Abstract
The 11-oxyandrogens, particularly 11-ketotestosterone, have been recognized as a biologically important gonadal androgen in teleost (bony) fishes for decades, and their presence in human beings has been known but poorly understood. Today, we recognize that 11-oxyandrogens derive from the human adrenal glands and are major bioactive androgens, particularly in women and children. This article will review their biosynthesis and metabolism, abundance in normal and pathologic states, and potential as biomarkers of adrenal developmental changes and disease. Specifically, 11-oxyandrogens are the dominant active androgens in many patients with 21-hydroxylase deficiency.
Keywords: adrenal, androgen, adrenarche, 21-hydroxylase deficiency, 11-hydroxylase
I. Introduction
The archetypal circulating androgen in humans is testosterone (T). Dihydroteststerone (DHT), which is the most potent recognized androgen, is synthesized within target cells, such as in the prostate or skin, by the 5α-reduction of T, with little amounts in the circulation. In healthy men, the testes are the main source of T, but T also derives from the ovary and the adrenal glands, primarily via peripheral conversion of androstenedione (A4). Adrenal-derived androgen precursors, including dehydroepiandrosterone (DHEA), androstenediol (Adiol) and their respective sulfates (DHEAS and AdiolS) are much more abundant than T in women and children, yet the peripheral pathways for T formation from these steroids are inefficient. Several disorders are impacted by inappropriate androgen production, including congenital adrenal hyperplasia (CAH), polycystic ovary syndrome (PCOS), and premature adrenarche. Distinguishing the source of androgen synthesis in such conditions is particularly important to guiding therapy. While 11-oxygenated C19 steroids (11-oxyandrogens) have been long identified and recognized as major androgens in teleost fishes, until recently their role(s) in human beings has been largely neglected. This review provides a historical perspective of 11-oxyandrogens and underlines their contributions, as currently understood, in disorders of androgen excess relevant to children and adolescents: CAH, PCOS and premature adrenarche.
II. Enzymatic machinery of 11-oxyandrogens synthesis in humans
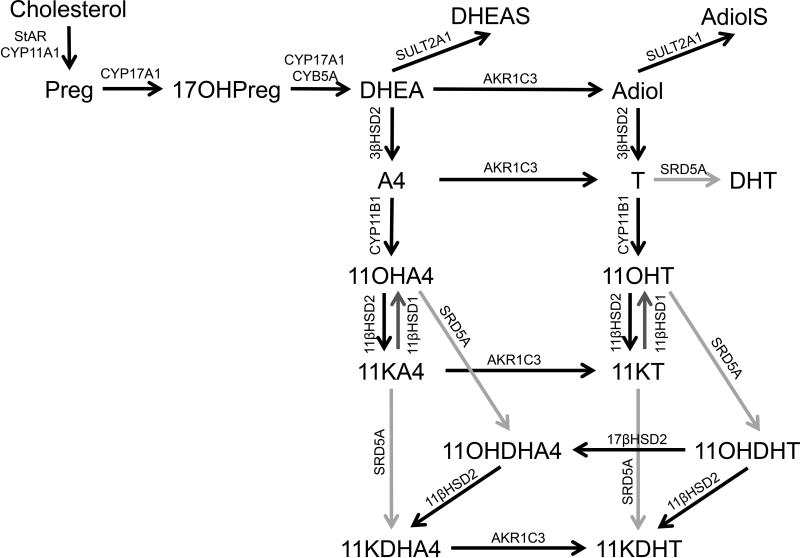
The synthesis of all 11-oxyandrogens relies on the adrenal enzyme cytochrome P450 11β-hydroxylase (CYP11B1), and patients with CYP11B1 deficiency or adrenal insufficiency have negligible amounts of 11-oxyandrogens [1–3]. CYP11B1 is expressed in the zonae fasciculata (ZF) and reticularis (ZR) of the adrenal gland [4] and its main function is to catalyze the last step in cortisol synthesis, under the regulation of adrenocorticotropin (ACTH). CYP11B1 can also use A4 and T as substrates, yielding 11β-hydroxyandrostenedione (11OHA4) and 11β-hydroxytestosterone (11OHT) (Figure 1). Alternatively, 11OHA4 synthesis can occur from cortisol, although this pathway is relatively minor [5]. 11OHA4 and 11OHT can be oxidized by the enzyme 11β-hydroxysteroid dehydrogenase ype 2 (11βHSD2) to 11-ketoandrostenedone (11KA4) and 11-ketotetsosterone (11KT), both in the adrenal gland and in periphery. In addition, 11KT can derive from reduction of 11KA4, via the enzyme aldo-keto reductase 1C3 (AKR1C3, or 17β-hydroxysteroid dehydrogenase type 5, 17βHSD5), which is expressed in the ZR and in many peripheral tissues [4]. Furthermore, Storbeck performed a series of in vitro studies demonstrating that 11OHA4 and 11OHT, as well as their cognate 11-ketosteroids, can serve as substrates for steroid 5α-reductase (SRD5A) [6]. In addition, 11-ketodihydrotestosterone (11KDHT) can be generated either from 11β-hydroxydihydrotestosterone (11OHDHT), via 11βHSD2, or from 11-keto-5α-androstanedione (11KDHA4), via AKR1C3 (Figure 1).
