Abstract
Aims/Introduction
To evaluate whether there is disparity of the efficacy and all‐cause mortality and other adverse effects between Asian and non‐Asian patients with sodium–glucose cotransporter 2 (SGLT2) inhibitors treatment.
Materials and Methods
Randomized clinical trials publicly available before January 2017, comparing SGLT2 inhibitors treatment with a placebo in type 2 diabetes patients were identified. The association between treatment and outcomes was estimated by computing the weighted mean difference for glycated hemoglobin level, blood pressure level, lipid profile levels and bodyweight, and the odds ratios for adverse events.
Results
A total of 17 trials with Asian patients were included and 39 trials with non‐Asian patients were included. Comparison of the glycated hemoglobin decreases corrected by a placebo between Asian and non‐Asian patients showed that there was a non‐significant difference of 0.05% between groups (P > 0.05). Comparisons of the bodyweight changes and blood pressure changes corrected by a placebo between Asian and non‐Asian patients did not show a significant difference between groups (P > 0.05). The risk of all‐cause mortality was not increased when compared with a placebo both in Asian and non‐Asian populations, and the risk of genital infection in Asian and non‐Asian populations were both significant increased.
Conclusions
Overall, according to the present meta‐analysis, comparison of the efficacy in SGLT2 inhibitors treatment between Asian and non‐Asian type 2 diabetes patients showed no significant difference in glycated hemoglobin reduction and bodyweight reduction. Furthermore, no disparity was found in the risk of all‐cause mortality or hypoglycemia in SGLT2 inhibitors treatment between Asian and non‐Asian patients.
Keywords: Asian, Sodium–glucose cotransporter 2 inhibitors, Type 2 diabetes
Introduction
The sodium–glucose cotransporter 2 (SGLT2) inhibitors are a new class of oral antidiabetic drugs, which reduce hyperglycemia by increasing urinary glucose excretion independently of insulin secretion or action. Recently, many randomized controlled trials have shown that SGLT2 inhibitors improved glycemic control in patients with type 2 diabetes both as monotherapy and as add‐on therapy1, 2, 3, 4. So far, approximately six kinds of SGLT2 inhibitors have been approved as dapagliflozin, canagliflozin, empagliflozin, ipragliflozin and ertugliflozin in the Western market, and another two kinds of SGLT2 inhibitors as tofogliflozin and luseogliflozin have been approved in Japan.
With the increasing prevalence of type 2 diabetes in the Asian population, and the unmet need for both good glucose control and low incidence of adverse effects, the efficacy of novel agents, such as SGLT2 inhibitors, in the Asian population are expected to be as effective as the efficacy in the non‐Asian population when treating patients with type 2 diabetes. Although several studies of SGLT2 inhibitors treatment have been carried out in Asian type 2 diabetes patients1, 2, 3, 4, comparisons between Asian and non‐Asian populations on glucose control, bodyweight control and blood pressure control, as well as the risk of all‐cause mortality, hypoglycemia and other adverse effects have not been well studied.
Because of the lower body mass index (BMI) of Asian patients compared with that of non‐Asian patients5, 6 and the different genetic and pharmacogenetics ethnic background7, 8, we hypothesize that the efficacy of glucose control and bodyweight control or the adverse effects between the two ethnicities might be different. Therefore, the aim of the present meta‐analysis was to evaluate the efficacy, as well as the adverse effects, between Asian and non‐Asian type 2 diabetes patients with SGLT2 inhibitors.
Methods
Search strategy
Randomized clinical trials publicly available before January 2017, comparing SGLT2 inhibitors treatment with a placebo in type 2 diabetes patients were identified. The following terms were used for searching: type 2 diabetes, sodium–glucose cotransporter 2 inhibitors, dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, luseogliflozin, ertugliflozin and randomized controlled trials. The databases of MEDLINE and the Cochrane Library Central Register of Controlled Trials were searched, first in December 2015 and second in January 2017. The registration number of this meta‐analysis is CRD42016047666.
Inclusion criteria and data extraction
The inclusion criteria were as follows: (i) placebo‐controlled trial of SGLT2 inhibitor treatment; (ii) type 2 diabetes patients; (iii) study length >12 weeks; (iv) the efficacy of glucose control was the primary outcome of the study; and (v) the trials were randomized controlled trials. The exclusion criteria were as follows: (i) active controlled study; (ii) non‐randomized trial; (iii) trials in type 1 diabetes patients; (iv) study length <12 weeks; and (v) the efficacy of glucose control could not be collected from the trial.
Two investigators (XYG and YFC) screened the titles and abstracts independently to identify potentially eligible trials. Any potentially relevant citation was then evaluated in full‐text. Any discrepancies were discussed with a third author (WJY) until a consensus was reached. The two investigators independently reviewed the main reports and supplementary materials, and extracted study and patient characteristics and treatment strategies. Each study included in this meta‐analysis was categorized as an Asian study or non‐Asian study according to whether the percentage of Asian participants in each study was ≥50% or not. The quality of each study and the risk of bias were evaluated using the Cochrane instrument.
End‐points
The primary end‐point of the present meta‐analysis was the efficacy of glucose control in SGLT2 inhibitors treatment between Asian and non‐Asian type 2 diabetes patients. The secondary end‐points were the placebo‐corrected changes from baseline of bodyweight, blood pressure, lipid profile between Asian and non‐Asian populations, as well as the risk of adverse effects between Asian and non‐Asian type 2 diabetes patients.
Statistical analysis
Treatment effects were estimated by random effects or fixed effects pairwise meta‐analysis. The association between treatment and outcomes was estimated by computing the weighted mean difference (WMD) for glycated hemoglobin (HbA1c) level, blood pressure level, lipid profile levels and bodyweight, and by computing the odds ratios (ORs) for all‐cause mortality, adverse events and hypoglycemia, together with 95% confidence intervals (95% CI). Higgins I 2 statistics were used to quantify the percentage of the total variance in the summary estimate as a result of between‐study heterogeneity. Publication bias was assessed through a funnel plot and Egger's test analysis. Statistical testing was two‐sided, with P < 0.05 considered statistically significant. Most statistical analyses were carried out with the Review Manager statistical software package (version 5.2; Nordic Cochrane Centre, Copenhagen, Denmark). Meta‐regression analysis was carried out with STATA (version 11.0; StataCorp, College Station, TX, USA). The present meta‐analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines for carrying out and reporting meta‐analyses of randomized controlled trials.
