Abstract
Aim/Introduction
Patients with type 1 diabetes are classified into three subtypes in Japan: acute onset, fulminant and slowly progressive. Acute‐onset type 1 diabetes would be equivalent to type 1A diabetes, the typical type 1 diabetes in Western countries. The insulin secretion capacity in Japanese patients with long‐standing type 1A diabetes is unclear. The aim of the present study was to clarify the course of endogenous insulin secretion during long‐term follow up and the factors associated with residual insulin secretion in patients with acute‐onset type 1 diabetes (autoimmune).
Materials and Methods
We retrospectively investigated endogenous insulin secretion capacity in 71 patients who fulfilled the diagnostic criteria for acute‐onset type 1 diabetes (autoimmune) in Japan. To assess the residual insulin secretion capacity, we evaluated randomly measured C‐peptide levels and the results of glucagon stimulation test in 71 patients.
Results
In the first year of disease, the child‐ and adolescent‐onset patients had significantly more in residual insulin secretion than the adult‐onset patients (34 patients in total). C‐peptide levels declined more rapidly in patients whose age of onset was ≤18 years than in patients whose age of onset was ≥19 years. Endogenous insulin secretion capacity stimulated by glucagon was completely lost in almost all patients at >15 years after onset (61 patients in total).
Conclusions
Most patients with acute‐onset type 1 diabetes (autoimmune) completely lose their endogenous insulin secretion capacity during the disease duration in Japan. Age of onset might affect the course of insulin secretion.
Keywords: Acute‐onset type 1 diabetes, Endogenous insulin secretion capacity, Long‐standing
Introduction
Type 1 diabetes results from pancreatic β‐cell destruction and insulin deficiency. Pathophysiologically, the American Diabetes Association and the World Health Organization divide type 1 diabetes into autoimmune diabetes (type 1A) and idiopathic diabetes (type 1B). Type 1A is characterized by the presence of islet‐related antibodies at the initial detection of hyperglycemia, including anti‐glutamic acid decarboxylase (GAD) antibodies, insulin autoantibodies and anti‐insulinoma‐associated antigen 2 antibodies, whereas type 1B is characterized by insulin dependence without evidence of autoimmunity1. The clinical features of Caucasian patients with type 1 diabetes, that of rapid onset of symptoms in a young lean patient with ketoacidosis, are virtually homogenous, prompting the proposal that almost all Caucasian type 1 diabetes patients can be classified as type 1A2. In such patients, insulin secretion capacity diminishes to 52% of the normal level within 10 weeks of diagnosis3.
Clinically, Japanese patients with type 1 diabetes are classified into three subtypes: acute‐onset, fulminant and slowly progressive4, 5, 6, 7. Acute‐onset type 1 diabetes would be equivalent to type 1A diabetes, the typical type 1 diabetes in Western countries; whereas fulminant type 1 diabetes is characterized by a rapid onset and an absence of islet‐related autoantibodies; and slowly progressive type 1 diabetes is characterized by the preservation of residual β‐cell function and persistent detection of islet‐related autoantibodies, thus resembling latent autoimmune diabetes in adults8. In addition, specific criteria have been established for both fulminant and slowly progressive type 1 diabetes, and it is estimated that the former subtype accounts for approximately 20% of cases with ketosis‐onset type 1 diabetes, whereas the latter accounts for 10% of patients who were diagnosed with type 2 diabetes8, 9. Thus, endogenous insulin secretion capacity must be evaluated carefully in cases of type 1A diabetes, except for those with fulminant type 1 diabetes, in which endogenous insulin secretion is completely abolished just after the onset, and those with slowly progressive diabetes, in which residual insulin secretion capacity is similar to that of patients with type 2 diabetes.