Figure 1. Synthesis of 11-oxyandrogens.
Preg, pregnenolone; 17OHPreg, 17α-hydroxypregnenolone; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; Adiol, 5-androstenediol; AdiolS, 5-androstenediol-3-sulfate; A4, androstenedione; T, testosterone; DHT, 5α-dihydrotestosterone; 11OHA4, 11β-hydroxyandrostenedione; 11KA4, 11-ketoandrostenedione; 11OHT, 11β-hydroxytestosterone; 11KT, 11-ketotestosterone; 11OHDHA4, 11β-hydroxy-5α-androstanedione; 11KDHA4, 11-keto-5α-androstanedione; 11OHDHT, 11β-hydroxydihydrotestosterone; 11KDHT, 11-ketodihydrotestosterone; StAR, steroidogenic acute regulatory protein; CYP11A1, cytochrome P450 cholesterol side-chain cleavage; CYP17A1, cytochrome P450 17α-hydroxylase/17,20-lyase; CYB5A, cytochrome b5; SULT2A1, sulfotransferase family 2A member 1; 3βHSD2, 3β-hydroxysteroid dehydrogenase type 2; CYP11B1, cytochrome P450 11β-hydroxylase; 11βHSD2, 11β-hydroxysteroid dehydrogenase type 2; 11βHSD1, 11β-hydroxysteroid dehydrogenase type 1; SRD5A, steroid 5α-reductase; AKR1C3, aldo-keto reductase 1C3.
III. Androgenic potency of 11-oxyandrogens
Considerable evidence has now demonstrated that 11KT and its 5α-reduced metabolite, 11KDHT are potent and clinically relevant agonists of the human androgen receptor (AR, NR3C4). Using an MDA-kb2 cell model expressing the human AR, Rege et al found that the maximum androgenic activity of 11KT was similar to that of T, and that the EC50 of 11KT was only 5-fold higher than T [7]. The maximal androgenic activity of 11OHT was below that of 11KT and T. In a subsequent study, using an optimized in vitro reporter system of human AR activation, without the co-expression of glucocorticoid, mineralocorticoid or progesterone receptors, 11KT demonstrated reproducible androgen potency, while 11OHT was inferior [8]. Storbeck and colleagues obtained similar results in a COS-1 cell system expressing human AR [6]. In a separate study, 11KT and 11KDHT were shown to bind to the human AR with affinities similar to that of T and DHT, and all four steroids promoted AR-regulated gene expression and cell growth in two androgen-dependent prostate cancer cell lines [9]. In contrast, as demonstrated decades ago [10, 11], 11OHA4 and 11KA4 showed minimal androgenic activity in all of the more recent models.
IV. 11-oxyandrogens across species
In the 1960s, 11KT and 11OHT were isolated from salmon plasma [12, 13]. Since then, 11KT has been extensively studied in teleost fishes, where it was shown to act as the major androgen, contributing to sexual maturation [14, 15] and to various functions, including male-type sexual behavior [16], induction of spermatogenesis [17], sperm motility [18], epidermal and dermal thickening [19].
The synthesis of 11KT in fish occurs by 11β-hydroxylation of A4 to 11OHA4, which is then oxidized to 11KA4 by an 11βHSD, prior to reduction by a 17β-hydroxysteroid dehydrogenase [20, 21]. 11KT can also be generated from T, via 11OHT [21].
In fish, 11KT is much higher in males than females and can induce female-to-male sex reversal in some teleost species [16]. In contrast, 11KT was found to be similar in both sexes in mice, despite significantly larger amounts of T in males [22]. Murine Cyp11b1 and Hsd11b2 were detected in both testicular Leydig and ovarian theca cells, although the latter is more highly expressed in the ovary [22, 23]. The mechanisms of this sexual dimorphism remains unclear, but it has been postulated that Hsd11b2 might regulate ovarian steroidogenesis by limiting the local access of active glucocorticoids.
11OHA4 was identified as a major adrenal product in primates over 40 years ago [5, 24, 25], but its contributions to physiology were poorly understood. In vitro studies have shown that 11OHA4 is abundantly produced by the human adrenal cells, both under basal and cosyntropin-stimulated conditions [26]. Similarly, steroid studies with serum obtained from human adrenal veins have demonstrated that 11OHA4 is produced in higher amounts than A4, both at baseline and after cosyntropin stimulation [3, 7]. The adrenal gland also produces 11OHT, 11KA4, and 11KT in an ACTH-dependent manner, although less robustly than 11OHA4.
While it has been proposed that the gonads might contribute to the synthesis of 11-oxyandrogens in humans, gonadal CYP11B1 expression was found to be minimal compared to that in the adrenal cortex [27]. Moreover, like in mice, 11KT and 11OHT have been found to circulate in comparable concentrations between sexes in human beings, despite dramatically larger amounts of T in men [3, 27], suggesting that the gonadal synthesis of 11-oxyandrogens is trivial relative to the adrenal component.
