Abstract
Aims/Introduction
To evaluate the efficacy and safety of combining insulin therapy with dipeptidyl peptidase‐4 (DPP‐4) inhibitors compared with combining insulin therapy with a placebo or other antihyperglycemic agents.
Materials and Methods
A literature search was carried out via electronic databases. The inclusion criteria were randomized controlled trials comparing the addition of DPP‐4 inhibitors to insulin with the addition of a placebo or other active hypoglycemic agents to insulin therapy, study duration of no less than 12 weeks carried out in type 2 diabetes patients and the availability of outcome data to evaluate a change in the glycated hemoglobin.
Results
The glycated hemoglobin‐lowering efficacy was significantly greater with DPP‐4 inhibitor/insulin (DPP‐4i/INS) than with placebo/insulin (weighted mean difference −0.53%, 95% confidence interval −0.63, −0.43, P < 0.01). The postprandial plasma glucose‐lowering efficacies was also significantly greater with DPP‐4i/INS than with placebo/insulin (weighted mean difference −1.65 mmol/L, 95% CI: −2.34, −0.96, P < 0.05). The risk of hypoglycemia or severe hypoglycemia was similar for DPP4i/INS and placebo/insulin treatments. There was no significant difference in the glycemia‐lowering efficacy between DPP‐4i/INS and alpha‐glucosidase inhibitors/insulin, thiazolidinedione/insulin and glucagon‐like peptide‐1 receptor agonist/insulin. Sodium–glucose cotransporter 2 inhibitor/insulin treatment achieved better placebo‐corrected efficacy in lowering postprandial plasma glucose, with less weight gain and no higher risk of hypoglycemia.
Conclusions
Treatment with DPP‐4 inhibitors combined with insulin improved glycemic control without an increased risk of hypoglycemia or weight gain compared with insulin treatment alone.
Keywords: Dipeptidyl peptidase‐4 inhibitor, Insulin, Type 2 diabetes
Introduction
Type 2 diabetes mellitus is a progressive disease characterized by insulin deficiency and insulin resistance. Guidelines recommend insulin therapy be initiated when patients with progressive type 2 diabetes mellitus do not achieve or maintain proper glycemic control on oral antihyperglycemic agents alone and add‐on therapies1, 2.
Dipeptidyl peptidase‐4 (DPP‐4) inhibitors prevent the degradation of gastrointestinal incretins glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP), resulting in improved glycemic control by increasing levels of GLP‐1 and GIP3, 4, 5. The efficacy and safety of DPP‐4 inhibitors and insulin combination therapy have been evaluated in several previous studies, including systematic reviews and meta‐analyses based on findings from randomized controlled trials (RCTs)6, 7. However, there are only limited data comparing DPP‐4 inhibitors with other antihyperglycemic agents in combination with insulin, especially head‐to‐head comparisons, and no systemic reviews or meta‐analyses have been published.
Therefore, the aim of the current systematic review and meta‐analysis was to evaluate the efficacy and safety of DPP‐4 inhibitors compared with a placebo or other antihyperglycemic agents in combination with insulin therapy.
Methods
Data collection and searching strategy
Studies were identified by a literature search of MEDLINE® (PubMed), the Cochrane Central Register of Controlled Trials (CENTRAL) and EMBASE® until December 2016. Documents for approved medications were searched for trials at http://www.clinicalstudyresults.org and http://www.clinicaltrials.gov.
The following terms were searched: type 2 diabetes; DPP‐4 inhibitors (DPP‐4i); metformin (MET); alpha‐glucosidase inhibitors (AGI); thiazolidinediones (TZD); glucagon‐like peptide‐1 receptor agonist (GLP‐1RA); sodium–glucose cotransporter 2 inhibitors (SGLT‐2i); acarbose; miglitol; voglibose; rosiglitazone; pioglitazone; troglitazone; sitagliptin; vildagliptin; saxagliptin; alogliptin; linagliptin; liraglutide; lixisenatide; exenatide; dapagliflozin; canagliflozin; empagliflozin; and RCTs.