The diagnostic criteria of acute‐onset type 1 diabetes mellitus were also proposed in Japan in 2012, as follows: (i) development of diabetic ketosis or ketoacidosis <3 months after the appearance of hyperglycemia‐related clinical features (thirst, polydipsia, polyuria and weight loss); (ii) the need for continuous insulin therapy after the diagnosis of diabetes mellitus; and (iii) positivity for anti‐islet autoantibodies (matching the American Diabetes Association/World Health Organization criteria for type 1A diabetes)6. Thus, the endogenous insulin secretion capacity of Japanese patients with long‐standing acute‐onset type 1 diabetes can be assessed and compared with that of Caucasian patients with type 1A diabetes. The significance of such a comparison both enables clarification of the pathology in patients with type 1 diabetes and provides a base for clinical decisions about the strategy of interventional therapy.
For determination of endogenous insulin secretion capacity, the glucagon stimulation test or mixed‐meal tolerance test are commonly carried out. These have been shown to be highly reproducible and correlated well with residual β‐cell function10. Glucagon‐stimulated C‐peptide is not affected by endogenously secreted incretin, and reflects a direct effect on the β‐cells11, 12.
To clarify the course of endogenous insulin secretion in patients who satisfied the diagnosis criteria of acute‐onset type 1 diabetes, we retrospectively investigated β‐cell function after long‐term follow up in such patients.
Methods
We recruited 71 patients who visited or were hospitalized at the Department of Metabolic Medicine Osaka University School of Medicine, Suita, Japan, between January 2000 and December 2014, and fulfilled the diagnostic criteria for acute‐onset type 1 diabetes. They had no family history of diabetes through three generations. Exclusion criteria were the following: absence of serum C‐peptide data, presence of chronic renal failure, presence of gestation and presence of other diseases (malignant lymphoma, idiopathic thrombocytopenic purpura, uterine cancer). Patients’ characteristics are shown in Table1. A total of 16 patients (22.5%) were aged ≤18 years at onset. The median age of onset was 28 years (interquartile range [IQR] 19–35 years), and the median blood glucose level at onset was 378 mg/dL (IQR 265–500 mg/dL). The median hemoglobin A1c at the onset was 10.6% (IQR 8.9–12.3%; Table1).
Table 1.
Participant characteristics
| n (women, %) | 71 (32, 54.9%) |
| Age at onset ≤18 years, n (%) | 16 (22.5%) |
| Age at onset (years) | 28 [19–35] |
| BMI (kg/m2) | 20.1 [19.0–23.9] |
| Blood glucose at onset (mg/dL) | 378 [265–500] |
| HbA1c at onset (%) | 10.6 [8.9–12.3] |
| ICA | 23/30 (76.7%) |
| GADAb | 65/70 (92.9%) |
| IA‐2Ab | 24/37 (64.9%) |
| Proliferative diabetic retinopathy | 2 (2.8%) |
| Nephropathy | 0 (0%) |
| Neuropathy | 2 (2.8%) |
| Thyroid disease | 47/67 (70.1%) |
Data are n (%) or median [IQR]. BMI, body mass index; GADAb, glutamic acid decarboxylase antibodies; HbA1c, hemoglobin A1c; IA‐2Ab, insulin autoantibodies and anti‐insulinoma‐associated antigen 2 antibodies; ICA, islet cell antibodies.
Patients were tested for the following autoantibodies: islet cell antibodies by indirect immunofluorescence method at onset; GAD antibodies and anti‐insulinoma‐associated antigen 2 antibodies by radioimmunoassay at onset or during the course of the disease. Islet cell antibody, GAD antibodies or anti‐insulinoma‐associated antigen 2 antibodies was detected in 23 of 30 patients (76.7%), in 65 of 70 patients (92.9%) or in 24 of 37 patients (64.9%), respectively. All patients tested positive for at least one of the three autoantibodies.
Patients had been treated with intensive insulin therapy after the onset of overt diabetes (64 with multiple daily injections and seven with continuous subcutaneous insulin infusion), and were usually seen monthly or bimonthly at our outpatient clinic. All patients were instructed on self‐monitoring of blood glucose. The insulin injection dose was adjusted to keep the pre‐meal glucose level <130 mg/dL by self‐monitoring of blood glucose levels and to maintain the hemoglobin A1c level <7.9% at every visit. Bodyweight, insulin injection dosage, hemoglobin A1c level, serum C‐peptide level (fasting or postprandial) and the status of each diabetic complication were monitored continuously over the study period in all patients.