V. Clinical applications of 11-oxyandrogens
V.1. Congenital adrenal hyperplasia
CAH encompass a heterogeneous group of autosomal recessive disorders in which one or more enzymes required for cortisol synthesis are defective. These inherited defects range from severe (also called classic) to mild (non-classic, with normal cortisol production but elevated precursor/product ratios), and from a single to multiple enzymatic steps. The negative feedback to the hypothalamus and the pituitary gland normally executed by cortisol becomes disrupted, leading to an over-activation of the hypothalamic-pituitary-adrenal axis. While this mechanism is meant to compensate for cortisol insufficiency, it leads to excessive production of upstream and off-target steroids.
The vast majority of CAH cases result from defects in 21-hydroxylase (CYP21A2). Classic 21-hydroxylase deficiency (21OHD) affects ~1:16,000 newborns [28]. Non-classic 21OHD is one of the most common inherited disorders, occurring in approximately 1:1,000 Caucasians and more commonly in Ashkenazi Jews, Hispanics and Mediterraneans [29]. In addition to the active CYP21A2 gene, human beings have a highly homologous pseudogene (CYP21A1P), which does not encode an active enzyme. The frequent occurrence of mutations in the CYP21A genes is favored by their close proximity to each other in a duplicated locus within the HLA major histocompatibility complex and which includes the genes for the fourth component of complement. [30–32] This locus undergoes frequent recombinations and/or gene conversion events, which disrupt the CYP21A2 gene with sequences from the CYP21A1P pseudogene.
The main substrate of CYP21A2, 17-hydroxyprogesterone (17OHP), which accumulates in 21OHD, has traditionally been used for both diagnosis and monitoring. Additionally, the CYP21A2 blockage and ACTH elevation promote the diversion of 17OHP towards formation of 19-carbon (C19) steroids (androgens and precursors). Although the catalytic efficiency of the human 17,20-lyase is much higher when using 17-hydroxypregnenolone as substrate [33], the high concentrations of 17OHP in 21OHD allow some conversion to A4, but CYP11B1 converts much of nascent 17OHP to 21-deoxycortisol in the adrenal. In parallel, intra-adrenal A4 and T can serve as substrates for CYP11B1, leading to 11OHA4 and 11OHT, respectively, which are then further metabolized to 11KA4 and 11KT, predominantly in the periphery.
While 17OHP has been the main steroid for the diagnosis of 21OHD, 17OHP is a poor indicator of disease control. Serum 17OHP fluctuates rapidly and prominently in relation to glucocorticoid dosing. For adults, a 17OHP in the normal range indicates over-treatment with glucocorticoids, which is associated with a multitude of side effects. Similarly, DHEA and DHEAS, the characteristic adrenal C19 steroids, are often paradoxically low in patients with classic 21OHD, even when treated conservatively [34]. In a cross-sectional study of 38 patients (19 men) with classic 21OHD between 3 and 59 years of age, we found that DHEAS and DHEA were 6- to 7-fold lower than in sex- and age-matched controls [3]. Surprisingly, however, pregnenolone sulfate was 3-fold higher in 21OHD patients than in their unaffected counterparts. While the mechanisms of DHEAS deficiency in 21OHD remain unclear, the utility of both DHEAS and DHEA as biomarkers of androgen excess in 21OHD is limited.
The most widely used biomarkers of androgen excess in 21OHD have been A4 and, in women, T. Clinical studies have shown that both A4 and T correlate poorly with phenotypic evidence of hyperandrogenism; moreover, both steroids are also produced from the gonads. Thus, in postpubertal males, T also reflects testicular function. Due to its adrenal origin, 11OHA4 has been previously proposed as a biomarker of androgen excess in 21OHD, but studies have been small and results inconclusive. In 1992, Carmina and colleagues studied the response of a set of androgens to 7-day dexamethasone suppression and found that the ratio of A4/11OHA4 was lower in women with non-classic 21OHD and higher in women with chronic anovulation as compared to controls [35]. Huerta et al evaluated the response of C19 steroids to ACTH stimulation in women with untreated non-classic 21OHD and normal age- and BMI-matched controls. While 11OHA4 was higher at baseline in 21OHD women, it did not differ between the two groups after ACTH stimulation, questioning its clinical relevance in such patients [36].
More recently, we have shown that all four 11-oxyandrogens are 3 to 4-fold higher in treated patients with classic 21OHD of both sexes than in age- and sex-paired controls [3]. Notably, while 11KT correlated directly with T in females and prepupertal boys, 11KT and T correlate inversely in post-pubertal males [3, 37]. Furthermore, while T correlated positively with LH, 11OHT and 11KT displayed a negative correlation with LH in sexually mature males. These findings suggest that 11KT synthesis relies predominantly on adrenal precursors and that its high concentrations in poorly controlled males with 21OHD suppress the hypothalamic-pituitary-gonadal axis, and subsequently testicular-derived T. Another important caveat is that 11KT was approximately twice as high as T in women and children with 21OHD, making 11KT the dominant circulating androgen in these patients. Collectively, these reports support the utility of 11KT as an adrenal-specific androgen, with applicability in both sexes.