Results
Studies included in the meta‐analysis and qualifications
The electronic searching began from December 2015 to January 2017 and retrieved 1989 citations. The flowchart of included studies is summarized in Figure 1. Overall, 56 randomized clinical trials were eligible for inclusion in the review. In Asian populations, 17 trials were included1, 2, 3, 4, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, among which two trials with dapagliflozin treatment compared with a placebo, three trials with canagliflozin treatment compared with a placebo, three trials with ipragliflozin treatment compared with a placebo, two trials with tofogliflozin treatment compared with a placebo, two trials with empagliflozin treatment compared with a placebo and five trials with luseogliflozin treatment compared with a placebo (shown in Table S1). In non‐Asian populations, 39 trials were included22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, among which 18 trials with dapagliflozin treatment compared with a placebo22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, seven trials with canagliflozin treatment compared with a placebo41, 42, 43, 44, 45, 46, 47, 10 trials with empagliflozin treatment compared with a placebo48, 49, 50, 51, 52, 53, 54, 55, 56, 57, two trials with ipragliflozin treatment compared with a placebo58, 59 and one trial with ertugliflozin treatment compared with a placebo60 (shown in Table S2).
Figure 1.
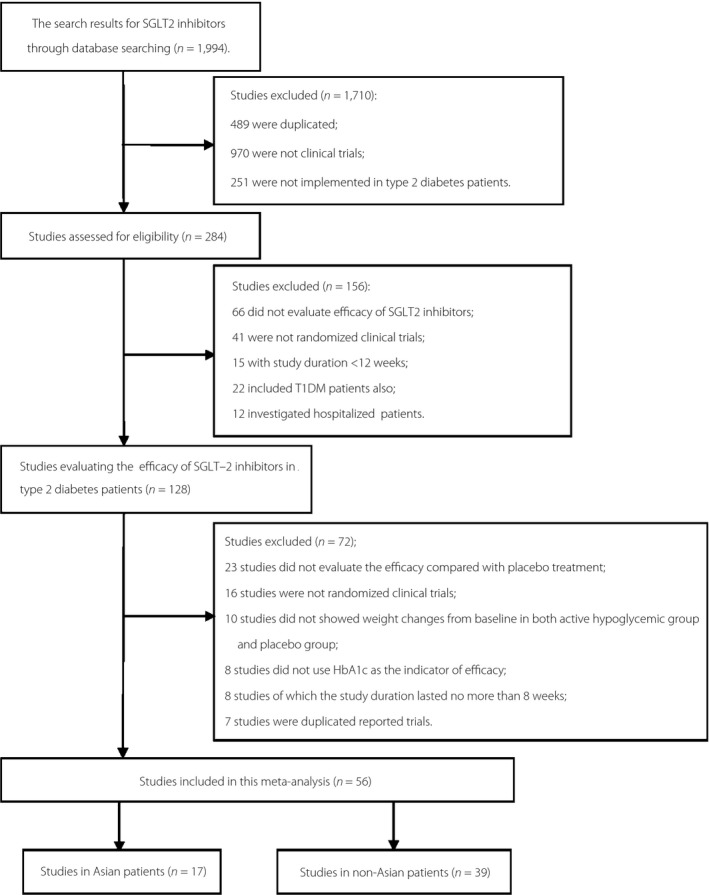
Flowchart of studies in Asian and non‐Asian type 2 diabetes patients included in the present meta‐analysis. SGLT2, sodium–glucose cotransporter 2; T1DM, type 1 diabetes mellitus.
By using the Cochrane instrument, the risk of bias was evaluated. Overall, the risk of bias was low, the random sequence generation, the allocation concealment, the blinding of participants and personnel, the blinding of outcome assessment, and the incomplete outcome data were all well reported, and the selective reporting was low (Figures S1 and S2).
Baseline characteristics between Asian and non‐Asian patients
For Asians, 2,600 patients were included in SGLT2 inhibitors treatment, whereas for non‐Asians, 12,272 patients were included in SGLT2 inhibitors treatment. Comparisons of the baseline characteristics between Asians and non‐Asians showed that baseline weight and baseline BMI were significantly different (BMI 26.4 kg/m2 vs 31.4 kg/m2, P < 0.05; weight 71.2 kg vs 87.0 kg, P < 0.05). Details are given in Table 1.
Table 1.
Baseline characteristics between Asian and non‐Asian type 2 diabetes patients receiving sodium–glucose cotransporter 2 inhibitors treatment
| Variables | Asian | Non‐Asian |
|---|---|---|
| No. studies | 17 | 39 |
| Age (years) | 56.6 ± 3.3 | 57.1 ± 4.6 |
| Male (%) | 33.9 | 47.1 |
| BMI (kg/m2) | 26.4 ± 1.6 | 31.4 ± 1.8a |
| Weight (kg) | 71.2 ± 4.1 | 87.0 ± 5.5a |
| DM duration (years) | 6.4 ± 2.3 | 7.4 ± 4.6 |
| HbA1c (%) | 8.00 ± 0.00 | 8.13 ± 0.41 |
P‐value <0.05. BMI, body mass index; DM, diabetes mellitus; HbA1c, glycated hemoglobin.
HbA1c changes in SGLT2 inhibitors treatment
Overall, in Asian patients, compared with a placebo, the SGLT2 inhibitors treatment resulted in a significantly greater change in HbA1c (WMD −0.65%, 95% CI −0.73% to −0.56%, P < 0.01). In dapagliflozin treatment, the HbA1c change from baseline was −0.74% compared with a placebo with statistical significance (95% CI −0.77% to −0.71%, P < 0.01). In canagliflozin treatment, tofogliflozin treatment, ipragliflozin treatment and luseogliflozin treatment, the HbA1c changes also significantly decreased (WMD −0.77%, 95% CI, −1.16% to −0.38%; −0.70%, 95% CI −0.98% to −0.43%; −0.65%, 95% CI −1.24% to −0.07%; and −0.49%, 95% CI −0.73% to −0.25%, respectively; all P < 0.01).
In non‐Asian patients, compared with a placebo, the SGLT2 inhibitors treatment was associated with a significantly greater decrease in HbA1c (WMD −0.60%, 95% CI −0.64% to −0.56%, P < 0.01). The dapagliflozin treatment, canagliflozin treatment, empagliflozin treatment and ipragliflozin treatment led to HbA1c decrease from baseline in 0.58%, 0.71%, 0.57% and 0.62%, respectively, when compared with a placebo with statistical significance.
Comparison of the HbA1c decreases corrected by a placebo between Asians and non‐Asians showed that there was a non‐significant difference of 0.05% between groups (P > 0.05). Details are shown in Table 2. In dapagliflozin treatment, the difference between groups was 0.16% with statistical significance in favor of Asian populations, but in canagliflozin treatment and ipragliflozin treatment, the differences between groups were not significant.
Table 2.
