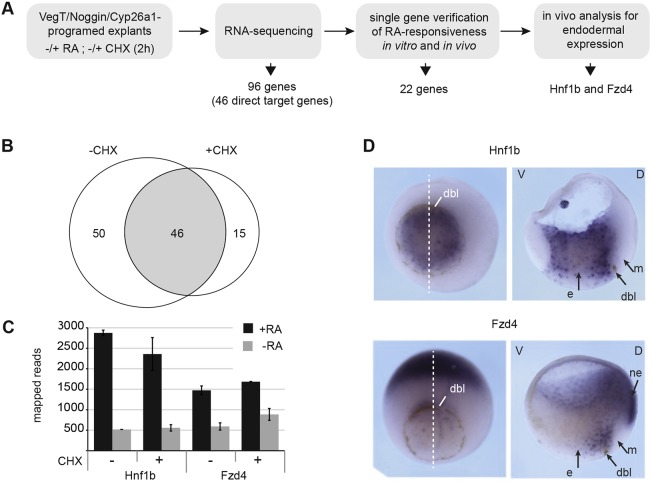
In an attempt to identify RA-induced genes that function in pancreas development, we further investigated the pancreatic explant system by using RNA-seq analysis. For the distinction of direct and indirect RA target genes, explants were treated with the translational inhibitor cycloheximide (CHX) (Fig. 2A). It was previously demonstrated that the gene encoding the RA-degrading enzyme, Cyp26a1, which we also found to be induced in the explant system, is a direct RA target gene (Ray et al., 1997; Abu-Abed et al., 1998). Cyp26a1 was induced in the presence of CHX within 1 h of the addition of RA, and its levels increased further 2 h after treatment initiation (Fig. S9A). Therefore, we performed transcriptome analysis for the identification of other RA target genes under the same conditions. In total, 96 genes were classified as RA targets, 46 of these as direct targets (Fig. 2B, Tables S2 and S3). Using Nanostring analysis, 82 candidate genes from the RNA-seq analysis were tested individually for their RA inducibility in whole embryos as well as in explants. Of these candidate genes, 41 were confirmed in both systems (Fig. S2A,C) and 22 of these were found to be reduced upon inhibition of RA signalling (Fig. S2B,C, Table S4).
Fig. 2.
Identification of Hnf1b and Fzd4 as direct endodermal RA target genes. (A) Experimental procedure for the identification of early direct RA target genes in the context of pancreas development. (B) Venn diagram comparing genes differentially expressed within 2 h of RA addition in the absence or presence of CHX. A total of 46 putative direct RA target genes were induced under both conditions. (C) RNA-seq results for RA-mediated Hnf1b and Fzd4 induction. The number of mapped reads 2 h after the addition of RA in the presence or absence of CHX is indicated. The data result from two biological replicates with ∼50 explants per condition. Average values are given as mean and error bars as s.e.m. (D) WMISH for Hnf1b and Fzd4 in gastrula-stage embryos. Whole embryos are depicted on the left-hand side and bisected embryos on the right-hand side. dbl, dorsal blastopore lip; e, endoderm; m, mesoderm; ne, neuroectoderm.
Previous microarray-based studies searching for RA-responsive genes have not made the distinction between direct and indirect target genes or different germ layers. Chen and colleagues (Zhang et al., 2013a) made use of whole embryos with downregulated RA signalling. In the same study, Hnf1b was listed as one of 138 genes downregulated by at least 2-fold in either stage 12, 23 or 34, although no further analyses were reported. The global screen for RAR-responsive genes performed by Blumberg and colleagues (Arima et al., 2005) used stage 18 embryos. There is an obvious overlap between the genes identified in these two previous screens and the current results, for example in respect to the Hox gene family.
RA target genes involved in pancreas specification are expected to be expressed in the dorsal endoderm of gastrula-stage embryos (Chalmers and Slack, 2000; Chen et al., 2004). WMISH analysis of the expression characteristics of the 22 primary candidate genes defined above revealed two genes with these expression characteristics, namely Hnf1b and Fzd4 (Fig. 2C,D). The other RA target genes were found to be expressed in the outer and/or internal involuting mesoderm, with some also expressed in the prospective neuroectoderm (Fig. S3).
Hnf1b was detected throughout the endoderm, with a slight enrichment in the dorsal area, as also confirmed by Nanostring analysis (Fig. 2D, Fig. S4). Fzd4 was most predominantly expressed in the territory of the endodermal pancreatic precursor cell population, as well as in the prospective neuroectoderm (Fig. 2D). Upon decreased endogenous RA signalling by Cyp26a1 RNA injection or by treatment with the RA-signalling inhibitor BMS453, both genes showed strongly reduced endodermal expression, upon treatment with exogenous RA increased expression (Fig. S5), confirming the in vivo relevance of RA signalling for the transcriptional control of both genes.
In summary, the screen for RA-induced genes with possible relevance for early events in pancreas development identified genes encoding the putative Wnt-receptor Fzd4 and the homeobox transcription factor Hnf1β. Both genes were expressed in the territory of gastrula-stage embryos where pancreatic precursor cells form and both were positively regulated by RA both in vitro and in vivo.
Hnf1β is required for pancreas development in vitro and in vivo
To examine whether Hnf1β mediates RA signalling in pancreas specification, we used an antisense Morpholino oligonucleotide (MO) affecting the splicing of Hnf1b heterogenous nuclear RNA (hnRNA), resulting in the production of a shortened protein that lacked both the DNA-binding and transactivation domains (Fig. S6). The downregulation of Hnf1b in the explant system led to a strong decrease in the expression of the pancreatic progenitor marker genes Ptf1a and Pdx1, as detected by RT-PCR analysis (Fig. 3A). Transcripts of the endo- and exocrine differentiation marker genes Insulin and Pdia2 were also strongly downregulated. Surprisingly, the expression of the endodermal marker gene Darmin was also significantly decreased, whereas that of another endodermal marker, Sox17a, was only slightly decreased.
Fig. 3.
Hnf1b is required for pancreas development in vitro and in vivo. (A) MO-mediated knockdown of Hnf1b in pancreatic explants. To demonstrate the specificity of the MO effect, RNA encoding a hormone-inducible version of Hnf1β (Hnf1b-GR) was co-injected and explants were treated with the GR inducer dexamethasone (DEX) together with RA at the equivalent of the gastrula stage. At the equivalent of stages 31 and 39, total RNA was isolated from ∼30 explants per condition and subjected to RT-PCR. Detection was of endogenous (endo) and injected Hnf1b (inj.), as well as for the marker genes indicated. The Hnf1b loss-of-function phenotype and its rescue was observed for four independent biological replicates. (B) Four-cell stage embryos were injected with RNA encoding β-galactosidase (glb1) and either Hnf1b-MO or a control-MO. At stage 32, embryos from two independent biological replicates were used for WMISH against Pdx1 and Ptf1a and a real-time PCR analysis for Pdx1, Ptf1a and Insulin. The graph indicates the fold change in tested markers in relation to Odc. ctr, uninjected embryos. (C) Four-cell stage embryos were injected with RNA encoding β-galactosidase alone or in combination with Hnf1b-GR RNA. At the gastrula stage, embryos were treated with dexamethasone (DEX) to induce Hnf1β function. WMISH against Pdx1 and Ptf1a at stage 32 is shown. Boxplots display the range of the percentage area of Pdx1 and endodermal Ptf1a domains in the endoderm observed in embryos from two independent biological replicates (see Fig. S7). By the use of ImageJ (https://imagej.net), Pdx1 and Ptf1a-positive areas were measured (orange dotted line) as a ratio of the whole endoderm (green dotted line). Values above the upper whisker, which is set at 1.5× interquartile range above the third quartile, are indicated as maximum outliers (°). (P-values in an unpaired Student's t-test **<0.01, ***<0.001).