Serum C‐peptide level was determined by radioimmunoassay methods before 2000, and by chemical luminescence immunoassay after 2001. To transform radioimmunoassay data, we used the equation y = 1.24 × −0.7. The minimal detection level of radioimmunoassay and chemical luminescence immunoassay was 0.1 nmol/L and 0.03 nmol/L, respectively. An insulin secretion defect was represented by a serum C‐peptide level of <0.03 nmol/L.
Optic fundi were examined by an ophthalmologist through dilated pupils and classified as: (i) no evidence of diabetic retinopathy; (ii) simple diabetic retinopathy; or (iii) pre‐proliferative retinopathy or proliferative retinopathy. Nephropathy was defined by urinary albumin excretion ≥30 mg/g creatinine from consecutive random sterile samples. The presence of neuropathy was defined by a decreased or absent Achilles tendon reflex.
Statistical analysis
First, we analyzed random C‐peptide levels within 1 year after the onset in 34 of 71 patients (19 women) whose data were available. Detailed demographic data are shown in Table S1. Multiple linear regression analyses, adjusted for age of onset and sex, were carried out to determine factors associated with C‐peptide levels.
Second, we evaluated changes in residual insulin secretion after onset using each patient's random C‐peptide data over time in 71 patients. A linear mixed model adjusted for age of onset and sex was used to explore clinical parameters associated with C‐peptide levels over time.
Third, the glucagon stimulation test was carried out in 61 of 71 patients to evaluate stimulated insulin secretion capacity. That was assessed by measuring serum C‐peptide levels at 0 and 6 min after intravenous administration of 1 mg of glucagon. Detailed demographic data are shown in Table S2. A Spearman's correlation test was used to clarify for a correlation between C‐peptide levels and duration of diabetes. An ordered logistic regression, adjusted for age of onset, sex, duration, body mass index (BMI) and blood pressure level, was used to estimate factors associated with C‐peptide levels.
A P‐value <0.05 was accepted as statistically significant. All analyses were carried out using the SPSS software package (version 22.0; IBM, Armonk, NY, USA).
This study protocol was approved by the Human Ethics Review Committee of Osaka University, and carried out according to the Declaration of Helsinki.
Results
Residual insulin secretion during the first year after onset
Multiple linear regression analyses adjusted sex showed that there was a significant difference between patients whose age of onset was ≤18 years and those aged ≥19 years in random C‐peptide level (P = 0.04). The random C‐peptide level in patients whose age of onset was ≤18 years was 0.26 nmol/L (IQR 0.17–0.34 nmol/L) and higher than that of the others 0.13 nmol/L (IQR 0.05–0.20 nmol/L). Mean blood glucose levels measured simultaneously were not different between the two groups. There was no correlation between C‐peptide level and blood glucose level or hemoglobin A1c level at onset. There was also no correlation between C‐peptide level and BMI, systolic and diastolic blood pressure levels, serum lipid levels, total daily insulin dosage, presence or absence of thyroid disease, or positivity of islet antibodies (Table2).
Table 2.