Efficacy changes with sodium–glucose cotransporter 2 inhibitors treatment between Asian and non‐Asian type 2 diabetes patients
| SGLT2 inhibitors | Asian | Non‐Asian | Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. participants (SGLT2i vs placebo) | WMD from baseline | 95% CI | I 2 | No. participants (SGLT2i vs placebo) | WMD from baseline | 95% CI | I 2 | ||
| HbA1c (%) | |||||||||
| Total | 1,592/1,333 | −0.65a | −0.73, −0.56 | 97% | 6,518/5,758 | −0.60a | −0.64, −0.56 | 99% | −0.05 |
| Dapagliflozin | 185/186 | −0.74a | −0.77, −0.71 | 0% | 2,312/2,316 | −0.58a | −0.65, −0.50 | 99% | −0.16a |
| Canagliflozin | 302/301 | −0.77a | −1.16, −0.38 | 89% | 1,731/1,521 | −0.71a | −0.77, −0.65 | 96% | −0.06 |
| Ipragliflozin | 317/185 | −0.65a | −1.24, −0.27 | 95% | 140/135 | −0.62a | −0.95, −0.30 | 86% | −0.03 |
| Tofogliflozin | 125/122 | −0.70a | −0.98, −0.43 | 98% | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 2,280/1,732 | −0.57a | −0.62, −0.52 | 99% | − |
| Luseogliflozin | 439/311 | −0.49a | −0.73, −0.25 | 99% | − | − | − | − | − |
| Bodyweight (kg) | |||||||||
| Total | 1,592/1,333 | −1.86a | −2.03, −1.70 | 97% | 5,866/5,547 | −1.94a | −2.24, −1.65 | 100% | 0.08 |
| Dapagliflozin | 185/186 | −1.92a | −2.04, −1.80 | 79% | 2,312/2,316 | −1.77a | −2.05, −1.49 | 99% | −0.15 |
| Canagliflozin | 302/301 | −2.01a | −2.80, −1.21 | 100% | 1,731/1,521 | −2.47a | −2.92, −2.01 | 100% | 0.46 |
| Ipragliflozin | 317/185 | −1.55a | −1.94, −1.17 | 57% | 140/135 | −1.73a | −1.92, −1.54 | 0% | 0.18 |
| Tofogliflozin | 125/122 | −2.35a | −2.87, −1.83 | 95% | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,628/1,521 | −1.93a | −2.10, −1.76 | 99% | − |
| Luseogliflozin | 439/311 | −1.68a | −1.95, −1.41 | 95% | − | − | − | − | − |
| SBP (mmHg) | |||||||||
| Total | 1,442/1,262 | −3.55a | −5.93, −1.17 | 100% | 5,419/4,995 | −4.38a | −5.01, −3.75 | 99% | 0.83 |
| Dapagliflozin | 185/186 | −0.22 | −5.86,5.43 | 100% | 2,152/2,156 | −3.75a | −4.40, −3.11 | 97% | 3.53 |
| Canagliflozin | 302/301 | −5.50a | −9.42, −1.58 | 100% | 1,731/1,521 | −5.19a | −6.29, −4.09 | 100% | −0.31 |
| Ipragliflozin | 317/185 | −3.69a | −6.07, −1.31 | 0% | − | − | − | − | − |
| Tofogliflozin | 125/122 | −2.53 | −8.95,3.90 | 88% | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,536/1,318 | −4.50a | −5.76, −3.24 | 100% | − |
| Luseogliflozin | 289/240 | −4.75a | −6.01, −3.49 | 83% | − | − | − | − | − |
| DBP (mmHg) | |||||||||
| Total | 1,442/1,262 | −2.39a | −3.23, −1.54 | 98% | 5,071/4,758 | −1.79a | −2.10, −1.48 | 99% | −0.60 |
| Dapagliflozin | 185/186 | −4.44 | −9.24,0.36 | 100% | 2,019/2,025 | −1.41a | −1.76, −1.05 | 95% | −3.03 |
| Canagliflozin | 302/301 | −1.75 | −4.79,1.29 | 100% | 1,731/1,522 | −2.01a | −2.41, −1.62 | 99% | 0.26 |
| Ipragliflozin | 317/185 | −2.71a | −4.29, −1.14 | 0% | − | − | − | − | − |
| Tofogliflozin | 125/122 | −1.93a | −2.60, −1.27 | 0% | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,321/1,211 | −2.27a | −3.05, −1.49 | 99% | − |
| Luseogliflozin | 289/240 | −2.00a | −3.25, −0.76 | 91% | − | − | − | − | − |
| T‐CHO (mg/dL) | |||||||||
| Total | 863/730 | 4.26a | 2.07,6.45 | 97% | 1,936/1,706 | 3.03a | 1.16,4.90 | 99% | 1.23 |
| Dapagliflozin | 185/186 | 3.36a | 2.75,3.98 | 51% | 649/641 | 2.99a | 1.81,4.18 | 80% | 0.37 |
| Canagliflozin | − | − | − | − | − | − | − | − | − |
| Ipragliflozin | 317/185 | 0.03 | −3.88,3.94 | 0% | − | − | − | − | − |
| Tofogliflozin | − | − | − | − | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,215/999 | 3.29 | 0.64,5.94 | 100% | − |
| Luseogliflozin | − | − | − | − | − | − | − | − | − |
| TG (mg/dL) | |||||||||
| Total | 1,375/1,196 | −17.66a | −23.63, −11.70 | 97% | 3,129/2,713 | −5.14a | −9.94, −0.34 | 99% | −12.52a |
| Dapagliflozin | 185/186 | −13.73a | −22.00, −5.46 | 98% | 757/749 | −9.95a | −16.29, −3.62 | 96% | −3.78 |
| Canagliflozin | 302/301 | −25.58a | −50.28, −0.89 | 100% | 1,003/817 | −6.00 | −17.33,5.32 | 100% | −9.58 |
| Ipragliflozin | 317/185 | −14.44a | −27.30, −1.58 | 59% | − | − | − | − | − |
| Tofogliflozin | − | − | − | − | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,297/1,081 | 0.07 | −6.52,6.67 | 99% | − |
| Luseogliflozin | 289/240 | −18.69a | −33.51, −3.87 | 95% | − | − | − | − | − |
| LDL‐C (mg/dL) | |||||||||
| Total | 1,314/1,142 | 4.10a | 1.66,6.54 | 99% | 3,129/2,713 | 3.03a | 2.92,3.14 | 99% | 1.07 |
| Dapagliflozin | 185/186 | 3.70 | −5.03,12.44 | 99% | 757/749 | 4.22a | 1.51,6.93 | 98% | −0.52 |
| Canagliflozin | 302/301 | 7.89a | 5.00,10.78 | 98% | 1,003/817 | 3.45 | −0.50,7.40 | 100% | 4.44 |
| Ipragliflozin | 317/185 | 0.07 | −3.85,3.98 | 0% | − | − | − | − | − |
| Tofogliflozin | − | − | − | − | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,297/1,081 | 2.10 | −0.26,4.47 | 100% | − |
| Luseogliflozin | 228/186 | 3.72 | −2.15,9.58 | 97% | − | − | − | − | − |
| HDL‐C (mg/dL) | |||||||||
| Total | 1314/1142 | 4.22a | 3.46,4.99 | 98% | 3,129/2,713 | 1.70a | 1.28,2.13 | 99% | 3.34a |
| Dapagliflozin | 185/186 | 8.13a | 6.53,9.72 | 90% | 760/749 | 1.75a | 1.46,2.05 | 63% | 6.38a |
| Canagliflozin | 302/301 | 4.00a | 2.63,5.37 | 99% | 1,003/817 | 2.28a | 1.50,3.06 | 99% | 1.72 |
| Ipragliflozin | 317/185 | 2.03 | 0.59,3.47 | 21% | − | − | − | − | − |
| Tofogliflozin | − | − | − | − | − | − | − | − | − |
| Empagliflozin | − | − | − | − | 1,297/1,081 | 1.49a | 0.81,2.16 | 100% | − |
| Luseogliflozin | 228/186 | 3.10a | 2.20,4.00 | 85% | − | − | − | − | − |
| ALT (U/L) | |||||||||
| Total | 352/344 | −7.99 | −15.59, −0.40 | 84% | 1,106/898 | −3.24 | −4.26, −2.22 | 82% | −4.75 |
| AST (U/L) | |||||||||
| Total | 140/139 | −1.76 | −5.58,2.05 | 68% | 633/454 | −3.43 | −6.49, −0.37 | 80% | 1.67 |
| GGT (U/L) | |||||||||
| Total | 140/139 | −9.54 | −16.13, −2.94 | 0% | − | − | − | − | − |
P‐value <0.05. ALT, alanine aminotransferase; AST, aminotransferase; CI, confidence interval; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; GGT, gamma‐glutamyl transferase; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SGLT2, sodium–glucose cotransporter 2; SGLT2i, sodium–glucose cotransporter 2 inhibitor; T‐CHO, total cholesterol; TG, triglyceride; WMD, weighted mean difference.