To test for the specificity of these effects, we performed a rescue experiment by co-injecting RNA encoding a dexamethasone-inducible variant of HNF1β, namely Hnf1β-GR. Upon dexamethasone treatment at the equivalent of the gastrula stage, expression of Pdx1, Insulin, Pdia2, Darmin, and the known direct Hnf1b target gene Hnf4a (Thomas et al., 2001) was fully restored in MO-injected embryos (Fig. 3A). The rescue of Ptf1a expression resulted in weaker and more variable effects in independent experiments. The requirement of Hnf1β for the pan-endodermal marker Darmin correlated with the pan-endodermal expression of Hnf1β.
In whole embryos, downregulation of Hnf1b revealed correlating effects. Upon MO injection, endodermal expression of Pdx1 and Ptf1a was almost completely ablated, as revealed by WMISH analysis (Fig. 3B). Quantitative real-time PCR analysis confirmed the strongly reduced Ptf1a and Pdx1 expression and revealed a similar decrease in Insulin expression. Hence, endodermal expression of Hnf1b is required for pancreas specification. We also assayed for effects of Hnf1b loss on other endodermal organ primordia. Maintenance of Hnf1β function was critical not only for pancreas development, but also for liver and lung development (Fig. S11A). Furthermore, we examined the effect of Hnf1b overexpression on Ptf1a and Pdx1. WMISH analysis revealed a marked expansion of the endodermal Pdx1 and Ptf1a expression domains (Fig. 3C, Fig. S7). In terms of the effects of Hnf1b overexpression on the development of other endodermal organs, a liver marker was expanded and a lung marker was reduced, whereas Darmin expression was not affected (Fig. S11B). Given that both loss and gain of Hnf1β function influence the pancreatic progenitor field, we also asked whether Hnf1β can substitute for RA in pancreas specification. Thus, Hnf1β function was induced in programmed explants with blocked endogenous RA signalling. Under these conditions, Hnf1b rescued Darmin and Hnf4a expression, but none of the pancreatic marker genes were detected (Fig. S8).
Taken together, functional analysis of Hnf1b revealed that it is essential for specification of endodermal organs, including the pancreas, but that it is not the only gene that mediates the RA response in this context.
Fzd4 is required for pancreas development in vitro and in vivo
Fzd4 occurs as two alternative splice variants: Fzd4 and Fzd4s (Yam et al., 2005; Swain et al., 2005). Fzd4 is a putative transmembrane receptor in the Wnt pathway, whereas Fzd4s lacks a transmembrane domain and, therefore, is considered a secreted protein that contains a Wnt-binding domain. Results from our RNA-seq and RT-PCR analyses suggested that both variants are directly induced by RA (Fig. S9). However, reads specific for Fzd4s were found at 20-fold lower levels compared with nondiscriminatory Fzd4/Fzd4s reads (Table S5). Nanostring and WMISH probes against Fzd4 transcripts should detect both variants; however, WMISH probes specific for Fzd4s stained the entire gastrula embryo, probably reflecting unspecific binding events (data not shown).
To examine whether Fzd4/Fzd4s have a regulatory function in pancreas development, we downregulated the expression of both variants by using an MO antisense oligonucleotide that blocks translation (as described by Gorny et al., 2013). Upon Fzd4/Fzd4s knockdown in pancreatic explants, Ptf1a, Pdx1, Insulin and Sox17a expression was lost compared with mismatch control MO-injected samples (Fig. 4A). Reduced pancreatic progenitor as well as differentiation marker gene expression was also observed in embryos and confirmed by real-time PCR (Fig. 4C). Furthermore, CRISPR/Cas-mediated gene mutations were used as an alternative loss-of-function approach in the explant system. Upon Fzd4-gRNA co-injection, a phenotype similar to that resulting from MO-mediated knockdown was observed (Fig. 4B). DNA sequence analysis revealed a 100% mutation rate in the Fzd4 exon1, resulting in deletions and sequence alterations in the Fzd4/Fzd4s proteins (Fig. S10A). Potential off-targets were predicted as described (Stemmer et al., 2015) (Table S6) and mutations were not detected (Fig. S10B). We also tested the effects of downregulating Fzd4 function on the development of other endodermal organs; this resulted in the reduced expression of lung and liver markers, whereas the expression of a stomach/intestine marker expanded into more anterior territories (Fig. S12).
Fig. 4.
Fzd4/Fzd4s is required for pancreas development in vitro and in vivo. (A) Fzd4-mo or the corresponding mismatch-MO (mmo) were co-injected along with Vegt- and Noggin-encoding RNAs. At the equivalent of stage 28, total RNA was isolated from the programmed explants and subjected to RT-PCR as indicated. (B) Fzd4-gRNA was co-injected along with RNAs encoding Cas9, Vegt, and Noggin into one-cell stage embryos. Explants were cultivated until the equivalent of stage 35. RT-PCR was for the genes indicated. Mutation rate is given for Cas9 only or for Cas9 in combination with Fzd4-gRNA. For both loss-of-function approaches, ∼30 explants per condition from two independent biological replicates were used. (C) Downregulation of Fzd4/Fzd4s by Fzd4-morpholino injection. 4-cell stage embryos were injected with RNA coding for β-galactosidase (glb1) and either Fzd4/Fzd4s-mo or the corresponding mmo. At stage 35/39, embryos from two independent biological replicates were used for WMISH against the indicated pancreatic markers and a real-time PCR analysis for Pdx1, Ptf1a and Insulin. The graph indicates the fold change in tested markers in relation to Odc. Average values are given as mean and error bars as s.e.m. ctr, uninjected embryos.
Taken together, both loss-of-function approaches revealed a requirement of Fzd4 and/or Fzd4s for endodermal patterning for the formation of different organs, including the pancreas, in Xenopus.
DISCUSSION
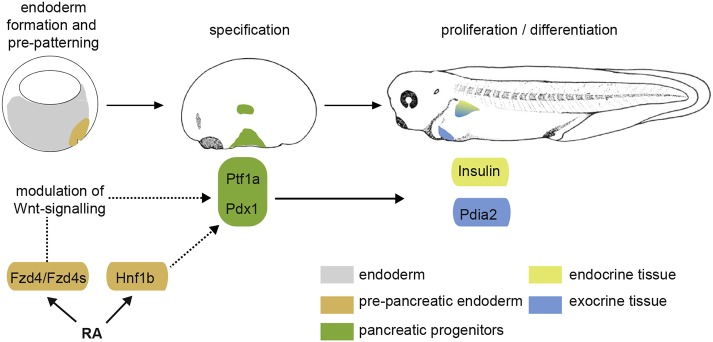
VegT/Noggin-programmed ectodermal explants from Xenopus embryos recapitulate the molecular events of pancreas specification and differentiation in a RA-dependent manner. By using this system, Hnf1b and Fzd4 were identified as direct RA target genes in the context of pancreas specification. Both genes appear to be involved in pre-patterning events of the embryonic endoderm that allow for pancreas formation. Hnf1β is a homeobox transcription factor functioning upstream of Pdx1 and Ptf1a, whereas Fzd4/Fzd4s appears to serve as a Wnt modulator, establishing adequate levels of Wnt signalling to allow for pancreas development (as shown schematically in Fig. 5).
