Introduction
Coccidioidomycosis, also known as San Joaquin Valley Fever, is a fungal disease endemic to Southwestern United States, parts of Mexico, and Central and South America.1, 2 Coccidioides immitis is the major species in Southern California and Northern Mexico, whereas Coccidioides posadasii causes disease in other endemic areas. Both Coccidioides species are dimorphic, soil-dwelling fungi capable of causing systemic disease through inhalation of airborne arthroconidia from the soil.3 There are an estimated 100,000 to 150,000 cases annually in the United States.2, 4, 5 Clinically, approximately 60% of individuals with primary coccidioidomycosis are asymptomatic, whereas the remaining 40% may experience mild to moderate influenzalike symptoms including cough, arthralgias, and profound fatigue. Primary cutaneous coccidioidomycosis is rare with only about 30 reported cases in the literature, and it is thought to be acquired by direct inoculation of the fungus through contact with plants.2, 6 Disseminated disease occurs in 0.5% to 1% of patients and can affect the skin, central nervous system, musculoskeletal system, and any other organ. If left untreated, central nervous system infections can be fatal and necessitate lifelong therapy. Disseminated disease is more commonly seen in the setting of immunosuppression. Thus, patients with human immunodeficiency virus, organ transplantation, or malignancy are at increased risk of severe disease.7 Other risk factors include late-stage pregnancy, diabetes mellitus, and Filipino or African-American descent.8, 9, 10
The many underlying factors that determine the manifestation of coccidioidomycosis are not well understood. We present a case series of 3 primary cutaneous coccidioidomycosis patients who were evaluated between March 2016 and April 2017 in Southern California Orange County. This timeframe marks a period of extended drought followed by record-breaking rainfall in late 2016. This case series raises the possibility of climate change being a contributing factor to presentation of cutaneous coccidioidomycosis and highlights the changing epidemiology of coccidioidomycosis that dermatologists face.
Case 1
A 19-year-old healthy white male student presented to our clinic with a 6-week history of enlarging ulcerated lesions on the left shoulder and right upper neck (Fig 1). Both lesions were asymptomatic. He otherwise felt well and did not recall any trauma to these areas. He denied cough, shortness of breath, fevers, or chills. He had not travelled recently. The patient lived at home with his parents and 4 siblings, who were all feeling well. On examination, 2 ulcerated pink plaques were noted: one on his left anterior shoulder and one on the right side of his upper neck. A skin biopsy found necrotizing granulomatous inflammation with presence of fungal microorganisms suggestive of coccidioidomycosis (Fig 2). Culture of the tissue specimen grew coccidioidomycosis. Additional workup found positive coccidioidomycosis IgM and IgG titers, and human immunodeficiency virus test was negative. Chest radiograph ruled out pulmonary disease, and the diagnosis of primary cutaneous coccidioidomycosis was made. He was started on fluconazole, 200 mg twice a day, and had significant improvement of his skin lesions at follow-up.
Fig 1.
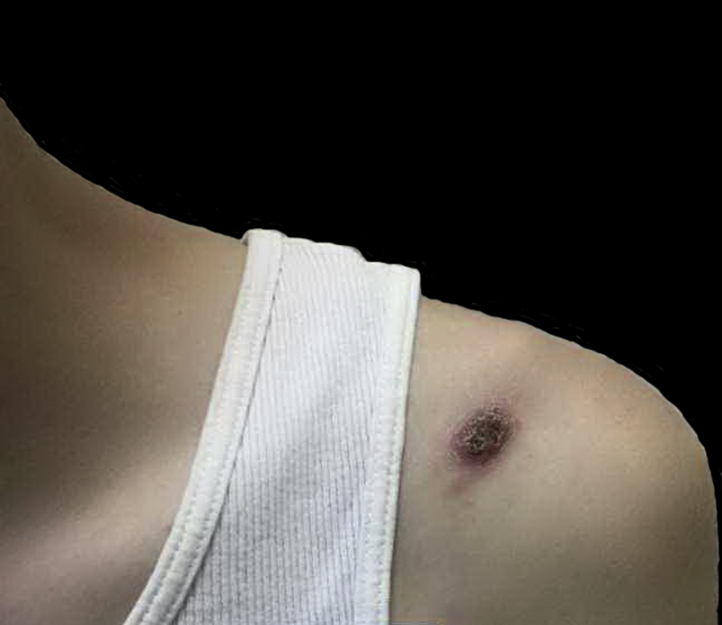
Ulcerating erythematous nodule on the left anterior shoulder.
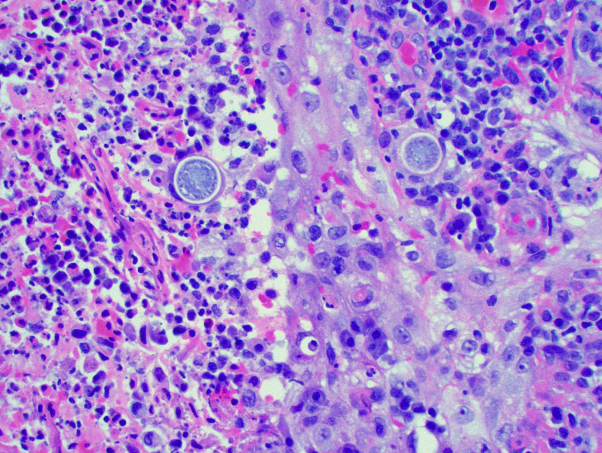
Fig 2.