Meta‐regression analysis showed that the HbA1c decrease from baseline was not associated with baseline HbA1c, baseline weight or baseline BMI, baseline age or sex, study duration, or duration of diabetes in both Asian and non‐Asian populations.
Weight changes in SGLT2 inhibitors treatment
In Asians, compared with a placebo, treatment with SGLT2 inhibitors led to a significant decrease in bodyweight (WMD −1.86 kg, 95% CI −2.03 to −1.70 kg, P < 0.01). Treatments with dapagliflozin, canagliflozin, ipragliflozin, tofogliflozin and luseogliflozin also led to significant decreases in bodyweight respectively (WMD −1.92 kg, 95% CI −2.04 to −1.80 kg; −2.01 kg, 95% CI −2.80 to −1.21 kg; −1.55 kg, 95% CI −1.94 to −1.17 kg; −2.35 kg, 95% CI −2.87 to −1.83 kg; −1.68 kg, 95% CI −1.95 to −1.41 kg; all P < 0.01). In non‐Asians, compared with a placebo, treatment with SGLT2 inhibitors was associated with a significant decrease in bodyweight (WMD −1.94 kg, 95% CI −2.24 to −1.65 kg, P < 0.01). Treatments with dapagliflozin, canagliflozin, empagliflozin and ipragliflozin also led to significant decreases in bodyweight, respectively. Comparison of the bodyweight changes corrected by a placebo between Asians and non‐Asians showed that there was no difference between groups (P > 0.05). Details are shown in Table 2.
Meta‐regression analysis showed that the bodyweight decrease from baseline was not associated with the HbA1c changes from baseline, baseline weight or BMI, baseline age or sex, study duration, or duration of diabetes in both Asians and non‐Asians.
Blood pressure changes in SGLT2 inhibitors treatment
Compared with a placebo, in Asians, treatment with SGLT2 inhibitors led to a significant decrease in the level of systolic blood pressure (SBP; WMD −3.55 mmHg, 95% CI −5.93 to −1.17 mmHg, P < 0.01) and a significant decrease in the level of diastolic blood pressure (DBP; WMD −2.39 mmHg, 95% CI −3.23 to −1.54 mmHg, P < 0.01). In non‐Asians, treatment with SGLT2 inhibitors was associated with a significant decrease both in the level of SBP (WMD −4.38 mmHg, 95% CI −5.01 to −3.75 mmHg, P < 0.01) and in the level of DBP (WMD −1.79 mmHg, 95% CI −2.10 to −1.48 mmHg, P < 0.01, respectively). Details are shown in Table 2.
Comparison of SBP change from baseline between Asian and non‐Asian patients showed that the difference was 0.83 mmHg without significance (P > 0.05). Comparison of DBP change from baseline between Asians and non‐Asians also showed a non‐significant difference (P > 0.05). Comparisons between Asians and non‐Asians in each kind of SGLT2 inhibitor treatment are also shown in Table 2.
Meta‐regression analysis showed that the SBP changes from baseline were not associated with baseline SBP, baseline weight or BMI, baseline age or sex, or study duration, in both Asians and non‐Asians. The DBP changes were not associated with baseline characteristics either.
Lipid profile changes in SGLT2 inhibitors treatment
In Asians, compared with a placebo, treatment with SGLT2 inhibitors was associated with a significant decrease in triglyceride (TG) level (WMD −17.66 mg/dL, 95% CI −23.63 to −11.70 mg/dL, P < 0.01), but a significant increase in the level of total cholesterol (T‐CHO) and high‐density lipoprotein cholesterol (HDL‐C; WMD 4.26 mg/dL, 95% CI 2.07 to 6.45 mg/dL, P < 0.01 and 4.22 mg/dL, 95% CI 3.46 to 4.99 mg/dL, P < 0.01, respectively). The level of low‐density lipoprotein cholesterol (LDL‐C) was also significant increased with SGLT2 inhibitors (WMD 4.10 mg/dL 95% CI 1.66 to 6.54 mg/dL, P < 0.01). In non‐Asians, the level of TG was also significantly decreased, and the levels of T‐CHO, LDL‐C and HDL‐C were significantly increased. Details are also shown in Table 2.
Comparison of the efficacy on lipid profiles in SGLT2 inhibitors treatment between Asians and non‐Asians showed that the decrease of TG level from baseline in Asians was significantly superior to that in non‐Asians with a 12.52‐mg/dL (P < 0.05) difference between groups; the increase of HDL‐C in Asians was also significantly superior to that in non‐Asians with a 3.34‐mg/dL (P < 0.05) difference between groups, and the increase of T‐CHO and LDL‐C level from baseline in Asians was comparable with that in non‐Asians. (1.29 mg/dL and 1.01 mg/dL, respectively, P > 0.05). Details are shown in Table 2.