Fig. 5.
Diagrammatic representation reflecting the role of RA signalling in pancreas specification during early Xenopus embryogenesis. During gastrulation, the expression of Fzd4 and Hnf1b is directly induced by RA. The overlapping activity of Fzd4 and Hnf1b establishes a pre-pancreatic domain within the dorsal endoderm. Fzd4/Fzd4s is a regulator of Wnt signalling within the dorsal endoderm, which modulates Wnt signalling to a level that allows the specification of pancreatic progenitors characterised by the co-expression of Ptf1a and Pdx1. Pancreatic progenitors subsequently proliferate and differentiate into endocrine and exocrine tissue.
Programming ectodermal explants to a pancreatic fate
The Xenopus explant system has been used to generate pancreatic tissue by the utilisation of simple protocols, including an endoderm-inducing factor and RA (Moriya et al., 2000b; Chen et al., 2004). Protocols for the generation of pancreatic tissue from hESCs or iPSCs similarly include RA, even though such multistep protocols are more complex and include multiple signalling molecules (Pagliuca et al., 2014; Shaer et al., 2015).
In the current study, we used the maternal transcription factor VegT to promote endoderm formation and created a dorsal endodermal environment by application of the BMP inhibitor Noggin. With the addition of RA, we aimed to recapitulate in vivo RA secretion from the dorsal mesoderm during gastrulation. The temporal profile of pancreatic progenitor and differentiation marker gene expression in such explants correlated with the pattern observed for pancreas development in whole embryos. These findings open the way for the use of pancreatic explants in studies aiming to elucidate the molecular mechanisms behind pancreas specification induced by RA.
However, there are some limitations of this explant system. As revealed by WMISH, the yield of pancreatic cells varied significantly and was never complete. A major portion of the cells either adopted a different endodermal state or developed a mesodermal or neuroectodermal fate, which could be indirectly induced by the activity of VegT. In line with such results, we observed robust expression of mesodermal and neuroectodermal marker genes, even though their level of expression was lower than that of general endodermal and pancreatic genes.
Hnf1b is a direct RA target required for pancreas development
We identified Hnf1b as a direct RA-responsive gene expressed in the early embryonic endoderm. Induction of Hnf1b by RA had previously been described for the murine hindbrain (Sirbu and Duester, 2006). In the explant system used in the current study, we found Hnf1b to be expressed in a RA-dependent manner. The idea of RA responsiveness was further supported by the prediction of two RA-responsive elements (RAREs) in the genomic locus of the mouse Hnf1b gene (Power and Cereghini, 1996; Pouilhe et al., 2007).
RA-induced expression of Hnf1b in the dorsal endoderm of gastrula-stage embryos appears to have a role in endoderm patterning. Our functional analyses in programmed explants and whole embryos revealed an essential role for Hnf1b in pancreas development, correlating with a loss of Ptf1a and Pdx1 expression. These findings are consistent with observations in HNF1b-mutant mice (Haumaitre et al., 2005). Interestingly, Maestro et al. (2003) defined Hnf1b-positive cells as a cellular stage distinct from Pdx1/p48 (Acot10 – Mouse Genome Informatics) multipotent pre-pancreatic cells in the mouse. Although we agree with defining this endodermal field as containing multipotent pancreatic cells in Xenopus, on the basis of the overlap of Hnf1b with Pdx1 expression domains in gastrula-stage endoderm, Hnf1β-positive cells do not yet express p48 (Ptf1a). It also appears that, in Xenopus, the onset of expression of Pdx1 and Hnf1b occurs simultaneously, in principle. Humans with a monogenetic form of diabetes, referred to as maturity onset diabetes of the young 5 (MODY 5) (Horikawa et al., 1997), are mutant in HNF1B. A previous study identified Hnf1β as a downstream target for RA in the context of zebrafish pancreas development (Song et al., 2007). However, direct induction of Hnf1β by RA and the early requirement of Hnf1β during gastrula and/or neurula stages were not revealed in this previous study.
In gastrula-stage embryos, RA-responsive Hnf1b expression was restricted to the dorsal endoderm. Hnf1b also exhibits RA-independent, pan-endodermal expression during early embryogenesis (Demartis et al., 1994); Hnf1β was previously identified as a direct downstream target of Sox17α, a regulator of endoderm development, which is expressed in an overlapping manner (Hudson et al., 1997; Clements et al., 1999; Sinner et al., 2004). Pan-endodermal expression has also been described for Darmin, a putative metalloprotease (Pera et al., 2003). Similar to Hnf1β, expression of Darmin was RA responsive in the explant system. We further observed a loss of Darmin expression upon Hnf1β downregulation, as well as Hnf1β-triggered induction of Darmin in RA-ablated explants. These observations clearly revealed Darmin to be a gene that occurs downstream of Hnf1b.
Hnf1b overexpression resulted in a modest expansion of the pancreatic progenitor domain, but this was not sufficient for ectopic pancreatic progenitor gene expression elsewhere in the endoderm, comparable to what has been previously described following combined ectopic expression of Ptf1a and Pdx1 (Afelik et al., 2006). Furthermore, Hnf1β alone was not sufficient to substitute RA in the induction of pancreatic marker genes in the explant system, suggesting the requirement for one or more additional RA-regulated factors to allow for induction of a pancreatic fate. We also detected effects of modulating Hnf1β activity on the development of other endodermal organs, perhaps reflecting a more general role for Hnf1β in this context. Such a function correlates with the pan-endodermal expression of Hnf1b in gastrula-stage embryos.
Fzd4/Fzd4s is a direct RA target gene required for pancreas development
Expression of Fzd4 during gastrulation was initially described for the prospective neuroectoderm (Shi and Boucaut, 2000). We observed endodermal expression of Fzd4 in RA-treated embryos and also in untreated embryos upon prolonged staining. In the pancreatic explant system, we identified Fzd4 as a direct RA target gene. Co-expression of two alternative splice variants, the transmembrane form Fzd4 and the secreted soluble form Fzd4s, has been described for Xenopus embryos (Sagara et al., 2001; Yam et al., 2005; Swain et al., 2005). In our RNA-seq experiments, a significant number of mapped reads for Fzd4/Fzd4s were induced by RA in the presence of a translational inhibitor, indicating that the expression of both Fzd4 variants is directly regulated by RA. Fzd4s-specific reads appeared at a lower level compared with the number of nondiscriminatory Fzd4/Fzd4s reads, indicating that the transmembrane version of Fzd4 is predominantly expressed. It has been reported that Fzd4 mRNA is provided maternally with increasing expression during gastrulation, whereas Fzd4s expression is only initiated during gastrulation (Swain et al., 2005). Remarkably, Fzd4/Fzd4s expression overlapped with the territory of putative pancreatic precursor cells in the dorsal endoderm, adjacent to the expression domain of the RA-generating enzyme RALDH2 in the dorsal mesoderm (Chen et al., 2001).