In a more recent study of 114 children and adults with classic 21OHD ages 2 to 67 years (59% younger than 18 years), we explored the association of 23 steroids with surrogates of poor 21OHD control, such as adrenal volume, testicular a drenal rest tumors (TART), bone age, menstrual irregularities and hirsutism [37]. We found that all four 11-oxyandrogens correlated tightly with adrenal volume (in adults) and with ACTH (r=~0.7, p<0.0001 for all). In addition, all four 11-oxyandrogens, and in particular 11OHA4, were 3–7 fold higher in males with TART as compared to those without TART of similar ages. On the other hand, we and others [38] found no differences in T between males with and without TART. Neither the 11-oxyandrogens nor any of the other steroids measured demonstrated a correlation with TART volume, in agreement with a study from Reisch and colleagues [39]. Nonetheless, patients with TART had larger adrenal volume than those without TART, suggesting poorer long-term control. While poor disease control is thought to contribute to TART growth [40–42], their mere presence and ultimate size is influenced by embryological factors and remain incompletely understood [38, 39, 43–46].
In females, 11OHT and 11KT were found to be higher in the group with menstrual disturbances and hirsutism than in those without. It is worth mentioning that none of the steroids studied predicted bone age advancement. Because bone age is impacted not only by sex-hormone dysregulation, primarily estrogens, but glucocorticoid therapy as well, it is not surprising that single-point steroid biomarkers cannot provide clinical insight into bone age derangements. Nonetheless, taken together, our recent studies support 11-oxyandrogens as better indicators of poor 21OHD control than 17OHP and T.
V.2. Polycystic ovary syndrome
PCOS is the most common ovarian disorder, affecting 5–15% of reproductive age women [47–49]. PCOS is a complex heterogeneous condition, characterized by reproductive abnormalities, ovulatory dysfunction, polycystic ovarian morphology, and hyperandrogenism [50, 51], but its pathogenesis remains poorly understood. Importantly, patients with PCOS have an increased risk of metabolic abnormalities, such as obesity, insulin resistance, type 2 diabetes mellitus, cardiovascular disease and dyslipidemia.
Hyperandrogenism is the cardinal clinical feature of PCOS and can be defined by either hirsutism and/or excess of serum androgens [50]; however, not all patients with PCOS have hirsutism or elevated T [52]. The source(s) of androgen excess in PCOS has been debated. While the ovary was originally considered the primary source of androgen excess in PCOS women, elevated adrenal androgens, particularly DHEA and DHEAS, have been observed in many patients with PCOS [53, 54]. Thus the adrenal gland has also been recognized as a source of androgen excess. Nonetheless, the mechanism of adrenal androgen excess in PCOS remains unclear. Some investigators have observed an excessive response of adrenal androgens and cortisol after cosyntropin stimulation in patients with PCOS [55, 56], suggesting that the adrenal androgen excess in PCOS might result from generalized adrenocortical hyper-reactivity to ACTH.
Recently, A4 has been used as a more sensitive marker of hyperandrogenism in PCOS than T [57–59]. In addition, A4 was proposed as a predictor of metabolic risk in PCOS [57]. Studies from early 1990s demonstrated that 11OHA4 is also elevated in PCOS [35, 60]. In a recent study of 114 women with PCOS and 49 healthy controls, traditional androgens, precursor steroids and 11-oxyandrogens were measured in peripheral serum, as well as their metabolites in 24h urine collections using mass spectrometry [61]. Serum 11OHA4, 11KA4, 11OHT, and 11KT were significantly higher in patients with PCOS than in controls, as was the urinary 11-oxyandrogen metabolite 11β-hydroxyandrosterone. Indeed, the relative contribution of the 11-oxyandrogens to the total circulating androgenic steroids was significantly greater in patients with PCOS than in controls. The authors also investigated the association of 11-oxyandrogens with markers of metabolic risk in patients with PCOS and found that serum 11OHA4 and 11KA4, but not the urinary 11-oxygenated T metabolites, correlated significantly with BMI, insulin and HOMA-IR.
While additional large scale studies in lean and obese women with PCOS are needed, these early findings suggest that the 11-oxyandrogens contribute significantly to the total androgen burden in PCOS and might explain clinical evidence of androgen excess in women with normal serum T. In addition, 11-oxyandrogen measurements might improve subtyping of PCOS and serve as biomarkers of metabolic risk.
V.3. Premature adrenarche
Adrenarche is a developmental process clinically defined by the appearance of axillary and pubic hair around age 8 years [62]. This phenomenon is marked by an increase in adrenal C19 steroid production, such as DHEA and DHEAS, which reflect the expansion of the ZR [62–64]. After regression of the fetal adrenal, infant adrenal glands display a distinct zona glomerulosa and ZF, but a small ZR [63]. Focal islands of ZR cells gradually appear in the adrenals of children around age 3 years and expand at age 4–5 years [63, 65]. A continuous layer of ZR cells is observed by age 6 years, and the ZR attains its maximum thickness around age 12–13 years [63, 65]. The expression of the steroidogenic enzymes and cofactors required for C19 steroid production, such as 17α-hydroxylase/17,20-lyase (CYP17A1) and cytochrome b5 (CYB5A), increases along with the changes of adrenal morphology that occur during adrenarche [66–68].