Analysis of factors affecting C‐peptide levels within 1 year after onset
| Factor | β coefficient (95% CI) | P‐value |
|---|---|---|
| Blood glucose level (at onset) | −0.166 (−0.001, 0.001) | 0.38 |
| HbA1c (at onset) | 0.014 (−0.069, 0.074) | 0.94 |
| BMI | −0.029 (−0.061, 0.052) | 0.87 |
| Systolic/diastolic blood pressure |
0.107 (−0.007, 0.013) −0.006 (−0.013, 0.012) |
0.56 0.98 |
| Serum lipid | ||
| Total cholesterol | −0.079 (−0.005, 0.003) | 0.67 |
| LDL cholesterol | −0.142 (−0.007, 0.003) | 0.47 |
| HDL cholesterol | −0.108 (−0.014, 0.008) | 0.58 |
| Triglycerides | 0.103 (−0.002, 0.003) | 0.57 |
| Total daily insulin dosage | −0.338 (−1.094, 0.018) | 0.06 |
| Age at onset: ≤18 years/≥19 years | −0.347 (−0.677, −0.002) | 0.04* |
| Thyroid disease (±) | 0.001 (−0.356, 0.354) | 0.99 |
| ICA (±) | −0.230 (−0.741, 0.265) | 0.33 |
| GADAb (±) | −0.041 (−0.477, 0.382) | 0.82 |
| IA‐2Ab (±) | 0.040 (−0.528, 0.602) | 0.89 |
Multiple linear regression analyses was performed to determine the association of serum random C‐peptide level within one year after onset with blood glucose level, hemoglobin A1c (HbA1c), body mass index (BMI), blood pressure, serum lipid level, total daily insulin dosage, age at onset, presence of thyroid disease or positivity of islet antibodies. Analysis showed that the patients whose age of onset was ≤18 years had significantly more in residual insulin secretion than the patients whose age of onset was ≥19 years in the first year of diabetes. CI, confidence interval; GADAb, glutamic acid decarboxylase antibodies; HDL, high‐density lipoprotein; IA‐2Ab, insulin autoantibodies and anti‐insulinoma‐associated antigen 2 antibodies; ICA, islet cell antibodies; LDL, low‐density lipoprotein. *P < 0.05.
Long‐Term insulin secretion by random C‐peptide measurement
C‐peptide levels declined more rapidly in patients whose age of onset was ≤18 years than in patients whose age of onset was ≥19 years over time (difference in slope −0.27, P = 0.02; Figure1). The rate of decline of the C‐peptide levels was not related to positivity of each islet‐related autoantibody, either islet cell antibodies, GAD antibodies or anti‐insulinoma‐associated antigen 2 antibodies. It was not also related to the presence or absence of autoimmune thyroid disease of Graves’ disease and Hashimoto's thyroiditis.
Figure 1.
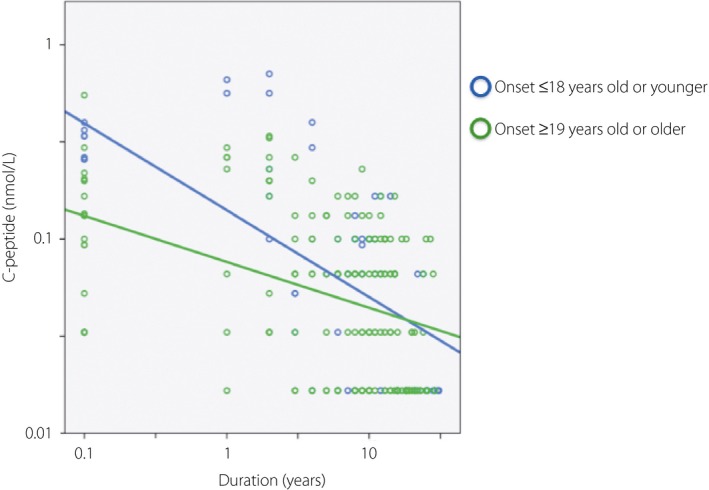
Effect of age at onset on C‐peptide levels after diagnosis. Patients whose age of onset was ≤18 years had a higher rate of decline than patients whose age of onset was ≥19 years.
Long‐Term insulin secretion in glucagon stimulation test
Both the stimulated C‐peptide levels and increment of C‐peptide levels after glucagon stimulation decreased according to the duration of disease. There was a significant negative correlation between the duration of diabetes and each of stimulated C‐peptide levels and increment of C‐peptide levels after glucagon stimulation (P < 0.001 each). Endogenous insulin secretion capacity was completely lost in almost all patients at >15 years after onset (Figure2). Ordinal logistic regression analysis showed that daily insulin dosage was an independent factor associated with stimulated C‐peptide levels (P = 0.02). Any other factors than daily insulin dosage did not associate with C‐peptide levels themselves, and any factors did not associate with the increment of C‐peptide level after glucagon stimulation (Table3).
Figure 2.