Previous loss-of-function studies with Fzd4/Fzd4s in Xenopus revealed defects in fin formation and neural crest migration (Gorny et al., 2013). Comparative transcriptome analyses in the mouse identified Fzd4 transcripts to be enriched in pancreatic progenitor cells compared with liver progenitors, although a function of Fzd4/Fzd4s in pancreas development was not demonstrated experimentally (Rodriguez-Seguel et al., 2013). Using two independent loss-of-function approaches, we found that Fzd4/Fzd4s is required for pancreas specification and differentiation in programmed Xenopus explants. Results obtained upon downregulation of Fzd4/Fzd4s in whole embryos provided further support for this notion, because significantly reduced levels of pancreatic progenitor and differentiation marker gene expression were observed.
Fzd4/Fzd4s as modulators of Wnt signalling in pancreas development
It was proposed by several previous studies that both canonical and noncanonical Wnt-signalling activities must be precisely controlled in the anterior endoderm of early embryos to maintain foregut identity and, thus, allow endodermal patterning appropriate for endodermal organ formation (McLin et al., 2007; Li et al., 2008; Damianitsch et al., 2009; Zhang et al., 2013b; Rodriguez-Seguel et al., 2013). Fzd4 appears to define an activity that is also involved in this process. It encodes a transmembrane protein described to function as a receptor in both canonical and noncanonical Wnt signalling downstream of Wnt5a (Umbhauer et al., 2000; Mikels and Nusse, 2006). Conversely, Fzd4s exhibits structural similarities with secreted frizzled related proteins (Rattner et al., 1997) and was demonstrated to bind Wnt ligands, thereby functioning as an activator and/or inhibitor of canonical Wnt signalling, depending on the identity of a given Wnt ligand (Sagara et al., 2001; Swain et al., 2005; Gorny et al., 2013). Another endodermally expressed Wnt receptor, Fzd7, was shown to modulate canonical as well as noncanonical Wnt signalling positively or negatively in a concentration-dependent manner, promoting foregut fate and thereby pancreas development. The loss of Fzd7 was shown to result in agenesis of liver and pancreas, with an overall loss of foregut identity (Zhang et al., 2013b). We observed a similar foregut phenotype upon Fzd4/Fzd4s downregulation in vivo. Thus, a precisely regulated concentration of the transmembrane receptor Fzd4 and/or secreted Fzd4s might be required to allow for pancreas development in the foregut. To obtain such concentration levels at the appropriate time during endoderm development, a time-controlled expression system for Fzd4/Fzd4s would be required. However, our initial attempts using a Tet-inducible system have not yet been successful. In summary, we propose that Fzd4/Fzd4s functions as a modulator of Wnt signalling downstream of RA in the context of endodermal patterning that allows for pancreas development in Xenopus.
MATERIALS AND METHODS
Microinjections and ectodermal explants
Xenopus laevis embryos were obtained by in vitro fertilisation and staged according to Nieuwkoop and Faber (1967). Capped sense RNAs were transcribed using the mMessage mMachine kits (Ambion). The following linearisation and transcription conditions were used with indicated amounts of RNA injected per embryo: VegtT (NotI/Sp6; 500 pg; Zhang and King, 1996), Noggin (NotI/Sp6; 500 pg; Smith et al., 1993), Cyp26a1 (MluI/T3; 2 ng; Hollemann et al., 1998), Hnf1β-GR (NotI/Sp6; 800 pg; Vignali et al., 2000), GFP (NotI/Sp6; 200 pg; Rubenstein et al., 1997) and β-galactosidase (NotI/Sp6; 200 pg; Chitnis et al., 1995). RNAs were injected in a volume of 4 nl as follows: one-cell stage animally (Fig. 4B; Fig. S10), two of two blastomeres animally (Fig. 1; Fig. 2; Fig. 3A; Fig. 4A; Fig. S1; Fig. S2A; Fig. S6; Fig. S8; Fig. S9), two of four blastomeres vegetally dorsal (Fig. 3B/C; Fig. S7; Fig. S11) or animally and vegetally dorsal (Fig. S2B; Fig. S4) or marginally dorsal (Fig. S5A), and two of eight vegetally dorsal (Fig. 4C; Fig. S12). Ectodermal explants were dissected from the blastocoel roof of stage 8/9 embryos and cultured in salt solution [88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 2.4 mM NaHCO3, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 10 mM HEPES, pH 7.8] with antibiotics [ampicillin (100 µg/ml), kanamycin (10 µg/ml) and gentamycin (10 µg/ml), ROTH] on 0.7% agarose at 14°C until control embryos had reached the desired stage.
Morpholino oligonucleotides and CRISPR/Cas
For knockdown experiments, antisense HNF1β-MO (CCTCGCTGTGAACAAAA CACAAA; 25 ng/embryo for WMISH/qPCR and 55 ng/embryo for explants), control-MO (CCTCTTACCTCAGTTACAATTTATA; same amounts as for HNF1β-MO), Fzd4/Fzd4s-MO (Gorny et al., 2013; ATTATTCTTCTTCTGTTGCCG CTGA; 5 ng/embryo for WMISH/qPCR and 45 ng/embryo for explants) and Fzd4-mismatch-MO (ATTATTaTTaTTCTaTTGCaGCTaA; same amounts as for Fzd4/Fzd4s-MO). For knockout experiments using CRISPR/Cas, 3 ng/embryo capped sense RNA of Cas9 (Blitz et al., 2013), prepared by Acc651 linearisation and transcribed with the mMessage mMachine T7 kit (Ambion), was injected animally at the one-cell stage alone or together with 300 pg/embryo uncapped sense Fzd4-gRNA, linearised with DraI and transcribed with a MEGAscript T7 kit (Invitrogen). Fzd4-gRNA was generated by cloning the oligonucleotides 5′phosp-TAGGCACATGGTGATCCTGATG and 5′phosp-AAACCATCAGGATCACCATGTG into pDR274 (Addgene). For target- and predicted potential off-target (CRISPR/Cas Target online predictor; CCTop; Stemmer et al., 2015) mutation analysis, genomic DNA from 50 explants, per condition and biological replicate, was isolated by using a ‘DNeasy Blood and Tissue Kit’ (Qiagen). The region around the target site and predicted off-target sites was amplified and cloned into the pGem®-T Easy vector (Promega) for sequence analysis.
Chemical treatments of embryos and explants
Treatments with 5 µM RA (all-trans RA, SIGMA) (variations are indicated) were done in the corresponding buffer at embryonic stage 11 for 1 h at 12°C under light protection. After the treatment, embryos and explants were cultivated until the controls reached the desired developmental stage. Treatments with 10 µg/ml Cyclohexamide (SIGMA) started 30 min prior to additional treatments. Whole embryos were incubated with 0.25 µM BMS453 (a gift from Bristol Myers Squibb; Chen et al., 1995) at stage 8/9 until stage 12. Afterwards, embryos were cultivated until they reached the desired stage. Injected embryos or ectodermal explants were cultivated in the dark in the presence of 10 µM dexamethasone (Sigma) at the equivalent of stage 11 until the desired stage was reached.