Histology shows necrotizing granulomatous inflammation with presence of fungal microorganisms suggestive of coccidioidomycosis.
Case 2
A 56-year-old white woman presented to the clinic with a 1-week history of an inflamed papule on the left cheek (see Table I for comparison of patient characteristics). Despite multiple courses of antibiotics, the lesion continued to grow. The patient is a domestic flight attendant and frequently worked in her garden. She had chills when the lesion first developed, but symptoms had resolved when she presented. She remained asymptomatic without any respiratory symptoms. A punch biopsy found necrotizing granulomatous inflammation containing organisms with morphologic features consistent with coccidioidomycosis. In addition, fungal cultures were positive for C immitis. Chest x-ray was normal without evidence of pulmonary disease, and the patient was started fluconazole, 400 mg daily, with improvement in her skin lesions at follow-up.
Table I.
Patient characteristics
| Patient | Age | Sex | Ethnicity | Site | Travel History | Other activities | Immunosuppresion | Chest radiograph |
|---|---|---|---|---|---|---|---|---|
| 1 | 19 | M | White | Shoulder and neck | None | None | None | Normal |
| 2 | 56 | F | White | Cheek | Yes | Gardening | None | Normal |
| 3 | 54 | M | Asian | Ring finger | None | Gardening | None | Normal |
Case 3
A 54-year-old Asian man presented with a several-month history of an erythematous plaque with surrounding pustules on his right dorsal ring finger (Fig 3). The lesion felt itchy, and the patient described some discharge, but there were no fever, chills, or pulmonary symptoms. The patient worked in an office and denied recent travel but did spend some time gardening. A skin biopsy found necrotizing granulomatous dermatitis with ulceration and abscess formation, and culture of the specimen was positive for C immitis and Pantoea agglomerans. Chest radiograph was negative for any pulmonary involvement. The patient was started on itraconazole and ampicillin, which helped initially. However, on follow-up, his lesion reappeared. A second biopsy was performed, which once again confirmed coccidioidomycosis infection, and the patient was switched to fluconazole. The plaque showed a significant reduction in size with the new treatment regimen.
Fig 3.
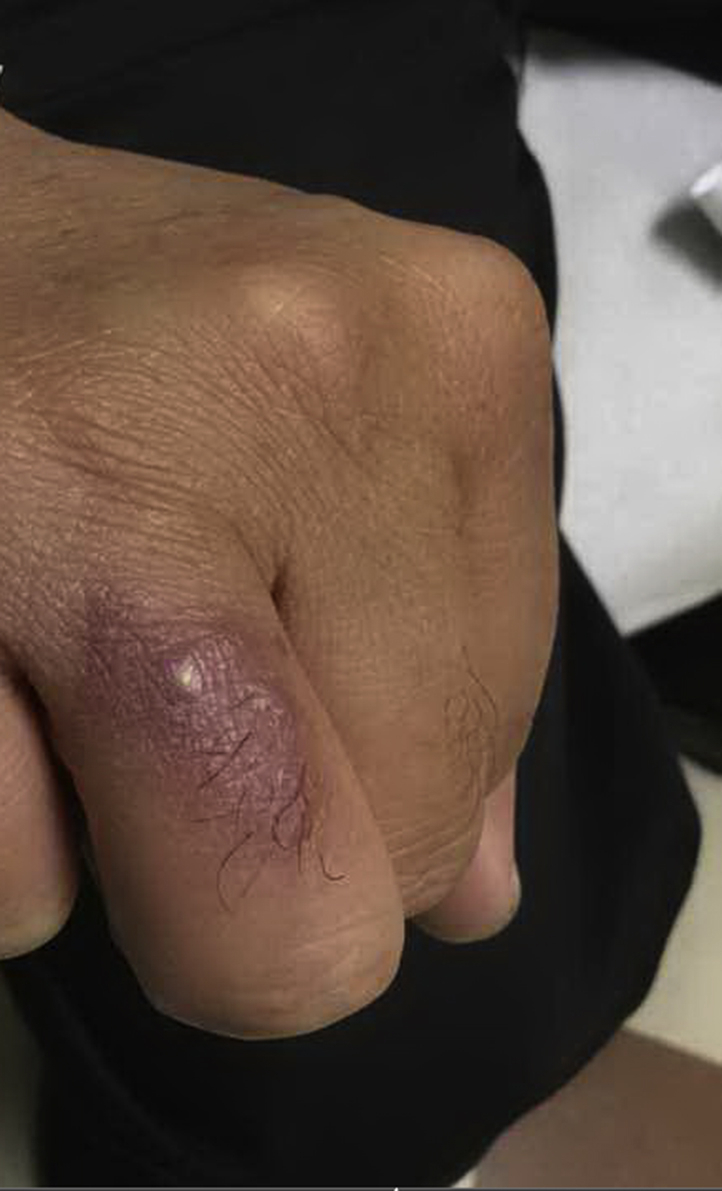
Erythematous plaque on right dorsal ring finger.