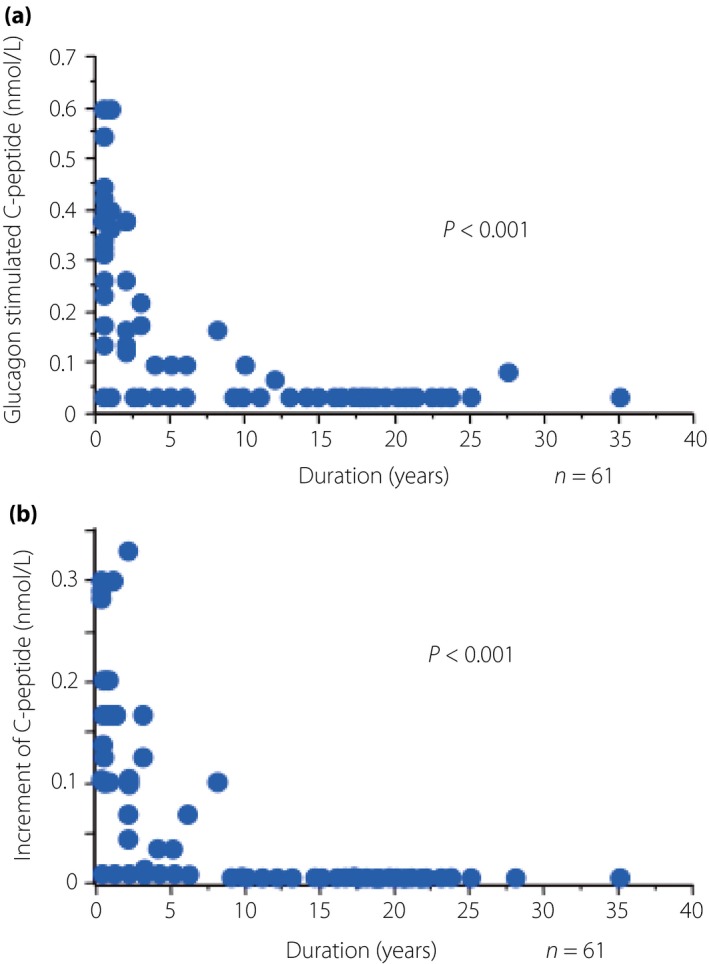
(a) Stimulated serum C‐peptide levels and (b) increment of C‐peptide levels and the duration of type 1 diabetes. Both stimulated serum C‐peptide levels and increment of C‐peptide levels showed a significant negative correlation with the disease duration.
Table 3.
Analysis of factors affecting C‐peptide levels in glucagon stimulation test
| Factor | Stimulated C‐peptide level | Increment of CPR level | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P‐value | Odds ratio (95% CI) | P‐value | |
| HbA1c | 0.91 (0.66–1.26) | 0.58 | 0.72 (0.49–1.107) | 0.11 |
| Total daily insulin dosage | 0.03 (0.00–0.61) | 0.02* | 0.03 (0.00–1.26) | 0.07 |
| Treatment: CSII | 0.07 (0.00–1.63) | 0.09 | 0.10 (0.03–3.64) | 0.34 |
| Total cholesterol | 0.99 (0.97–1.01) | 0.15 | 0.99 (0.97–1.01) | 0.16 |
| LDL cholesterol | 0.99 (0.97–1.01) | 0.41 | 1.00 (0.97–1.02) | 0.54 |
| HDL cholesterol | 0.96 (0.93–1.00) | 0.07 | 0.95 (0.91–1.01) | 0.08 |
| Triglycerides | 1.00 (0.99–1.00) | 0.47 | 1.00 (0.99–1.01) | 0.75 |
| GADAb | 1.61 (0.17–15.1) | 0.67 | 2.24 (0.19–25.7) | 0.51 |
| IA‐2Ab | 0.65 (0.06–7.08) | 0.72 | 3.16 (0.05–19.1) | 0.58 |
| ICA | 4.15 (0.18–93.0) | 0.37 | 0.30 (0.00–15.0) | 0.54 |
| Thyroid disease | 1.11 (0.19–6.71) | 0.90 | 1.47 (0.15–14.1) | 0.73 |
Ordinal logistic regression adjusted for age of onset, sex, duration, body mass index and blood pressure level was carried out to estimate factors associated with C‐peptide levels. Any other factors than daily insulin dosage did not associate with C‐peptide levels themselves, and any factors did not associate with the increment of C‐peptide level after glucagon stimulation. CI, confidence interval; CPR, C‐peptide immunoreactivity; CSII, continuous subcutaneous insulin infusion; GADAb, glutamic acid decarboxylase antibodies; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; IA‐2Ab, insulin autoantibodies and anti‐insulinoma‐associated antigen 2 antibodies; ICA, islet cell antibodies; LDL, low‐density lipoprotein. *P < 0.05.