Eligibility criteria
The inclusion criteria were: (i) RCTs comparing the addition of DPP‐4 inhibitors with insulin, with the addition of a placebo or other active hypoglycemic agents to insulin therapy; (ii) study duration ≥12 weeks; (iii) studies carried out in type 2 diabetes patients; and (iv) the efficacy of glucose control was the primary outcome of the study. Non‐RCTs carried out in type 2 diabetes patients, studies in type 1 diabetes patients and studies with duration <12 weeks were excluded.
Data extraction
Two review authors (GXY and YWJ) independently extracted the following data from each study: publication data (title, first author, year), study design, baseline characteristics of the study population (sample size, age, sex, duration of type 2 diabetes mellitus, body mass index [BMI] and glycated hemoglobin [HbA1c]), description of the study drugs, treatment duration and primary outcome measures (changes from baseline to study end‐point for HbA1c). Discrepancies were resolved by a third investigator (CL).
Assessment of study quality and risk of bias
A visual inspection of the funnel plot and the Egger's test of the funnel plot were used to assess the publication bias. The quality of all included studies was assessed by the Cochrane risk of bias tool including selection bias, performance bias, detection bias, attrition bias, reporting bias and others8.
Statistical analysis
The weighted mean difference (WMD) and 95% confidence interval (CI) were calculated for continuous variables, including the change in HbA1c, fasting plasma glucose (FPG), postprandial plasma glucose (PPG), bodyweight and daily dosage of insulin from baseline. The risk ratio (RR) and 95% confidence interval (CI) were calculated to analyze the risk of hypoglycemia and severe hypoglycemia. The between‐study heterogeneity was distributed as the χ2 statistic, and statistical significance was reached when P < 0.05. The Higgins I 2 statistics were used to quantify the percentage of the total variance in the summary estimate due to between‐study heterogeneity. Fixed effects and random effects models were used with low and high levels of heterogeneity, respectively. Review Manager statistical software package (version 5.3; The Nordic Cochrane Center, Copenhagen, Denmark) was used for statistical analyses. This meta‐analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines.
Results
Characteristics of enrolled studies
Ultimately, 36 studies that met the inclusion criteria were analyzed, including 7 studies that compared a placebo with a combination of a DPP‐4 inhibitor and insulin (DPP4i/INS)9, 10, 11, 12, 13, 14, 15, 3 studies that compared a placebo with a combination of metformin and insulin (MET/INS)16, 17, 18, 7 studies that compared a placebo with a combination of thiazolidinedionesin and insulin (TZD/INS)17, 19, 20, 21, 22, 23, 24, 5 studies that compared a placebo with a combination of an alpha‐glucosidase inhibitor and insulin (AGI/INS)25, 26, 27, 28, 29, 7 studies that compared a placebo with a combination of a GLP‐1 receptor agonist and insulin (GLP‐1RA/INS)30, 31, 32, 33, 34, 35, 36 and 8 studies that compared a placebo with a combination of SGLT‐2i and insulin (SGLT‐2i/INS)37, 38, 39, 40, 41, 42, 43, 44. Details are presented in a flow chart (Figure 1). Characteristics of the included studies are outlined in Table S1. Figure S1 summarizes the risks of bias of the included studies.
Figure 1.
Flow chart of included studies.
Methodological quality
All the included studies were randomized, placebo‐controlled trials. Most studies reported age, sex, diabetes duration, HbA1c, BMI, bodyweight between the comparison groups at baseline. Overall, the risk of bias was low. Figure S1 summarizes the risks of bias of the included studies. Funnel plots assessing the precision of the data suggested a low risk of publication bias (data not shown).
Efficacy outcomes
Changes in HbA1c
The HbA1c‐lowering efficacy was significantly greater with DPP‐4i/INS than with PBO/INS (WMD −0.53%, 95% CI: −0.63, −0.43, P < 0.01; Figure 2; Table 1). The placebo‐corrected HbA1c change from baseline was greater with MET/INS than with DPP‐4i/INS (P < 0.05). There was no significant difference in the placebo‐corrected HbA1c change from baseline between DPP‐4i/INS and AGI/INS, TZD/INS, GLP‐1RA/INS and SGLT‐2i/INS (P > 0.05).