Whole-mount in situ hybridisation
WMISH of whole embryos and explants was done as previously described (Harland, 1991) with modifications (Hollemann and Pieler, 1999). The probes were prepared as follows: Cebpd (EcoRI/T7; Ikuzawa et al., 2005), Cyp26a1 (ClaI/T7; Hollemann et al., 1998), Darmin (EcoRI/T7; Pera et al., 2003), Dhrs3 (EcoRI/SP6; Kam et al., 2010), Foxh1 (EcoRI/T7; Kofron et al., 2004), IFABP (NcoI/Sp6; Henry et al., 1996), Fst (SalI/T7; Tashiro et al., 1991), Fzd4 (BamHI/T7; Swain et al., 2005), Gbx2 (ApaI/SP6; Maczkowiak et al., 2010), hHex (NotI/T7; Newman et al., 1997), Hnf1β (BamHI/T7; Vignali et al., 2000), Hoxa1-b (SalI/T7; Sive and Cheng, 1991), Hoxb1 (EcoRI/SP6; Nieto et al., 1992), Hoxd1 (EcoRI/T7; Sive and Cheng, 1991), Hoxd4 (EcoRI/T7; Klein et al., 2002), Igf3 (SalI/T7; Richard-Parpaillon et al., 2002), Ins (NotI/T7; Shuldiner et al., 1989), Lhx1 (XhoI/T7; Taira et al., 1994), Meis3a (ClaI/T3; Salzberg et al., 1999), Nkx2.1 (NotI/T7; Hollemann and Pieler, 2000), Nkx6.2 (XhoI/T7; Dichmann and Harland, 2011), Pdia2 (BamHI/T7; Sogame et al., 2003), Pdx1 (ApaI/SP6; Wright et al., 1989), Prph (SalI/T7; Sharpe et al., 1989), Ptf1a (NotI/T7; Afelik et al., 2006), Xl.45046 (SalI/T7; BioScience IMAGp998J07121170Q), Xl.47239 (SmaI/T7; BioScience IRBHp990G0486), Xl.51509 (SalI/T7; BioScience IMAGp998L11929 6Q) and Xl.57926 (ClaI/T7; BioScience IMAGp998C1718900Q).
RT-PCR and real-time RT-PCR
Total RNA from whole embryos and explants was isolated using the peqGOLD Trifast reagent (peQlab) and reverse transcribed by the use of random hexamer oligonucleotides (Invitrogen) and MuLV reverse transcriptase (Roche). The following oligonucleotides were used for target amplification in RT-PCR: injected CYP26a1 (GTCGACCTGTGGATCCAAAGA/GATGCGTCTTGTAGATGCGAC); endogenous CYP26a1 (CCCGGAGATTCCTCGAGGTT/GACACCACGACCAAGACCCG); Darmin (GGTTACCGATTACTTGGAGG/AGCATCATCTGGTCCACCAA); Fzd4s (CATCAGGATCACCATGTGCCAG/GAAAGTAAACCCCCTGTGCTGAG); Glucagon (AGAATTTATTGAGTGGTTGA/ATCGGCATGTCTTCTGTCC); H4 (CGGGATAACATTCAGGGTATCACT/ATCCATGGCGGTAACTGTCTTCCT); injected HNF1β (CG GGGACATGTGCAAGTTCT/CAAGCTACTTGTTCTTTTTGC); HNF1β (AAAGGGCAGAAGT GGACAGG/ATGCAGCACGTTTTTGGGTC); Hnf4α (AGACTCCCCAACCATCTCCA/CGCTTTCCCAAAGAGGCAAC); Insulin (ATGGCTCTATGGATGCAGTG/AGAGAACATGTGCTGT GGCA); Odc (GCCATTGTGAAGACTCTCTCCATTC/TTCGGGTGATTCCTTGCCAC); Pdx1 (GTCCTCCAGACATCTCACCG/AGCATGACTGCCAGCTCTAC); Pdia2 (GGAGGAAAGAGG GACCAA/GCGCCAGGGCAAAAGTG); Ptf1a (GTTGTCAGAACGGCCAAAGT/GGTACCGAGTGGAACCAAAG); and Sox17α (CAAGAGACTGGCACAGCAGA/CTGCTTGGGGTTCCCTGTAG). Oligonucleotides that were different for real-time PCR were as follows: Pdx1 (GTCCTCCAGACATCTCA CCG/AGCATGACTGCCAGCTCTAC) and Ptf1a (GGTACAGTCCGATCTGCCGC/GGAGTCCAC ACTTTGGCCGT). The iQ SYBR Green Supermix (Bio-Rad) and the iCycler iQ detection system (Bio-Rad) were used for measurements. Relative expression was calculated by normalising to the expression levels of Odc. Both RT- and real-time RT-PCR were done for at least two independent biological replicates. Error bars result from the standard error of the mean (s.e.m.).
Library preparation for RNA sequencing
Library preparation for RNA sequencing was performed using the TruSeq Stranded Total RNA with Ribo-Zero Gold kit removing both cytoplasmic and mitochondrial rRNA (Illumina, Cat. No. RS-122-2201). RNA samples from two independent experiments with ∼50 explants for each condition and ∼200 ng of total RNA were used as starting material. Accurate quantitation of cDNA libraries was performed by using the QuantiFluor™ dsDNA System (Promega). The size range of final cDNA libraries was determined by applying the DNA 1000 chip on the Bioanalyzer 2100 from Agilent (280 bp). cDNA libraries were amplified and sequenced by using the cBot and HiSeq2000 from Illumina with 50 bp single-end chemistry.
Data pre-processing and bioinformatics analysis
The sequence intensity images were transformed to bcl files (BaseCaller) and were demultiplexed to fastq files with CASAVA (version 1.8.2). The quality of resulting sequence reads was checked by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Obtained sequence reads were aligned to the transcript reference sequences of Xenopus tropicalis (kindly provided by Michael J. Gilchrist; Gilchrist et al., 2004). Reads that could not be characterised were also aligned to selected X. laevis transcriptome sequences (UniGene). Alignment was performed using Bowtie2 (version 2.1.0) in local alignment mode allowing six mismatches within 50 bases (Langmead and Salzberg, 2012). Conversion of resulting alignment files, sorting, filtering of unique hits and counting were conducted with samtools (Li et al., 2009). Read count data were analysed in the R/Bioconductor environment (http://www.bioconductor.org) loading edgeR (Robinson et al., 2010). Counts were normalised to trimmed mean of M-values and the dispersion was estimated. For the detection of differentially expressed genes, a test based on a generalised linear model likelihood ratio assuming negative binominal data distribution was performed via edgeR. Candidate genes were filtered to a minimum of a 2-fold change difference from the control and a FDR-corrected P-value of <0.05. For transcript-specific determination of Fzd4/Fzd4s abundances, the reads were aligned to the X. laevis genome version Xenla9.1 using the STAR alignment software (Dobin et al., 2013) allowing for two mismatches. Reads mapping to the whole Fzd4 gene region (Fzd4/Fzd4s) and the annotated Fzd4 intron only (Fzd4s) were counted using the bedtools coverage command (http://bedtools.readthedocs.io).
Nanostring analysis
For the Nanostring analysis, 600 ng of total RNA from five embryos or 50 explants was used. The counts were normalised in two steps using the nSolver software (https://www.nanostring.com/products/analysis-software/nsolver). The counts were initially normalised with respect to the mean of positive control counts and then normalised to the geometric mean of the housekeeping gene encoding ornithine carboxylase (odc). Finally, to consider the background, the mean and 2-fold of the standard deviation of the eight negative controls were subtracted. Values less than 1 were set to 1. Data from two independent experiments (A and B) were used to calculate a mean value. Error bars indicate the s.e.m.