Discussion
The present study has showed the time‐course and the other characteristics of insulin secreting capacity in acute‐onset type 1 diabetes (autoimmune) by three analyses. First, in the first year of disease onset, the child‐ and adolescent‐onset patients had significantly more in residual insulin secretion than the adult‐onset patients. Second, the C‐peptide levels of the patients whose age of onset was ≤18 years declined more rapidly than those of the patients whose age of onset was ≥19 years in 29 years after the onset. Third, there was a significant negative correlation between the duration of diabetes and insulin secretion in the glucagon stimulation test. Almost all patients lose their endogenous insulin secretion capacity completely during the course of acute‐onset type 1 diabetes in 15 years.
First, multiple linear regression analyses showed more residual insulin secretion during the first year of diagnosis in patients whose age of onset was ≤18 years than in patients whose age of onset was ≥19 years. Karjalainen et al.13 reported that the adults whose age of onset was 20.0–55.8 years had higher serum C‐peptide concentration at diagnosis of type 1 diabetes in comparison with children whose age of onset was 1.3–18.2 years (0.29 vs 0.17 nmol/L). The reason for the different result from the previous study remains unclear. In Japan, higher residual insulin secretion capacity in children with type 1 diabetes might be caused by the earlier diagnosis resulting from the urine check system carried out during school health examinations14.
Second, a linear mixed model adjusted for age of onset and sex showed that the C‐peptide levels of the patients whose age of onset was ≤18 years declined more rapidly than those of the patients whose age of onset was ≥19 years. This finding accords with the previous reports in Caucasians. In the Diabetes Control and Complications Trial, C‐peptide levels more sharply declined in adolescents than in adults after 5 years from diagnosis, though both the population of adults and adolescents retained insulin secretory capacity during the first 5 years after onset15. Trial Net showed age of onset in type 1 diabetes was highly correlated with loss of C‐peptide over time. The patients whose age of onset was >21 years manifested a significantly slower rate of C‐peptide decline than others. Barker et al.16 reported the younger the age of type 1 diabetes onset, the more rapidly fasting C‐peptide declines during the 1 year after diagnosis.
Third, the longer the duration of disease, the lower the stimulated C‐peptide levels, and endogenous insulin secretion was completely lost in almost all patients >15 years after onset. In contrast, other studies of type 1A diabetes suggested possible residual endogenous insulin secretion capacity, even after a long disease duration in Caucasians. Keenan et al.17 reported that random serum C‐peptide levels could still be detected with a detection limit of 0.03 nmol/L in 67.4% of 411 patients at 50 years after onset or longer, whereas Oram et al.18 showed stimulated C‐peptide levels in response to mixed‐meal of >3.3 pmol/L in 25 of 37, and >30 pmol/L in four of 37 patients with diabetes after 30‐year disease duration.
The difference between studies in Caucasians and the present study might be attributed to several genetic factors related to specific immune reactions or β‐cell regeneration between Japanese and Caucasians. For example, with respect to INS VNTR influencing the expression level of insulin in the thymus, the class III allele was associated with significantly higher levels of stimulated C‐peptide at disease onset19. In Caucasian patients, the INS VNTR genotype class I/III or III/III allele frequency is 34%, whereas almost all Japanese patients show the class I/I genotype20. With regard to the genetic factors related to β‐cell regeneration or differentiation, the neuroD1/BETA2 and MAFA genes have been associated with susceptibility to type 1A diabetes, but the polymorphism of these genes is different between Japanese and Caucasian patients21, 22. Additionally, BMI was higher in the patients in the USA than in the present study, and it might affect the course of C‐peptide secretion, even if the detailed courses of BMI were not shown. In the present study, the median body mass index was 20.1 kg/m2 (IQR 19.0–23.9 kg/m2). In the Diabetes Control and Complications Trial, carried out in the USA, the baseline BMIs were 23.3–24.3 kg/m2 23. In the Josulin 50‐Year Medalist Study by Keenan et al.17 in the USA and another study by Oram et al.18 in the UK, the BMI was 26.0 ± 5.1 kg/m2 and 25 kg/m2 (IQR 23–28 kg/m2), respectively.