Meta‐regression analysis showed that the TG decrease from baseline was not associated with baseline TG, baseline weight or BMI, baseline age or sex, or HbA1c changes from baseline in both Asians and non‐Asians, and the increase of T‐CHO or LDL‐C or HDL‐C was not associated with baseline lipid level, baseline weight or BMI, baseline age or sex, or HbA1c changes from baseline in both Asians and non‐Asians.
Effects on liver enzymes in SGLT2 inhibitors treatment
In Asians, compared with the placebo, treatment with SGLT2 inhibitors led to a significant decrease in alanine aminotransferase level, as well as a significant decrease in gamma‐glutamyl transferase level. In non‐Asians, treatment with SGLT2 inhibitors resulted in a significant decrease in alanine aminotransferase level and also in aminotransferase level. No significant difference was found in aminotransferase and alanine aminotransferase between Asian and non‐Asian patients with SGLT2 inhibitors treatment. Data for gamma‐glutamyl transferase was not enough for comparison. Details are shown in Table 2.
All‐cause mortality with SGLT2 inhibitors treatment
In Asians, compared with a placebo, the risk of all‐cause mortality was not significantly increased (OR 1.05, 95% CI 0.11 to 9.77, P = 0.97). In non‐Asians, compared with a placebo, the risk of all‐cause mortality was not significantly increased (OR 0.85, 95% CI 0.52 to 1.39, P = 0.52). The between‐group difference of all‐cause mortality in SGLT2 inhibitors treatment in Asians and non‐Asians was not statistical significant (P > 0.05).
Adverse effects with SGLT2 inhibitors treatment
The risk of hypoglycemia in the treatment of SGLT2 inhibitors in Asians was significantly increased compared with a placebo (OR 1.78, 95% CI 1.14 to 2.79, P < 0.01), and also significantly increased in add‐on treatment (OR 2.05, 95% CI 1.11 to 3.81, P = 0.02), but not in monotherapy (OR 1.51, 95% CI 0.79 to 2.89, P = 0.21). The risk of hypoglycemia in non‐Asians was also significantly increased compared with a placebo (OR 1.25, 95% CI 1.13 to 1.39, P < 0.01), and in add‐on treatment (OR 1.26, 95% CI 1.13 to 1.39, P < 0.01), but not in monotherapy (OR 1.17, 95% CI 0.59 to 2.29, P = 0.66). The between‐group difference of SGLT2 inhibitors in Asians and non‐Asians was not statistically significant (P > 0.05).
The risk of urinary infection during treatment with SGLT2 inhibitors in Asians was not increased compared with a placebo (OR 1.01, 95% CI 0.67 to 1.54, P = 0.95). The risk of genital infection in Asians was significantly increased compared with a placebo (OR 2.24, 95% CI 1.18 to 4.25, P < 0.05). However, the risk of genital infection and urinary infection in non‐Asians with SGLT2 inhibitors treatment were both significantly increased compared with a placebo (OR 3.79, 95% CI 3.09 to 4.66, P < 0.01 and OR 1.14, 95% CI 1.01 to 1.29, P = 0.03, respectively). Details are shown in Table 3.
Table 3.
Adverse effects in sodium–glucose cotransporter 2inhibitors treatment between Asian and non‐Asian type 2 diabetes patients
| SGLT2 inhibitors | Asian | Non‐Asian | ||||||
|---|---|---|---|---|---|---|---|---|
| No. participants (SGLT2i vs placebo) | OR | 95% CI | I 2 | No. participants (SGLT2i vs placebo) | OR | 95% CI | I 2 | |
| All‐cause mortality | ||||||||
| Total | 3,126/1,426 | 1.05 | 0.11,9.77 | 0% | 12,175/5,852 | 0.85 | 0.52, 1.39 | 0% |
| Hypoglycemia | ||||||||
| Total | 2,889/1,272 | 1.78a | 1.14,2.79 | 1% | 12,272/5,791 | 1.25a | 1.13,1.39 | 0% |
| Mono | 1,999/924 | 1.51 | 0.79,2.89 | 0% | 1,545/540 | 1.17 | 0.59,2.29 | 0% |
| Add on | 890/348 | 2.05a | 1.11.3.81 | 79% | 10,727/5,251 | 1.26a | 1.13.1.39 | 0% |
| Add on + SU | – | – | – | – | 2,551/1,701 | 1.22a | 1.04,1.43 | 19% |
| Add on + MET/TZD | – | – | – | – | 5,818/2,507 | 1.44 | 1.00,2.07 | 0% |
| Add on + INS | – | – | – | – | 2,358/1,043 | 1.20 | 0.91,1.57 | 56% |
| Genital infection | ||||||||
| Total | 2,889/1,272 | 2.24a | 1.18,4.25 | 0% | 12,633/5,867 | 3.79a | 3.09,4.66 | 15% |
| Urinary infection | ||||||||
| Total | 2,889/1,272 | 1.01 | 0.67,1.54 | 0% | 12,633/5,867 | 1.14a | 1.01,1.29 | 0% |
| Ketone urine/ketosis | ||||||||
| Total | 595/242 | 3.30a | 1.27,8.59 | 0% | – | – | – | – |
P‐value <0.05. CI, confidence interval; INS, insulin; MET, metformin; SGLT2, sodium–glucose cotransporter 2; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione.
Compared with a placebo, treatment with SGLT2 inhibitors was associated with a significantly increased risk of ketosis in Asian patients (OR 3.30, 95% CI 1.27 to 8.59, P < 0.01).
Discussion
According to the present meta‐analysis, comparison of HbA1c decreases from baseline with SGLT2 inhibitors treatment between Asian and non‐Asian patients showed that HbA1c decreased more in Asians than that in non‐Asians, with the difference of 0.05% between groups without significance (P > 0.05). Although baseline characteristics of the present meta‐analysis as well as previous studies5, 6 showed that the baseline BMI and baseline bodyweight were significantly lower in Asians, meta‐regression analysis from this study showed that the HbA1c decrease from baseline was neither associated with baseline weight or baseline BMI, nor with baseline HbA1c, baseline age or sex, study duration, or duration of diabetes in both Asians and non‐Asians. The previous hypothesis that there might be a difference of the efficacy on glucose control was not confirmed by the present meta‐analysis.
The efficacy in SGLT2 inhibitors treatment on bodyweight decrease was also comparable between Asians and non‐Asians. Although there were significant differences of baseline BMI and bodyweight between Asians and non‐Asians, this meta‐regres sion analysis showed that the bodyweight decrease from baseline was not associated with the HbA1c changes from baseline, or baseline weight or BMI in both Asian and non‐Asian populations. The mechanisms of SGLT2 inhibitors treatment on bodyweight might be due to caloric loss through glucose excretion in the urine61, or due to reduced total body fat and lean body mass, as well as visceral adipose tissue62, or due to improvements in weight‐related quality of life63.