Supplementary Material
Acknowledgements
The authors would like to thank Herbert Steinbeisser and Lilian Kaufmann for providing Fzd4/Fzd4s DNA constructs and the Fzd4/Fzd4s-morpholino. Furthermore, we thank Anne-Helene Monsoro-Burq (gbx2), Hui Zhao (dhrs3), Monica Vettel (prph) and Richard Harland (nkx6.2) for making the corresponding DNA constructs available.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualisation: T.P.; Methodology: M.B.G.-B., C.P., T.L.; Software: C.P., T.L.; Validation: C.P., T.L.; Formal analysis: C.P., T.L.; Investigation: M.B.G.-B.; Writing - original draft: T.P., M.B.G.-B.; Writing - review & editing: T.P.; Supervision: T.P.; Project administration: T.P.; Funding acquisition: T.P.
Funding
This work was funded by the Georg-August-Universität Göttingen. Deposited in PMC for immediate release.
Data availability
RNA sequencing data have been submitted to the GEO repository under accession number GSE112718.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.161372.supplemental
References
- Abu-Abed S. S., Beckett B. R., Chiba H., Chithalen J. V., Jones G., Metzger D., Chambon P. and Petkovich M. (1998). Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha. J. Biol. Chem. 273, 2409-2415. 10.1074/jbc.273.4.2409 [DOI] [PubMed] [Google Scholar]
- Afelik S., Chen Y. and Pieler T. (2006). Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 20, 1441-1446. 10.1101/gad.378706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima K., Shiotsugu J., Niu R., Khandpur R., Martinez M., Shin Y., Koide T., Cho K. W., Kitayama A., Ueno N. et al. (2005). Global analysis of RAR-responsive genes in the Xenopus neurula using cDNA microarrays. Dev. Dyn. 232, 414-431. 10.1002/dvdy.20231 [DOI] [PubMed] [Google Scholar]
- Blitz I. L., Biesinger J., Xie X. and Cho K. W. Y. (2013). Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis 51, 827-834. 10.1002/dvg.22719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A. and Pieler T. (2010). Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (Basel) 1, 413-426. 10.3390/genes1030413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers A. D. and Slack J. M. (2000). The Xenopus tadpole gut: fate maps and morphogenetic movements. Development 127, 381-392. [DOI] [PubMed] [Google Scholar]
- Chen J. Y., Penco S., Ostrowski J., Balaguer P., Pons M., Starrett J. E., Reczek P., Chambon P. and Gronemeyer H. (1995). RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. EMBO J. 14, 1187-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Pollet N., Niehrs C. and Pieler T. (2001). Increased XRALDH2 activity has a posteriorizing effect on the central nervous system of Xenopus embryos. Mech. Dev. 101, 91-103. 10.1016/S0925-4773(00)00558-X [DOI] [PubMed] [Google Scholar]
- Chen Y., Pan F. C., Brandes N., Afelik S., Sölter M. and Pieler T. (2004). Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev. Biol. 271, 144-160. 10.1016/j.ydbio.2004.03.030 [DOI] [PubMed] [Google Scholar]
- Chitnis A., Henrique D., Lewis J., Ish-Horowicz D. and Kintner C. (1995). Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 375, 761-766. 10.1038/375761a0 [DOI] [PubMed] [Google Scholar]
- Clements D., Friday R. V. and Woodland H. R. (1999). Mode of action of VegT in mesoderm and endoderm formation. Development 126, 4903-4911. [DOI] [PubMed] [Google Scholar]
- Damianitsch K., Melchert J. and Pieler T. (2009). XsFRP5 modulates endodermal organogenesis in Xenopus laevis. Dev. Biol. 329, 327-337. 10.1016/j.ydbio.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Demartis A., Maffei M., Vignali R., Barsacchi G. and De Simone V. (1994). Cloning and developmental expression of LFB3/HNF1β transcription factor in Xenopus laevis. Mech. Dev. 47, 19-28. 10.1016/0925-4773(94)90092-2 [DOI] [PubMed] [Google Scholar]
- Dichmann D. S. and Harland R. M. (2011). Nkx6 genes pattern the frog neural plate and Nkx6.1 is necessary for motoneuron axon projection. Dev. Biol. 349, 378-386. 10.1016/j.ydbio.2010.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M. and Gingeras T. R. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15-21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist M. J., Zorn A. M., Voigt J., Smith J. C., Papalopulu N. and Amaya E. (2004). Defining a large set of full-length clones from a Xenopus tropicalis EST project. Dev. Biol. 271, 498-516. 10.1016/j.ydbio.2004.04.023 [DOI] [PubMed] [Google Scholar]
- Gorny A.-K., Kaufmann L. T., Swain R. K. and Steinbeisser H. (2013). A secreted splice variant of the Xenopus frizzled-4 receptor is a biphasic modulator of Wnt signalling. Cell Commun. Signal. 11, 89 10.1186/1478-811X-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland R. M. (1991). In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36, 685-695. 10.1016/S0091-679X(08)60307-6 [DOI] [PubMed] [Google Scholar]
- Haumaitre C., Barbacci E., Jenny M., Ott M. O., Gradwohl G. and Cereghini S. (2005). Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc. Natl. Acad. Sci. USA 102, 1490-1495. 10.1073/pnas.0405776102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. L., Brivanlou I. H., Kessler D. S., Hemmati-Brivanlou A. and Melton D. A. (1996). TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development 122, 1007-1015. [DOI] [PubMed] [Google Scholar]
- Hollemann T. and Pieler T. (1999). Xpitx-1: a homeobox gene expressed during pituitary and cement gland formation of Xenopus embryos. Mech. Dev. 88, 249-252. 10.1016/S0925-4773(99)00184-7 [DOI] [PubMed] [Google Scholar]
- Hollemann T. and Pieler T. (2000). Xnkx-2.1: a homeobox gene expressed during early forebrain, lung and thyroid development in Xenopus laevis. Dev. Genes Evol. 210, 579-581. 10.1007/s004270000098 [DOI] [PubMed] [Google Scholar]
- Hollemann T., Chen Y., Grunz H. and Pieler T. (1998). Regionalized metabolic activity establishes boundaries of retinoic acid signalling. EMBO J. 17, 7361-7372. 10.1093/emboj/17.24.7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa Y., Iwasaki N., Hara M., Furuta H., Hinokio Y., Cockburn B. N., Lindner T., Yamagata K., Ogata M., Tomonaga O. et al. (1997). Mutation in hepatocyte nuclear factor-1β gene (TCF2) associated with MODY. Nat. Genet. 17, 384-385. 10.1038/ng1297-384 [DOI] [PubMed] [Google Scholar]
- Hudson C., Clements D., Friday R. V., Stott D. and Woodland H. R. (1997). Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell 91, 397-405. 10.1016/S0092-8674(00)80423-7 [DOI] [PubMed] [Google Scholar]