Premature adrenarche is defined as the occurrence of pubic and axillary hair, adult type body odor, oily hair and skin, acne and accelerated growth before age 8 years in girls or 9 years in boys, accompanied by hormonal evidence of adrenal androgen production [69–72]. Importantly, girls with premature adrenarche are at increased risk for developing PCOS, infertility and features of metabolic syndrome. As a group, children with premature adrenarche exhibit elevated serum adrenal androgens for age, including DHEA, DHEAS, A4 and T; [73–75] however, some children with clinical signs typical of premature adrenarche have normal serum DHEAS concentrations [70, 73]. The secretion of 11-oxyandrogens during adrenarche has been explored as early as the 1970s, but initial results did not appear promising. Parker et al found no significant changes in 11OHA4 with age [76]. On the other hand, Holownia and colleagues observed a gradual rise in plasma concentrations of 11OHA4 from age 9 through 16 years, which was similar in both sexes [77]. In a recent study, Rege et al used liquid chromatography-tandem mass spectrometry to characterize the steroid metabolome in children with premature adrenarche and found that not only DHEAS, but also 11-oxyandrogens, including 11OHA4 and 11KT, were significantly higher in children with premature adrenarche as compared to controls [78]. Further studies are required to understand the role of 11-oxyandrgesn in normal and premature adrenarche.
VI. Summary
Although the presence of 11-oxyandrogens in human beings has been documented for several decades, their functions in normal physiology and pathologic states remain poorly understood. Sufficient evidence has appeared to identify 11OHA4 as a major product of the adrenal gland and 11KT as the dominant active androgen metabolite of 11OHA4. The 11-oxyandrogens are the predominant androgens in most patients with classic 21OHD, except for men with good disease control and normal testicular function. Ironically, while T and DHT have been recognized as the main androgens in human beings for many years, their prominence might be limited to normal adult males. The contributions of 11-oxyandrogens to androgen excess disorders in women and children looms large and awaits further definition.
Acknowledgments
Funding: AFT was supported by grants 1K08DK109116 and AG-024824/UL1TR000433 pilot grant from the Claude D. Pepper Older Americans Independence Centers/MICHR; RJA was supported by grant R01GM086596.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Polson DW, Reed MJ, Franks S, Scanlon MJ, James VH. Serum 11β-hydroxyandrostenedione as an indicator of the source of excess androgen production in women with polycystic ovaries. J Clin Endocrinol Metab. 1988;66(5):946–950. doi: 10.1210/jcem-66-5-946. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim F, Giton F, Boudou P, Villette JM, Julien R, Galons H, Fiet J. Plasma 11β-hydroxy-4-androstene-3,17-dione: comparison of a time-resolved fluoroimmunoassay using a biotinylated tracer with a radioimmunoassay using a tritiated tracer. The Journal of steroid biochemistry and molecular biology. 2003;84(5):563–568. doi: 10.1016/s0960-0760(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 3.Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. European journal of endocrinology / European Federation of Endocrine Societies. 2016;174(5):601–609. doi: 10.1530/EJE-15-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. The Journal of clinical endocrinology and metabolism. 2014;99(3):E518–527. doi: 10.1210/jc.2013-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axelrod LR, Kraemer DC, Burdett J, Jr, Goldzieher JW. Biosynthesis of 11 - hydroxyandrostenedione by human and baboon adrenals. Acta Endocrinol (Copenh) 1973;72(3):545–550. doi: 10.1530/acta.0.0720545. [DOI] [PubMed] [Google Scholar]
- 6.Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Molecular and cellular endocrinology. 2013;377(1–2):135–146. doi: 10.1016/j.mce.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. The Journal of clinical endocrinology and metabolism. 2013;98(3):1182–1188. doi: 10.1210/jc.2012-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campana C, Rege J, Turcu AF, Pezzi V, Gomez-Sanchez CE, Robins DM, Rainey WE. Development of a novel cell based androgen screening model. The Journal of steroid biochemistry and molecular biology. 2016;156:17–22. doi: 10.1016/j.jsbmb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-Ketotestosterone and 11-Ketodihydrotestosterone in Castration Resistant Prostate Cancer: Potent Androgens Which Can No Longer Be Ignored. PloS one. 2016;11(7):e0159867. doi: 10.1371/journal.pone.0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosemberg E, Dorfman RI. Biological activity of 9α-fluoro-11β-hydroxy-Δ4-androstene-3, 17-dione. Proc Soc Exp Biol Med. 1958;99(2):336–338. [PubMed] [Google Scholar]