The present study showed that endogenous insulin secretion capacity in acute‐onset type 1 diabetes reduces rapidly during a few years after onset, more rapidly in patients whose age of onset was ≤18 years, suggesting that immune intervention should be recommended shortly after diagnosis of type 1A diabetes, especially in younger‐onset patients. Preservation of endogenous insulin secretion capacity and maintenance of steady blood glucose levels also reduce the risk of development of diabetic complications24. Inhibition of β‐cell‐specific autoimmunity and promotion of β‐cell regeneration and neogenesis are necessary for the prevention of type 1 diabetes. For example, anti‐CD3 agents, teplizumab and abatacept, inhibit T‐cell immune responses against β‐cells and thus reduce the loss of C‐peptide secretion25, 26. A GLP‐1 receptor agonist increased formation of new β‐cells, suppressed β‐cell apoptosis and delayed the onset of diabetes27. Kielgast et al.28 studied the effect of liraglutide therapy on insulin dose reduction between type 1 diabetes patients with residual β‐cell function and those without. Their studies together show that residual insulin secretion capacity is important for successful immune or regenerative intervention. The present study suggests that immune intervention for type 1A diabetes is recommended to be introduced shortly after the onset of disease.
In conclusion, most Japanese patients with acute‐onset type 1 diabetes (autoimmune) completely lose their endogenous insulin secretion capacity during the course of the disease. The age of onset might affect the course of insulin secretion, as shown in Caucasian patients.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Characteristics of participants who had random C‐peptide levels examined within 1 year after the onset.
Table S2 ¦ Characteristics of participants who were evaluated for stimulated insulin secretion capacity.
Acknowledgments
The authors appreciate Professor Ayumi Shintani, Department of Clinical Epidemiology and Biostatistics, Osaka University, for her helpful advice on statistical analysis. There was no external funding for this study.
J Diabetes Investig 2018;9:806–812
References
- 1. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37: S81–S90. [DOI] [PubMed] [Google Scholar]
- 2. Rewers M. Challenges in diagnosing type 1 diabetes in different populations. Diabetes Metab J 2012; 36: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steele C, Hagopian WA, Gitelman S, et al Insulin secretion in type 1 diabetes. Diabetes 2004; 53: 426–433. [DOI] [PubMed] [Google Scholar]
- 4. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imagawa A, Hanafusa T, Awata T, et al Report of the committee of the Japan diabetes society on the research of fulminant and acute‐onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig 2012; 3: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kawasaki E, Maruyama T, Imagawa A, et al Diagnostic criteria for acute‐onset type 1 diabetes mellitus (2012): report of the committee of Japan diabetes society on the research of fulminant and acute‐onset type 1 diabetes mellitus. J Diabetes Investig 2014; 5: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanaka S, Ohmori M, Awata T, et al Diagnostic criteria for slowly progressive insulin‐dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the committee on slowly progressive insulin‐dependent (type 1) diabetes mellitus of the Japan diabetes society. Diabetol Int 2015; 6: 1–7. [Google Scholar]
- 8. Kobayashi T, Tamemoto K, Nakanishi K, et al Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care 1993; 16: 780–788. [DOI] [PubMed] [Google Scholar]
- 9. Imagawa A, Hanafusa T, Uchigata Y, et al Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 2003; 26: 2345–2352. [DOI] [PubMed] [Google Scholar]
- 10. Greenbaum CJ, Mandrup‐Poulsen T, McGee PF, et al Mixed‐meal tolerance test versus glucagon stimulation test for the assessment of beta‐cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008; 31: 1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet 1965; 2: 415–416. [DOI] [PubMed] [Google Scholar]