Furthermore, the efficacy on SBP of SGLT2 inhibitors treatment in Asians showed no significant decrease compared with a placebo, but in non‐Asians showed a significant decrease. However, comparisons between Asian and non‐Asian patients showed no significant difference. Comparisons of DBP change from baseline in Asians and non‐Asians also showed non‐significant difference; though, both in Asian and non‐Asian populations, the DBP levels were significantly decreased. The mechanism of SGLT2 inhibitors reducing blood pressure has not been well established. It was suggested that it might be related to improved glycemic control, diuretic effects, weight loss, reduced arterial stiffness or direct vascular effects61, 64, 65, 66. However, emerging data also suggested that SBP lowering was independent of weight loss in canagliflozin treatment67. According to the meta‐regression analysis of the present meta‐analysis, the SBP changes and DBP changes were not associated with baseline SBP, baseline weight or BMI or bodyweight decrease, baseline age or sex, or study duration, in both Asians and non‐Asians.
Comparison of the efficacy on lipid profiles between Asians and non‐Asians showed that the reduction of TG level in Asians was superior to that in non‐Asians, the increase of HDL‐C in Asians was also superior to that in non‐Asians, and the increase of T‐CHO and LDL‐C level from baseline in Asians was comparable with that in non‐Asians. It was discussed previously that the effects on lipid profile appeared limited and probably not clinically relevant68. The possible mechanism reported was by reducing LDL catabolism and lipid utilization69. Meta‐regression analysis from this the present showed that the TG reduction and HDL increase were not associated with baseline TG level or HDL level, baseline weight or BMI, baseline age or sex, or HbA1c changes from baseline in both Asian and non‐Asian populations, which suggested that the lipid profile changes might be independent from the glucose level changes.
No disparity of the risk of all‐cause mortality was found in the present meta‐analysis, which did not show the possible disparity found in the randomized placebo‐controlled trial of Cardiovascular Outcomes and Mortality of Empagliflozin in Type 2 Diabetes (EMPA‐REG) study70. In that trial, the prespecified subgroup analysis showed that in Asians, the hazard ratio for the primary outcome was lower than that in white or in black populations, though without significance. Therefore, the result from the present meta‐analysis confirmed that both Asians and non‐Asians might benefit from SGLT2 inhibitors treatment.
According to this analysis, treatment with SGLT2 inhibitors was not associated with the risk of hypoglycemia when taken as monotherapy or in combination with other antidiabetes agents not associated with hypoglycemia, but was associated with increased risk of hypoglycemia when used in combination with insulin or sulfonylureas, both in Asian and non‐Asian patients. The low risk of hypoglycemia with SGLT2 inhibitors was due to the mechanism of SGLT2 inhibitors – that they act independently of the glucose‐dependent secretion of insulin by the pancreatic β‐cells. Another possible reason was the renal threshold of glycemia, below which SGLT2 inhibitors would not be expected to cause further urinary glucose excretion.
Results from the present meta‐analysis showed that the risk of urinary infection with SGLT2 inhibitors treatment in Asians was not increased when compared with a placebo, but was significantly increased in non‐Asians. The risk of genital infection in Asians and non‐Asians was significantly increased. It is known that the possible reason for the increased risk of genital infection and urinary infection is because of the glycosuric effect of SGLT2 inhibitors71. However, in Asian patients, the risk of urinary infection was not significantly increased according to the present meta‐analysis. The reason for this is not clear so far. The number of patients involved in the analysis for urinary tract infection was 1,272 and 5,867 for Asians and non‐Asians, respectively. Therefore, one possible reason might be associated with the analysis for urinary infection in Asians being underpowered. To explain this phenomenon more clearly, large data of Asian populations are required in the future.
In terms of the risk of ketosis with SGLT2 inhibitors treatment, the present meta‐analysis showed that in Asians, the risk of ketosis was significantly increased with SGLT2 inhibitors treatment when compared with a placebo. Studies included in this meta‐analysis in non‐Asians have not reported on the risk of ketosis; therefore, comparison between these two populations on the risk of ketosis could be carried out. It is known that both the US Food and Drug Administration72 and the European Medicines Agency73 warned that SGLT2 inhibitors might result in euglycemic ketoacidosis. Though the adverse events reported in the randomized controlled trials included in the present meta‐analysis were not ketoacidosis but ketosis, the high risk of this adverse effect in SGLT2 inhibitors treatment compared with a placebo should be treated cautiously and studied further.
The present meta‐analysis compared the placebo‐corrected efficacy in glucose control, bodyweight control, blood pressure control, lipid profile control and the risk of adverse effects of SGLT2 inhibitors treatment between Asians and non‐ Asians in a large sample of type 2 diabetes patients. However, as a meta‐analysis, the study still had some limitations. Data from separate studies, especially between Asian and non‐ Asian populations, were combined for the evaluation of the efficacy, as well as adverse effects. The inclusion criteria and the variables, such as age, body mass index, HbA1c level, bodyweight and duration of diabetes, might be different across studies, which could cause a high level of heterogeneity. If the level of heterogeneity was high, we used the random effects model for analysis, and also carried out sensitivity analysis. Baseline data between Asians and non‐Asians were not comparable, but we used meta‐regression analysis to evaluate the association of the efficacy and baseline variables, and adjusted the covariates. Another limitation was that there are several doses of SGLT2 inhibitors. In the present analysis, if there were several doses of SGLT2 inhibitors in one study, only the standard dose of each SGLT2 inhibitor treatment arm was included. For example, 10 mg dapagliflozin, 300 mg canagliflozin, 25 mg empagliflozin, 300 mg ipragliflozin, 40 mg tofogliflozin and 5 mg luseogliflozin. If there was only one dose of SGLT2 inhibitors in one study, this dose of each SGLT2 inhibitor treatment arm was included. In Asian patients, there are three studies with 2.5 mg luseogliflozin treatment and three studies with 50 mg ipragliflozin treatment. In non‐Asian patients, all studies included were with the standard dose. Therefore, for most of studies included, there was no difference in the dosage between Asian and non‐Asian type 2 diabetes patients. We also carried out sensitive analysis to evaluate the efficacy with different dosages, but no significant difference was found. Furthermore, publication bias is possible, because positive results had a greater chance of being selected for publication than negative results. We used the funnel plot assessment to minimize this limitation, of course, the present results should be interpreted cautiously.
Overall, according to the present meta‐analysis, comparison of the efficacy in SGLT2 inhibitors treatment between Asian and non‐Asian type 2 diabetes patients showed no significant difference in HbA1c reduction and bodyweight reduction. Furthermore, no disparity was found in the risk of all‐cause mortality or hypoglycemia in SGLT2 inhibitors treatment between Asian and non‐Asian patients.