- 11.Dorfman RI, Rooks WH, 2nd, Jones JB, Leman JD. Androgenic activity of highly purified 5α-androstane and 5α-androstan-17β-ol. J Med Chem. 1966;9(6):930–931. doi: 10.1021/jm00324a030. [DOI] [PubMed] [Google Scholar]
- 12.Idler DR, Schmidt PJ, Ronald AP. Isolation and identification of 11-ketotestosterone in salmon plasma. Can J Biochem Physiol. 1960;38:1053–1057. [PubMed] [Google Scholar]
- 13.Idler DR, Macnab HC. The biosynthesis of 11-ketotestosterone and 11β-hydroxytestosterone by Atlantic salmon tissues in vitro. Can J Biochem. 1967;45(4):581–589. doi: 10.1139/o67-067. [DOI] [PubMed] [Google Scholar]
- 14.Kindler PM, Philipp DP, Gross MR, Bahr JM. Serum 11-ketotestosterone and testosterone concentrations associated with reproduction in male bluegill (Lepomis macrochirus: Centrarchidae) Gen Comp Endocrinol. 1989;75(3):446–453. doi: 10.1016/0016-6480(89)90180-9. [DOI] [PubMed] [Google Scholar]
- 15.Feist G, Schreck CB, Fitzpatrick MS, Redding JM. Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation. Gen Comp Endocrinol. 1990;80(2):299–313. doi: 10.1016/0016-6480(90)90174-k. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Nakanishi T. 11-ketotestosterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii. Gen Comp Endocrinol. 1999;115(2):178–187. doi: 10.1006/gcen.1999.7314. [DOI] [PubMed] [Google Scholar]
- 17.Nagahama Y, Miura T, Kobayashi T. The onset of spermatogenesis in fish. Ciba Found Symp. 1994;182:255–267. doi: 10.1002/9780470514573.ch14. discussion 267–270. [DOI] [PubMed] [Google Scholar]
- 18.Miura T, Yamauchi K, Takahashi H, Nagahama Y. The role of hormones in the acquisition of sperm motility in salmonid fish. J Exp Zool. 1992;261(3):359–363. doi: 10.1002/jez.1402610316. [DOI] [PubMed] [Google Scholar]
- 19.Pottinger TG, Pickering AD. The effects of 11-ketotestosterone and testosterone on the skin structure of brown trout, Salmo trutta L. Gen Comp Endocrinol. 1985;59(3):335–342. doi: 10.1016/0016-6480(85)90389-2. [DOI] [PubMed] [Google Scholar]
- 20.Mayer I, Borg B, Schulz R. Conversion of 11-ketoandrostenedione to 11-ketotestosterone by blood cells of six fish species. Gen Comp Endocrinol. 1990;77(1):70–74. doi: 10.1016/0016-6480(90)90207-3. [DOI] [PubMed] [Google Scholar]
- 21.Leitz T, Reinboth R. The biosynthesis of 11-ketotestosterone by the testis of the Siamese fighting fish Betta splendens Regan (Anabantoidei, Belontiidae) Gen Comp Endocrinol. 1987;66(1):145–157. doi: 10.1016/0016-6480(87)90359-5. [DOI] [PubMed] [Google Scholar]
- 22.Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A, Miyamoto K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology. 2008;149(4):1786–1792. doi: 10.1210/en.2007-1015. [DOI] [PubMed] [Google Scholar]
- 23.Wang GM, Ge RS, Latif SA, Morris DJ, Hardy MP. Expression of 11β-hydroxylase in rat Leydig cells. Endocrinology. 2002;143(2):621–626. doi: 10.1210/endo.143.2.8638. [DOI] [PubMed] [Google Scholar]
- 24.O'Hare MJ, Nice EC, Magee-Brown R, Bullman H. High-pressure liquid chromatography of steroids secreted by human adrenal and testis cells in monolayer culture. Journal of chromatography. 1976;125(1):357–367. doi: 10.1016/s0021-9673(00)93831-7. [DOI] [PubMed] [Google Scholar]
- 25.Hudson RW, Killinger DW. The in vitro biosynthesis of 11 -hydroxyandrostenedione by human adrenal homogenates. The Journal of clinical endocrinology and metabolism. 1972;34(1):215–224. doi: 10.1210/jcem-34-1-215. [DOI] [PubMed] [Google Scholar]
- 26.Xing Y, Edwards MA, Ahlem C, Kennedy M, Cohen A, Gomez-Sanchez CE, Rainey WE. The effects of ACTH on steroid metabolomic profiles in human adrenal cells. The Journal of endocrinology. 2011;209(3):327–335. doi: 10.1530/JOE-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imamichi Y, Yuhki KI, Orisaka M, Kitano T, Mukai K, Ushikubi F, Taniguchi T, Umezawa A, Miyamoto K, Yazawa T. 11-ketotestosterone is a major androgen produced in human gonads. The Journal of clinical endocrinology and metabolism. 2016 doi: 10.1210/jc.2016-2311. jc20162311. [DOI] [PubMed] [Google Scholar]
- 28.Therrell BL. Newborn screening for congenital adrenal hyperplasia. Endocrinology and metabolism clinics of North America. 2001;30(1):15–30. doi: 10.1016/s0889-8529(08)70017-3. [DOI] [PubMed] [Google Scholar]
- 29.Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. American journal of human genetics. 1985;37(4):650–667. [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll MC, Campbell RD, Porter RR. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White PC, Grossberger D, Onufer BJ, Chaplin DD, New MI, Dupont B, Strominger JL. Two genes encoding steroid 21-hydroxylase are located near the genes encoding the fourth component of complement in man. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(4):1089–1093. doi: 10.1073/pnas.82.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(14):5111–5115. doi: 10.1073/pnas.83.14.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fluck CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88(8):3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- 34.Rezvani I, Garibaldi LR, Digeorge AM, Artman HG. Disproportionate suppression of dehydroepiandrosterone sulfate (DHEAS) in treated patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatric research. 1983;17(2):131–134. doi: 10.1203/00006450-198302000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Carmina E, Stanczyk FZ, Chang L, Miles RA, Lobo RA. The ratio of androstenedione:11β-hydroxyandrostenedione is an important marker of adrenal androgen excess in women. Fertil Steril. 1992;58(1):148–152. doi: 10.1016/s0015-0282(16)55152-8. [DOI] [PubMed] [Google Scholar]