- Ikuzawa M., Kobayashi K., Yasumasu S. and Iuchi I. (2005). Expression of CCAAT/enhancer binding protein delta is closely associated with degeneration of surface mucous cells of larval stomach during the metamorphosis of Xenopus laevis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 140, 505-511. 10.1016/j.cbpc.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Kam R. K. T., Chen Y., Chan S.-O., Chan W.-Y., Dawid I. B. and Zhao H. (2010). Developmental expression of Xenopus short-chain dehydrogenase/reductase 3. Int. J. Dev. Biol. 54, 1355-1360. 10.1387/ijdb.092984rk [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. L., Strausberg R. L., Wagner L., Pontius J., Clifton S. W. and Richardson P. (2002). Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev. Dyn. 225, 384-391. 10.1002/dvdy.10174 [DOI] [PubMed] [Google Scholar]
- Kofron M., Puck H., Standley H., Wylie C., Old R., Whitman M. and Heasman J. (2004). New roles for FoxH1 in patterning the early embryo. Development 131, 5065-5078. 10.1242/dev.01396 [DOI] [PubMed] [Google Scholar]
- Kroon E., Martinson L. A., Kadoya K., Bang A. G., Kelly O. G., Eliazer S., Young H., Richardson M., Smart N. G., Cunningham J. et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443-452. 10.1038/nbt1393 [DOI] [PubMed] [Google Scholar]
- Langmead B. and Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357-359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Rankin S. A., Sinner D., Kenny A. P., Krieg P. A. and Zorn A. M. (2008). Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 22, 3050-3063. 10.1101/gad.1687308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G. and Durbin R. (2009). The sequence alignment/Map format and SAMtools. Bioinformatics 25, 2078-2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maczkowiak F., Matéos S., Wang E., Roche D., Harland R. and Monsoro-Burq A. H. (2010). The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Dev. Biol. 340, 381-396. 10.1016/j.ydbio.2010.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro M. A., Boj S. F., Luco R. F., Pierreux C. E., Cabedo J., Servitja J. M., German M. S., Rousseau G. G., Lemaigre F. P. and Ferrer J. (2003). Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum. Mol. Genet. 12, 3307-3314. 10.1093/hmg/ddg355 [DOI] [PubMed] [Google Scholar]
- Martín M., Gallego-Llamas J., Ribes V., Kedinger M., Niederreither K., Chambon P., Dollé P. and Gradwohl G. (2005). Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev. Biol. 284, 399-411. 10.1016/j.ydbio.2005.05.035 [DOI] [PubMed] [Google Scholar]
- McLin V. A., Rankin S. A. and Zorn A. M. (2007). Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207-2217. 10.1242/dev.001230 [DOI] [PubMed] [Google Scholar]
- Mikels A. J. and Nusse R. (2006). Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115 10.1371/journal.pbio.0040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya N., Komazaki S. and Asashima M. (2000a). In vitro organogenesis of pancreas in Xenopus laevis dorsal lips treated with retinoic acid. Dev. Growth Differ. 42, 175-185. 10.1046/j.1440-169x.2000.00498.x [DOI] [PubMed] [Google Scholar]
- Moriya N., Komazaki S., Takahashi S., Yokota C. and Asashima M. (2000b). In vitro pancreas formation from Xenopus ectoderm treated with activin and retinoic acid. Dev. Growth Differ. 42, 593-602. 10.1046/j.1440-169x.2000.00542.x [DOI] [PubMed] [Google Scholar]
- Newman C. S., Chia F. and Krieg P. A. (1997). The XHex homeobox gene is expressed during development of the vascular endothelium: overexpression leads to an increase in vascular endothelial cell number. Mech. Dev. 66, 83-93. 10.1016/S0925-4773(97)00092-0 [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Bradley L. C., Hunt P., Das Gupta R., Krumlauf R. and Wilkinson D. G. (1992). Molecular mechanisms of pattern formation in the vertebrate hindbrain. Ciba Found Symp. 165, 92-102; discussion 102-107. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D. and Faber J. (1967). Normal Table of Xenopus laevis, 2nd edn. Amsterdam: (Daudin) North Holland Publishing Co. [Google Scholar]
- Pagliuca F. W., Millman J. R., Gürtler M., Segel M., Van Dervort A., Ryu J. H., Peterson Q. P., Greiner D. and Melton D. A. (2014). Generation of functional human pancreatic beta cells in vitro. Cell 159, 428-439. 10.1016/j.cell.2014.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F. C. and Wright C. (2011). Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 240, 530-565. 10.1002/dvdy.22584 [DOI] [PubMed] [Google Scholar]
- Pan F. C., Chen Y., Bayha E. and Pieler T. (2007). Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech. Dev. 124, 518-531. 10.1016/j.mod.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Pera E. M., Martinez S. L., Flanagan J. J., Brechner M., Wessely O. and De Robertis E. M. (2003). Darmin is a novel secreted protein expressed during endoderm development in Xenopus. Gene Expr. Patterns 3, 147-152. 10.1016/S1567-133X(03)00011-5 [DOI] [PubMed] [Google Scholar]
- Pouilhe M., Gilardi-Hebenstreit P., Desmarquet-Trin Dinh C. and Charnay P. (2007). Direct regulation of vHnf1 by retinoic acid signaling and MAF-related factors in the neural tube. Dev. Biol. 309, 344-357. 10.1016/j.ydbio.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Power S. C. and Cereghini S. (1996). Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol. Cell. Biol. 16, 778-791. 10.1128/MCB.16.3.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner A., Hsieh J.-C., Smallwood P. M., Gilbert D. J., Copeland N. G., Jenkins N. A. and Nathans J. (1997). A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA 94, 2859-2863. 10.1073/pnas.94.7.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray W. J., Bain G., Yao M. and Gottlieb D. I. (1997). CYP26, a novel mammalian cytochrome P450, is induced by retinoic acid and defines a new family. J. Biol. Chem. 272, 18702-18708. 10.1074/jbc.272.30.18702 [DOI] [PubMed] [Google Scholar]
- Rezania A., Bruin J. E., Riedel M. J., Mojibian M., Asadi A., Xu J., Gauvin R., Narayan K., Karanu F., O'Neil J. J. et al. (2012). Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 61, 2016-2029. 10.2337/db11-1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Parpaillon L., Héligon C., Chesnel F., Boujard D. and Philpott A. (2002). The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev. Biol. 244, 407-417. 10.1006/dbio.2002.0605 [DOI] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J. and Smyth G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139-140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Seguel E., Mah N., Naumann H., Pongrac I. M., Cerda-Esteban N., Fontaine J.-F., Wang Y., Chen W., Andrade-Navarro M. A. and Spagnoli F. M. (2013). Mutually exclusive signaling signatures define the hepatic and pancreatic progenitor cell lineage divergence. Genes Dev. 27, 1932-1946. 10.1101/gad.220244.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A., Merriam J. and Klymkowsky M. W. (1997). Localizing the adhesive and signaling functions of plakoglobin. Dev. Genet. 20, 91-102. [DOI] [PubMed] [Google Scholar]
- Sagara N., Kirikoshi H., Terasaki H., Yasuhiko Y., Toda G., Shiokawa K. and Katoh M. (2001). FZD4S, a splicing variant of frizzled-4, encodes a soluble-type positive regulator of the WNT signaling pathway. Biochem. Biophys. Res. Commun. 282, 750-756. 10.1006/bbrc.2001.4634 [DOI] [PubMed] [Google Scholar]