Disclosure
LNJ has received fees for lecture presentations and for consulting from Abbott, AstraZeneca, Bristol‐Myers Squibb, Merck, Metabasis, Novartis, Eli Lilly, Roche, Sanofi‐Aventis and Takeda. There are no other disclosures for this research. The authors declare no conflict of interest.
Supporting information
Figure S1 ¦ Evaluation of the risk of bias in sodium–glucose cotransporter 2 inhibitors treatment in Asian type 2 diabetes patients.
Figure S2 ¦ Evaluation of the risk of bias in sodium–glucose cotransporter 2 inhibitors treatment in non‐Asian type 2 diabetes patients.
Table S1 ¦ Characteristics of randomized controlled trials in sodium–glucose cotransporter 2 inhibitors treatment in Asian type 2 diabetes patients.
Table S2 ¦ Characteristics of randomized controlled trials in sodium–glucose cotransporter 2 inhibitors treatment in non‐Asian type 2 diabetes patients.
Acknowledgments
We thank Dr Ling Chen, Dr Yumin Ma, Dr Siqian Gong, and other doctors and nurses for their practical work during the study at Peking University People's Hospital Endocrinology and Metabolism Department.
J Diabetes Investig 2018;9:850–861
Clinical Trial Registry
International Prospective Register of Systematic Reviews
CRD42016047666
References
- 1. Ji L, Ma J, Li H, et al Dapagliflozin as monotherapy in drug‐naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther 2014; 36: 84–100. [DOI] [PubMed] [Google Scholar]
- 2. Kaku K, Inoue S, Matsuoka O, et al Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: a phase II multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2013; 15: 432–440. [DOI] [PubMed] [Google Scholar]
- 3. Inagaki N, Kondo K, Yoshinari T, et al Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, 12‐week study. Diabetes Obes Metab 2013; 15: 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inagaki N, Kondo K, Yoshinari T, et al Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24‐week, randomized, double‐blind, placebo‐controlled, Phase III study. Expert Opin Pharmacother 2014; 15: 1501–1515. [DOI] [PubMed] [Google Scholar]
- 5. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan JC, Malik V, Jia W, et al Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 7. Cho YS, Lee JY, Park KS, et al Genetics of type 2 diabetes in East Asian populations. Curr Diab Rep 2012; 12: 686–696. [DOI] [PubMed] [Google Scholar]
- 8. Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet 2010; 375: 408–418. [DOI] [PubMed] [Google Scholar]
- 9. Ji L, Han P, Liu Y, et al Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab 2015; 17: 23–31. [DOI] [PubMed] [Google Scholar]
- 10. Kashiwagi A, Takahashi H, Ishikawa H, et al A randomized, double‐blind, placebo‐controlled study on long‐term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long‐term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab 2015; 17: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kashiwagi A, Kazuta K, Goto K, et al Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2015; 17: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu CH, Min KW, Chuang LM, et al Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of a phase 3 randomized, placebo‐controlled, double‐blind, multicenter trial. J Diabetes Investig 2016; 7: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaku K, Watada H, Iwamoto Y, et al Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol 2014; 13: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikeda S, Takano Y, Cynshi O, et al A novel and selective sodium‐glucose cotransporter‐2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2015; 17: 984–993. [DOI] [PubMed] [Google Scholar]
- 15. Roden M, Weng J, Eilbracht J, et al Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–219. [DOI] [PubMed] [Google Scholar]
- 16. Michael R, Ludwig M, Vedel CA, et al Safety, tolerability and effects on cardiometabolic risk factors of empagliflozin monotherapy in drug‐naïve patients with type 2 diabetes: a double‐blind extension of a Phase III randomized controlled trial. Cardiovasc Diabetol 2015; 14: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seino Y, Sasaki T, Fukatsu A, et al Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, phase 3 study. Curr Med Res Opin 2014; 30: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 18. Seino Y, Sasaki T, Fukatsu A, et al Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, placebo‐controlled, phase II study. Curr Med Res Opin 2014; 30: 1219–1230. [DOI] [PubMed] [Google Scholar]
- 19. Seino Y, Sasaki T, Fukatsu A, et al Dose‐finding study of luseogliflozin in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, double‐blind, placebo‐controlled, phase II study. Curr Med Res Opin 2014; 30: 1231–1244. [DOI] [PubMed] [Google Scholar]
- 20. Seino Y, Inagaki N, Haneda M, et al Efficacy and safety of luseogliflozin added to various oral antidiabetic drugs in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2015; 6: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haneda M, Seino Y, Inagaki N, et al Influence of renal function on the 52‐week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther 2016; 38: 66–88. [DOI] [PubMed] [Google Scholar]
- 22. Bailey CJ, Gross JL, Pieters A, et al Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 23. Bailey CJ, Iqbal N, T'Joen C, et al Dapagliflozin monotherapy in drug‐naive patients with diabetes: a randomized‐controlled trial of low‐dose range. Diabetes Obes Metab 2012; 14: 951–959. [DOI] [PubMed] [Google Scholar]
- 24. Bolinder J, Ljunggren O, Kullberg J, et al Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 25. Ljunggren O, Bolinder J, Johansson L, et al Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab 2012; 14: 990–999. [DOI] [PubMed] [Google Scholar]
- 26. Schummdraeger PM, Burgess L, Korányi L, et al Twice‐daily dapagliflozin co‐administered with metformin in type 2 diabetes: a 16‐week randomized, placebo‐controlled clinical trial. Diabetes Obes Metab 2015; 17: 42–51. [DOI] [PubMed] [Google Scholar]
- 27. Ferrannini E, Ramos SJ, Salsali A, et al Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care 2010; 33: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bailey CJ, Morales Villegas EC, Woo V, et al Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double‐blind placebo‐controlled 102‐week trial. Diabet Med 2015; 32: 531–541. [DOI] [PubMed] [Google Scholar]
- 29. Henry RR, Murray AV, Marmolejo MH, et al Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract 2012; 66: 446–456. [DOI] [PubMed] [Google Scholar]
- 30. Leiter LA, Cefalu WT, Bruin TW, et al Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. J Am Geriatr Soc 2014; 62: 1252–1262. [DOI] [PubMed] [Google Scholar]
- 31. Cefalu WT, Leiter LA, de Bruin TW, et al Dapagliflozin's effects on glycemia and cardiovascular risk factors in high‐risk patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. Diabetes Care 2015; 38: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. List JF, Woo V, Morales E, et al Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009; 32: 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matthaei S, Bowering K, Rohwedder K, et al Dapagliflozin improves glycemic control and reduces body weight as add‐on therapy to metformin plus sulfonylurea: a 24‐week randomized, double‐blind clinical trial. Diabetes Care 2015; 38: 365–372. [DOI] [PubMed] [Google Scholar]