- 12. Pozzilli P, Raz I, Peled D, et al Evaluation of long‐term treatment effect in a type 1 diabetes intervention trial: differences after stimulation with glucagon or a mixed meal. Diabetes Care 2014; 37: 1384–1391. [DOI] [PubMed] [Google Scholar]
- 13. Karjalainen J, Salmela P, Ilonen J, et al A comparison of childhood and adult type I diabetes mellitus. N Engl J Med 1989; 320: 881–886. [DOI] [PubMed] [Google Scholar]
- 14. Urakami T, Morimoto S, Nitadori Y, et al Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome‐incidence and clinical characteristics of childhood type 2 diabetes in Japan. Pediatr Res 2007; 61: 141–145. [DOI] [PubMed] [Google Scholar]
- 15. Effects of age, duration and treatment of insulin‐dependent diabetes mellitus on residual beta‐cell function: observations during eligibility testing for the diabetes control and complications trial (DCCT). The DCCT research group. J Clin Endocrinol Metab 1987; 65: 30–36. [DOI] [PubMed] [Google Scholar]
- 16. Barker A, Lauria A, Schloot N, et al Age‐dependent decline of β‐cell function in type 1 diabetes after diagnosis: a multi‐centre longitudinal study. Diabetes Obes Metab 2014; 16: 262–267. [DOI] [PubMed] [Google Scholar]
- 17. Keenan HA, Sun JK, Levine J, et al Residual insulin production and pancreatic ß‐cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010; 59: 2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oram RA, Jones AG, Besser RE, et al The majority of patients with long‐duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014; 57: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nielsen LB, Mortensen HB, Chiarelli F, et al Impact of IDDM2 on disease pathogenesis and progression in children with newly diagnosed type 1 diabetes: reduced insulin antibody titres and preserved beta cell function. Diabetologia 2006; 49: 71–74. [DOI] [PubMed] [Google Scholar]
- 20. Awata T, Kawasaki E, Ikegami H, et al Insulin gene/IDDM2 locus in Japanese type 1 diabetes: contribution of class I alleles and influence of class I subdivision in susceptibility to type 1 diabetes. J Clin Endocrinol Metab 2007; 92: 1791–1795. [DOI] [PubMed] [Google Scholar]
- 21. Iwata I, Nagafuchi S, Nakashima H, et al Association of polymorphism in the NeuroD/BETA2 gene with type 1 diabetes in the Japanese. Diabetes 1999; 48: 416–419. [DOI] [PubMed] [Google Scholar]
- 22. Noso S, Kawabata Y, Babaya N, et al Association study of MAFA and MAFB genes related to organ specific autoimmunity, with susceptibility to type‐1 diabetes in Japanese and Caucasians populations. J Genet Syndr Gene Ther 2013; 4: 204–210. [Google Scholar]
- 23. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the diabetes control and complications trial. The DCCT research group. Diabetes Care 2001; 24: 1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steffes MW, Sibley S, Jackson M, et al Beta‐cell function and the development of diabetes‐related complications in the diabetes control and complications trial. Diabetes Care 2003; 26: 832–836. [DOI] [PubMed] [Google Scholar]
- 25. Herold KC, Gitelman SE, Masharani U, et al A single course of anti‐CD3 monoclonal antibody hOKT3gamma1(Ala‐Ala) results in improvement in C‐peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005; 54: 1763–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orban T, Bundy B, Becker DJ, et al Costimulation modulation with abatacept in patients with recent‐onset type 1 diabetes: follow‐up 1 year after cessation of treatment. Diabetes Care 2014; 37: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadjiyanni I, Baggio LL, Poussier P, et al Exendin‐4 modulates diabetes onset in nonobese diabetic mice. Endocrinology 2008; 149: 1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kielgast U, Krarup T, Holst JJ, et al Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta‐cell function. Diabetes Care 2011; 34: 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Characteristics of participants who had random C‐peptide levels examined within 1 year after the onset.
Table S2 ¦ Characteristics of participants who were evaluated for stimulated insulin secretion capacity.