- Salzberg A., Elias S., Nachaliel N., Bonstein L., Henig C. and Frank D. (1999). A Meis family protein caudalizes neural cell fates in Xenopus. Mech. Dev. 80, 3-13. 10.1016/S0925-4773(98)00187-7 [DOI] [PubMed] [Google Scholar]
- Schiesser J. V., Micallef S. J., Hawes S., Elefanty A. G. and Stanley E. G. (2014). Derivation of insulin-producing beta-cells from human pluripotent stem cells. Rev. Diabet. Stud. 11, 6-18. 10.1900/RDS.2014.11.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T. C., Young H. Y., Agulnick A. D., Babin M. J., Baetge E. E., Bang A. G., Bhoumik A., Cepa I., Cesario R. M., Haakmeester C. et al. (2012). A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE 7, e37004 10.1371/journal.pone.0037004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaer A., Azarpira N., Vahdati A., Karimi M. H. and Shariati M. (2015). Differentiation of human-induced pluripotent stem cells into insulin-producing clusters. Exp. Clin. Transplant. 13, 68-75. 10.6002/ect.2013.0131 [DOI] [PubMed] [Google Scholar]
- Sharpe C. R., Pluck A. and Gurdon J. B. (1989). XIF3, a Xenopus peripherin gene, requires an inductive signal for enhanced expression in anterior neural tissue. Development 107, 701-714. [DOI] [PubMed] [Google Scholar]
- Shi D.-L. and Boucaut J.-C. (2000). Xenopus frizzled 4 is a maternal mRNA and its zygotic expression is localized to the neuroectoderm and trunk lateral plate mesoderm. Mech. Dev. 94, 243-245. 10.1016/S0925-4773(00)00294-X [DOI] [PubMed] [Google Scholar]
- Shih H. P., Wang A. and Sander M. (2013). Pancreas organogenesis: from lineage determination to morphogenesis. Annu. Rev. Cell Dev. Biol. 29, 81-105. 10.1146/annurev-cellbio-101512-122405 [DOI] [PubMed] [Google Scholar]
- Shuldiner A. R., Phillips S., Roberts C. T. Jr, LeRoith D. and Roth J. (1989). Xenopus laevis contains two nonallelic preproinsulin genes. cDNA cloning and evolutionary perspective. J. Biol. Chem. 264, 9428-9432. [PubMed] [Google Scholar]
- Sinner D., Rankin S., Lee M. and Zorn A. M. (2004). Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 131, 3069-3080. 10.1242/dev.01176 [DOI] [PubMed] [Google Scholar]
- Sirbu I. O. and Duester G. (2006). Retinoic-acid signalling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nat. Cell Biol. 8, 271-277. 10.1038/ncb1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H. L. and Cheng P. F. (1991). Retinoic acid perturbs the expression of Xhox.lab genes and alters mesodermal determination in Xenopus laevis. Genes Dev. 5, 1321-1332. 10.1101/gad.5.8.1321 [DOI] [PubMed] [Google Scholar]
- Smith W. C., Knecht A. K., Wu M. and Harland R. M. (1993). Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature 361, 547-549. 10.1038/361547a0 [DOI] [PubMed] [Google Scholar]
- Sogame A., Hayata T. and Asashima M. (2003). Screening for novel pancreatic genes from in vitro-induced pancreas in Xenopus. Dev. Growth Differ. 45, 143-152. 10.1034/j.1600-0854.2004.00683.x [DOI] [PubMed] [Google Scholar]
- Song J., Kim H. J., Gong Z., Liu N.-A. and Lin S. (2007). Vhnf1 acts downstream of Bmp, Fgf, and RA signals to regulate endocrine beta cell development in zebrafish. Dev. Biol. 303, 561-575. 10.1016/j.ydbio.2006.11.040 [DOI] [PubMed] [Google Scholar]
- Stafford D. and Prince V. E. (2002). Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 12, 1215-1220. 10.1016/S0960-9822(02)00929-6 [DOI] [PubMed] [Google Scholar]
- Stafford D., Hornbruch A., Mueller P. R. and Prince V. E. (2004). A conserved role for retinoid signaling in vertebrate pancreas development. Dev. Genes Evol. 214, 432-441. 10.1007/s00427-004-0420-6 [DOI] [PubMed] [Google Scholar]
- Stemmer M., Thumberger T., Del Sol Keyer M., Wittbrodt J. and Mateo J. L. (2015). CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE 10, e0124633 10.1371/journal.pone.0124633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain R. K., Katoh M., Medina A. and Steinbeisser H. (2005). Xenopus frizzled-4S, a splicing variant of Xfz4 is a context-dependent activator and inhibitor of Wnt/beta-catenin signalling. Cell Commun. Signal. 3, 12 10.1186/1478-811X-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Otani H., Jamrich M. and Dawid I. B. (1994). Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 120, 1525-1536. [DOI] [PubMed] [Google Scholar]
- Tashiro K., Yamada R., Asano M., Hashimoto M., Muramatsu M. and Shiokawa K. (1991). Expression of mRNA for activin-binding protein (follistatin) during early embryonic development of Xenopus laevis. Biochem. Biophys. Res. Commun. 174, 1022-1027. 10.1016/0006-291X(91)91521-D [DOI] [PubMed] [Google Scholar]
- Thomas H., Jaschkowitz K., Bulman M., Frayling T. M., Mitchell S. M., Roosen S., Lingott-Frieg A., Tack C. J., Ellard S., Ryffel G. U. et al. (2001). A distant upstream promoter of the HNF-4alpha gene connects the transcription factors involved in maturity-onset diabetes of the young. Hum. Mol. Genet. 10, 2089-2097. 10.1093/hmg/10.19.2089 [DOI] [PubMed] [Google Scholar]
- Umbhauer M., Djiane A., Goisset C., Penzo-Mendez A., Riou J. F., Boucaut J. C. and Shi D. L. (2000). The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 19, 4944-4954. 10.1093/emboj/19.18.4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali R., Poggi L., Madeddu F. and Barsacchi G. (2000). HNF1(beta) is required for mesoderm induction in the Xenopus embryo. Development 127, 1455-1465. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Schnegelsberg P. and De Robertis E. M. (1989). XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development 105, 787-794. [DOI] [PubMed] [Google Scholar]
- Yam J. W. P., Chan K. W., Ngan E. S. W. and Hsiao W. L. W. (2005). Genomic structure, alternative splicing and tissue expression of rFrp/sFRP-4, the rat frizzled related protein gene. Gene 357, 55-62. 10.1016/j.gene.2005.05.025 [DOI] [PubMed] [Google Scholar]
- Zhang J. and King M. L. (1996). Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development 122, 4119-4129. [DOI] [PubMed] [Google Scholar]
- Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., Shi Y. and Deng H. (2009). Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 19, 429-438. 10.1038/cr.2009.28 [DOI] [PubMed] [Google Scholar]
- Zhang T., Guo X. and Chen Y. (2013a). Retinoic acid-activated Ndrg1a represses Wnt/β-catenin signaling to allow Xenopus pancreas, oesophagus, stomach, and duodenum specification. PLoS ONE 8, e65058 10.1371/journal.pone.0065058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Rankin S. A. and Zorn A. M. (2013b). Different thresholds of Wnt-Frizzled 7 signaling coordinate proliferation, morphogenesis and fate of endoderm progenitor cells. Dev. Biol. 378, 1-12. 10.1016/j.ydbio.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.