Discussion
We present 3 patients with primary cutaneous coccidioidomycosis without systemic manifestations. All 3 patients were residents of Orange County, California, a nonendemic region for coccidioidomycosis. However, California had recently experienced a 5-year drought followed by a period of historic rainfall. The patients were neither immunosuppressed nor were they outdoor occupational workers, individuals more likely to be infected by coccidioidomycosis. However, 2 of 3 patients enjoyed gardening as a hobby, putting them at increased risk for direct inoculation via contact soil. All 3 patients responded well to treatment and had significant improvement of their cutaneous lesions.
This case series highlights the changing epidemiology of coccidioidomycosis and emphasizes the importance for dermatologists to be aware of possibly new endemic areas as climate continues to change. The incidence of coccidioidomycosis has increased in both Arizona and California recently, with changing climactic and environmental factors thought to be contributing elements.11 Previous studies have found that C immitis outcompetes other fungal organisms in drought periods and thrives in subsequent wet periods.12 Changes in the amount of rainfall accounted for changing coccidioidomycosis incidence during the tested 23-year period. Thus, we speculate that we are observing an increased number of cutaneous coccidioidomycosis cases caused by the extended drought period followed by heavy rainfall, which California experienced this year. Additionally, there have been recent reports of patients in eastern Washington state who acquired coccidioidomycosis without any travel history.11, 13 Subsequent studies of soil samples collected in that region confirmed the presence of C immitis, reflecting the changing demographics of endemic diseases in the Unites States.14, 15
Primary cutaneous coccidioidomycosis is a rare manifestation of C immitis, and the skin lesions can often be clinically nonspecific. Because dermatologists are at the forefront for evaluating skin lesions, we play an important role in the timely diagnosis and treatment of cutaneous coccidioidomycosis. This case series underlines the changing epidemiology of coccidioidomycosis because Southern California is not typically an endemic area for coccidioidomycosis. As rainfall and drought patterns continue to change, dermatologists who live in traditionally nonendemic areas need to include coccidioidomycosis in their differential diagnoses even when they approach patients without travel history, as new endemic areas continue to emerge.
References
- 1.Benedict K., Thompson G.R., 3rd, Deresinski S., Chiller T. Mycotic infections acquired outside areas of known endemicity, United States. Emerg Infect Dis. 2015;21(11):1935–1941. doi: 10.3201/eid2111.141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia Garcia S.C., Salas Alanis J.C., Flores M.G., Gonzalez Gonzalez S.E., Vera Cabrera L., Ocampo Candiani J. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015;90(5):610–619. doi: 10.1590/abd1806-4841.20153805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis E.R., Bowers J.R., Barker B.M. Dust devil: the life and times of the fungus that causes valley Fever. PLoS Pathog. 2015;11(5):e1004762. doi: 10.1371/journal.ppat.1004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiCaudo D.J. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55(6):929–942. doi: 10.1016/j.jaad.2006.04.039. quiz 943-925. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Valley Fever (Coccidioidomycosis) statistics. https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html Available at:
- 6.Chang A., Tung R.C., McGillis T.S., Bergfeld W.F., Taylor J.S. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49(5):944–949. doi: 10.1016/s0190-9622(03)00462-6. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter J.B., Feldman J.S., Leyva W.H., DiCaudo D.J. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62(5):831–837. doi: 10.1016/j.jaad.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Bercovitch R.S., Catanzaro A., Schwartz B.S., Pappagianis D., Watts D.H., Ampel N.M. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53(4):363–368. doi: 10.1093/cid/cir410. [DOI] [PubMed] [Google Scholar]
- 9.Louie L., Ng S., Hajjeh R. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5(5):672–680. doi: 10.3201/eid0505.990508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenstein N.E., Emery K.W., Werner S.B. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995-1996. Clin Infect Dis. 2001;32(5):708–715. doi: 10.1086/319203. [DOI] [PubMed] [Google Scholar]
- 11.Kaffenberger B.H., Shetlar D., Norton S.A., Rosenbach M. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76(1):140–147. doi: 10.1016/j.jaad.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Zender C.S., Talamantes J. Climate controls on valley fever incidence in Kern County, California. Int J Biometeorol. 2006;50(3):174–182. doi: 10.1007/s00484-005-0007-6. [DOI] [PubMed] [Google Scholar]
- 13.Marsden-Haug N., Goldoft M., Ralston C. Coccidioidomycosis acquired in Washington state. Clin Infect Dis. 2013;56(6):847–850. doi: 10.1093/cid/cis1028. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Rivera C., Bhatty M., Christie P.J. Mechanism and function of type IV secretion during infection of the human host. Microbiol Spectr. 2016;4(3) doi: 10.1128/microbiolspec.VMBF-0024-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsden-Haug N., Hill H., Litvintseva A.P. Coccidioides immitis identified in soil outside of its known range - Washington, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(20):450. [PMC free article] [PubMed] [Google Scholar]