- 36.Huerta R, Dewailly D, Decanter C, Knochenhauer ES, Boots LR, Azziz R. 11β-hydroxyandrostenedione and Δ5-androstenediol as markers of adrenal androgen production in patients with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 1999;72(6):996–1000. doi: 10.1016/s0015-0282(99)00402-1. [DOI] [PubMed] [Google Scholar]
- 37.Turcu AF, Mallappa A, Elman M, Avila NA, Marko J, Rao H, Tsodikov A, Auchus RJ, Merke DP. 11-Oxygenated Androgens are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency. The Journal of clinical endocrinology and metabolism. 2017 doi: 10.1210/jc.2016-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claahsen-van der Grinten HL, Sweep FC, Blickman JG, Hermus AR, Otten BJ. Prevalence of testicular adrenal rest tumours in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. European journal of endocrinology / European Federation of Endocrine Societies. 2007;157(3):339–344. doi: 10.1530/EJE-07-0201. [DOI] [PubMed] [Google Scholar]
- 39.Reisch N, Scherr M, Flade L, Bidlingmaier M, Schwarz HP, Muller-Lisse U, Reincke M, Quinkler M, Beuschlein F. Total adrenal volume but not testicular adrenal rest tumor volume is associated with hormonal control in patients with 21-hydroxylase deficiency. The Journal of clinical endocrinology and metabolism. 2010;95(5):2065–2072. doi: 10.1210/jc.2009-1929. [DOI] [PubMed] [Google Scholar]
- 40.Stikkelbroeck NM, Hermus AR, Suliman HM, Jager GJ, Otten BJ. Asymptomatic testicular adrenal rest tumours in adolescent and adult males with congenital adrenal hyperplasia: basal and follow-up investigation after 2.6 years. Journal of pediatric endocrinology & metabolism : JPEM. 2004;17(4):645–653. doi: 10.1515/jpem.2004.17.4.645. [DOI] [PubMed] [Google Scholar]
- 41.Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Hermus AR. Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertility and sterility. 2007;88(3):705, e705–708. doi: 10.1016/j.fertnstert.2006.11.148. [DOI] [PubMed] [Google Scholar]
- 42.Hamwi GJ, Gwinup G, Mostow JH, Besch PK. Activation of Testicular Adrenal Rest Tissue by Prolonged Excessive Acth Production. The Journal of clinical endocrinology and metabolism. 1963;23:861–869. doi: 10.1210/jcem-23-9-861. [DOI] [PubMed] [Google Scholar]
- 43.Smeets EE, Span PN, van Herwaarden AE, Wevers RA, Hermus AR, Sweep FC, Claahsen-van der Grinten HL. Molecular characterization of testicular adrenal rest tumors in congenital adrenal hyperplasia: lesions with both adrenocortical and Leydig cell features. The Journal of clinical endocrinology and metabolism. 2015;100(3):E524–530. doi: 10.1210/jc.2014-2036. [DOI] [PubMed] [Google Scholar]
- 44.Knudsen JL, Savage A, Mobb GE. The testicular 'tumour' of adrenogenital syndrome--a persistent diagnostic pitfall. Histopathology. 1991;19(5):468–470. doi: 10.1111/j.1365-2559.1991.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 45.Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. The Journal of clinical endocrinology and metabolism. 2001;86(12):5721–5728. doi: 10.1210/jcem.86.12.8090. [DOI] [PubMed] [Google Scholar]
- 46.Falhammar H, Nystrom HF, Ekstrom U, Granberg S, Wedell A, Thoren M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. European journal of endocrinology / European Federation of Endocrine Societies. 2012;166(3):441–449. doi: 10.1530/EJE-11-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 48.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38. e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 49.Lauritsen MP, Bentzen JG, Pinborg A, Loft A, Forman JL, Thuesen LL, Cohen A, Hougaard DM, Nyboe Andersen A. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum Reprod. 2014;29(4):791–801. doi: 10.1093/humrep/det469. [DOI] [PubMed] [Google Scholar]
- 50.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 51.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome--Part 1. Endocr Pract. 2015;21(11):1291–1300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 52.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman DI, Klove K, Lobo RA. The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil Steril. 1984;42(1):76–81. doi: 10.1016/s0015-0282(16)47961-6. [DOI] [PubMed] [Google Scholar]
- 54.Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. The Journal of clinical endocrinology and metabolism. 1998;83(7):2317–2323. doi: 10.1210/jcem.83.7.4948. [DOI] [PubMed] [Google Scholar]