- 34. Mathieu C, Ranetti AE, Li DS. Randomized, double‐blind, phase 3 trial of triple therapy with dapaglif lozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care 2015; 38: 2009–2017. [DOI] [PubMed] [Google Scholar]
- 35. Rosenstock J, Vico M, Wei L, et al Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012; 35: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenstock J, Hansen L, Zee P, et al Dual add‐on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double‐blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015; 38: 376–383. [DOI] [PubMed] [Google Scholar]
- 37. Strojek K, Yoon KH, Hruba V, et al Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2011; 13: 928–938. [DOI] [PubMed] [Google Scholar]
- 38. Wilding JP, Norwood P, T'Joen C, et al A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care 2009; 32: 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilding JP, Woo V, Soler NG, et al Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 2012; 156: 405–415. [DOI] [PubMed] [Google Scholar]
- 40. Weber MA, Mansfield TA, Cain VA, et al Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol 2016; 4: 211–220. [DOI] [PubMed] [Google Scholar]
- 41. Bode B, Stenlöf K, Harris S, et al Long‐term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab 2015; 17: 294–303. [DOI] [PubMed] [Google Scholar]
- 42. Forst T, Guthrie R, Goldenberg R, et al Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab 2014; 16: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lavalle‐Gonzalez FJ, Januszewicz A, Davidson J, et al Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013; 56: 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neal B, Perkovic V, De ZD, et al Efficacy and safety of canaglif lozin, an inhibitor of sodium‐glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 2015; 38: 403–411. [DOI] [PubMed] [Google Scholar]
- 45. Rosenstock J, Aggarwal N, Polidori D, et al Dose‐ranging effects of canagliflozin, a sodium‐glucose cotransporter 2 inhibitor, as add‐on to metformin in subjects with type 2 diabetes. Diabetes Care 2012; 35: 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stenlof K, Cefalu WT, Kim KA, et al Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yale JF, Bakris G, Cariou B, et al Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barnett AH, Mithal A, Manassie J, et al Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 49. Ferrannini E, Seman L, Seewaldt‐Becker E, et al A Phase IIb, randomized, placebo‐controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab 2013; 15: 721–728. [DOI] [PubMed] [Google Scholar]
- 50. Hadjadj S, Rosenstock J, Meinicke T, et al Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care 2016; 39: 1718–1728. [DOI] [PubMed] [Google Scholar]
- 51. Haring HU, Merker L, Seewaldt‐Becker E, et al Empagliflozin as add‐on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care 2013; 36: 3396–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Häring HU, Merker L, Seewaldtbecker E, et al Empagliflozin as add‐on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract 2015; 110: 82–90. [DOI] [PubMed] [Google Scholar]
- 53. Haring HU, Merker L, Seewaldt‐Becker E, et al Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care 2014; 37: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 54. Merker L, Haring HU, Christiansen AV, et al Empagliflozin as add‐on to metformin in people with type 2 diabetes. Diabet Med 2015; 32: 1555–1567. [DOI] [PubMed] [Google Scholar]
- 55. Kovacs CS, Seshiah V, Swallow R, et al Empagliflozin improves glycaemic and weight control as add‐on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab 2014; 16: 147–158. [DOI] [PubMed] [Google Scholar]
- 56. Rosenstock J, Jelaska A, Frappin G, et al Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014; 37: 1815–1823. [DOI] [PubMed] [Google Scholar]
- 57. Ross S, Thamer C, Cescutti J, et al Efficacy and safety of empagliflozin twice daily versus once daily in patients with type 2 diabetes inadequately controlled on metformin: a 16‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab 2015; 17: 699–702. [DOI] [PubMed] [Google Scholar]
- 58. Fonseca VA, Ferrannini E, Wilding JP, et al Active‐ and placebo‐controlled dose‐finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications 2013; 27: 268–273. [DOI] [PubMed] [Google Scholar]
- 59. Wilding JPH, Ferrannini E, Fonseca VA, et al Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose‐finding study. Diabetes Obes Metab 2013; 15: 403–409. [DOI] [PubMed] [Google Scholar]
- 60. Amin NB, Wang X, Jain SM, et al Dose‐ranging efficacy and safety study of ertugliflozin, a sodium‐glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes on a background of metformin. Diabetes Obes Metab 2015; 17: 591–598. [DOI] [PubMed] [Google Scholar]
- 61. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015; 75: 33–59. [DOI] [PubMed] [Google Scholar]
- 62. Cefalu WT, Leiter LA, Yoon KH, et al Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 noninferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 63. Traina S, Guthrie R, Slee A. The impact of weight loss on weight‐related quality of life and health satisfaction: results from a trial comparing canagliflozin with sitagliptin in triple therapy among people with type 2 diabetes. Postgrad Med 2014; 126: 7–15. [DOI] [PubMed] [Google Scholar]
- 64. Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in EMPA‐REG OUTCOME: comparison with classic diuretics. Diabetes Metab 2016; 42: 224–233. [DOI] [PubMed] [Google Scholar]
- 65. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015; 9: 48–53. [DOI] [PubMed] [Google Scholar]
- 66. Baker WL, Smyth LR, Riche DM, et al Effects of sodium‐glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens 2014; 8: 262–275. [DOI] [PubMed] [Google Scholar]
- 67. Cefalu WT, Stenlof K, Leiter LA, et al Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia 2015; 58: 1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ptaszynska A, Hardy E, Johnsson E, et al Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med 2013; 125: 181–189. [DOI] [PubMed] [Google Scholar]
- 69. Briand F, Mayoux E, Brousseau E, et al Empagliflozin, via switching metabolism towards lipid utilization, moderately increases LDL‐cholesterol levels through reduced LDL catabolism. Diabetes 2016; 65: 2032–2038. [DOI] [PubMed] [Google Scholar]
- 70. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 71. Scheen AJ. SGLT2 inhibitors: benefit/risk balance. Curr Diab Rep 2016; 16: 92. [DOI] [PubMed] [Google Scholar]
- 72. US Food and Drug Administration . Drug safety communications: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf 2015. Accessed June 7, 2016.
- 73. European Medicines Agency. EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes; Healthcare professionals should be aware of possible atypical cases . ENP Newswire. February 29, 2016. http://trove.nla.gov.au/version/224544755.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Evaluation of the risk of bias in sodium–glucose cotransporter 2 inhibitors treatment in Asian type 2 diabetes patients.
Figure S2 ¦ Evaluation of the risk of bias in sodium–glucose cotransporter 2 inhibitors treatment in non‐Asian type 2 diabetes patients.
Table S1 ¦ Characteristics of randomized controlled trials in sodium–glucose cotransporter 2 inhibitors treatment in Asian type 2 diabetes patients.
Table S2 ¦ Characteristics of randomized controlled trials in sodium–glucose cotransporter 2 inhibitors treatment in non‐Asian type 2 diabetes patients.