- 55.Lucky AW, Rosenfield RL, McGuire J, Rudy S, Helke J. Adrenal androgen hyperresponsiveness to adrenocorticotropin in women with acne and/or hirsutism: adrenal enzyme defects and exaggerated adrenarche. J Clin Endocrinol Metab. 1986;62(5):840–848. doi: 10.1210/jcem-62-5-840. [DOI] [PubMed] [Google Scholar]
- 56.Azziz R, Boots LR, Parker CR, Jr, Bradley E, Jr, Zacur HA. 11β-hydroxylase deficiency in hyperandrogenism. Fertil Steril. 1991;55(4):733–741. [PubMed] [Google Scholar]
- 57.O'Reilly MW, Taylor AE, Crabtree NJ, Hughes BA, Capper F, Crowley RK, Stewart PM, Tomlinson JW, Arlt W. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J Clin Endocrinol Metab. 2014;99(3):1027–1036. doi: 10.1210/jc.2013-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R, Pfeifer M, Pignatelli D, Pugeat M, Yildiz BO. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 59.Pasquali R, Zanotti L, Fanelli F, Mezzullo M, Fazzini A, Morselli Labate AM, Repaci A, Ribichini D, Gambineri A. Defining Hyperandrogenism in Women With Polycystic Ovary Syndrome: A Challenging Perspective. The Journal of clinical endocrinology and metabolism. 2016;101(5):2013–2022. doi: 10.1210/jc.2015-4009. [DOI] [PubMed] [Google Scholar]
- 60.Stanczyk FZ, Chang L, Carmina E, Putz Z, Lobo RA. Is 11β-hydroxyandrostenedione a better marker of adrenal androgen excess than dehydroepiandrosterone sulfate? Am J Obstet Gynecol. 1991;165(6 Pt 1):1837–1842. doi: 10.1016/0002-9378(91)90042-p. [DOI] [PubMed] [Google Scholar]
- 61.O'Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W. 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102(3):840–848. doi: 10.1210/jc.2016-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auchus RJ, Rainey WE. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 2004;60(3):288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- 63.Rege J, Rainey WE. The steroid metabolome of adrenarche. The Journal of endocrinology. 2012;214(2):133–143. doi: 10.1530/JOE-12-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- 65.Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. The Journal of endocrinology. 2009;203(2):241–252. doi: 10.1677/JOE-09-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995;317(2):343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 67.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71(1):83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53(6):739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 69.Utriainen P, Laakso S, Liimatta J, Jaaskelainen J, Voutilainen R. Premature adrenarche--a common condition with variable presentation. Horm Res Paediatr. 2015;83(4):221–231. doi: 10.1159/000369458. [DOI] [PubMed] [Google Scholar]
- 70.Voutilainen R, Jaaskelainen J. Premature adrenarche: etiology, clinical findings, and consequences. The Journal of steroid biochemistry and molecular biology. 2015;145:226–236. doi: 10.1016/j.jsbmb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Idkowiak J, Lavery GG, Dhir V, Barrett TG, Stewart PM, Krone N, Arlt W. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165(2):189–207. doi: 10.1530/EJE-11-0223. [DOI] [PubMed] [Google Scholar]
- 72.Utriainen P, Jaaskelainen J, Romppanen J, Voutilainen R. Childhood metabolic syndrome and its components in premature adrenarche. J Clin Endocrinol Metab. 2007;92(11):4282–4285. doi: 10.1210/jc.2006-2412. [DOI] [PubMed] [Google Scholar]
- 73.Utriainen P, Voutilainen R, Jaaskelainen J. Continuum of phenotypes and sympathoadrenal function in premature adrenarche. Eur J Endocrinol. 2009;160(4):657–665. doi: 10.1530/EJE-08-0367. [DOI] [PubMed] [Google Scholar]
- 74.Kaplowitz P, Soldin SJ. Steroid profiles in serum by liquid chromatography-tandem mass spectrometry in infants with genital hair. Journal of pediatric endocrinology & metabolism : JPEM. 2007;20(5):597–605. doi: 10.1515/jpem.2007.20.5.597. [DOI] [PubMed] [Google Scholar]
- 75.von Oettingen J, Sola Pou J, Levitsky LL, Misra M. Clinical presentation of children with premature adrenarche. Clin Pediatr (Phila) 2012;51(12):1140–1149. doi: 10.1177/0009922812456238. [DOI] [PubMed] [Google Scholar]
- 76.Parker LN, Sack J, Fisher DA, Odell WD. The adrenarche: prolactin, gonadotropins, adrenal androgens, and cortisol. J Clin Endocrinol Metab. 1978;46(3):396–401. doi: 10.1210/jcem-46-3-396. [DOI] [PubMed] [Google Scholar]
- 77.Holownia P, Owen EJ, Conway GS, Round J, Honour JW. Studies to confirm the source of 11β-hydroxyandrostenedione. The Journal of steroid biochemistry and molecular biology. 1992;41(3–8):875–880. doi: 10.1016/0960-0760(92)90441-k. [DOI] [PubMed] [Google Scholar]
- 78.Rege J, Kasa-Vubu JZ, Muth TA, Auchus RJ, Smith JM, White PC, Rainey WE. Endocrine Society Meeting: 2017. Orlando, FL: Oxford Academic; 2017. Premature Adrenarche Is Marked By Elevated Levels of Serum 11-Ketotestosterone and Δ5-Steroid Sulfates. [Google Scholar]