Figure 2.
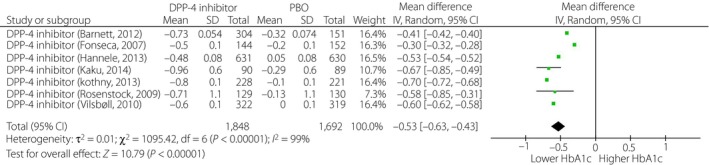
Change from baseline in glycated hemoglobin (HbA1c) of dipeptidyl peptidase‐4 (DPP‐4) inhibitor/insulin treatment. CI, confidence interval; PBO, placebo.
Table 1.
Comparisons of glycated hemoglobin change from baseline in different treatment groups
| No. studies | No. participants (active agents vs PBO) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| HbA1c change from baseline (%) | |||||
| DPP‐4i/INS | 7 | 1,848/1,692 | −0.53 | −0.63, −0.43 | <0.01a |
| MET/INS | 3 | 127/135 | −0.88 | −1.11, −0.64 | <0.01a |
| AGI/INS | 5 | 375/367 | −0.55 | −1.12, 0.01 | 0.06 |
| TZD/INS | 7 | 758/760 | −0.61 | −0.80, −0.41 | <0.01a |
| SGLT‐2i/INS | 8 | 1,658/1,585 | −0.66 | −0.79, −0.53 | <0.01a |
| GLP‐1RA/INS | 7 | 1,393/1,223 | −0.74 | −1.07, −0.41 | <0.01a |
P‐value < 0.05. AGI, alpha‐glucosidase inhibitors; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; INS, insulin; MET, metformin; PBO, placebo; TZD, thiazolidinediones; SGLT‐2i, sodium–glucose cotransporter 2 inhibitor; WMD, weighted mean difference.
Changes in FPG
When DPP4i/INS treatment was compared with PBO/INS treatment, the change in FPG was not significant (WMD −0.32 mmol/L, 95% CI: −0.75, 0.11, P = 0.14; I 2 = 100%, random effects model was used; Table 2). The difference in the placebo‐corrected FPG change from baseline between DPP‐4i/INS and MET/INS, AGI/INS, TZD/INS, GLP‐1RA/INS and SGLT‐2i/INS treatments was not significant (P > 0.05).
Table 2.
Comparisons of fasting plasma glucose change from baseline in different treatment groups
| No. studies | No. participants (active agents vs PBO) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| FPG change from baseline (mmol/L) | |||||
| DPP‐4i/INS | 7 | 1,848/1,692 | −0.32 | −0.75, 0.11 | 0.14 |
| MET/INS | 3 | 127/135 | −0.72 | −1.58, 0.14 | 0.10 |
| AGI/INS | 4 | 268/267 | −0.02 | −0.30, 0.26 | 0.87 |
| TZD/INS | 6 | 750/750 | −1.16 | −3.15, 0.83 | 0.25 |
| SGLT‐2i/INS | 6 | 800/753 | −0.63 | −1.39, 0.13 | 0.10 |
| GLP‐1RA/INS | 7 | 1,393/1,223 | −0.46 | −0.87, ‐0.05 | <0.05a |
P‐value < 0.05. AGI, alpha‐glucosidase inhibitors; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; FPG, fasting plasma glucose; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; INS, insulin; MET, metformin; PBO, placebo; SGLT‐2i, sodium–glucose cotransporter 2 inhibitor; SU, sulfonylurea; TZD, thiazolidinediones; WMD, weighted mean difference.
Changes in PPG
The PPG‐lowering efficacy was significantly greater with DPP‐4i/INS than with PBO/INS (WMD −1.65 mmol/L, 95% CI: −2.34, −0.96, P < 0.01; I 2 = 100%, random effects model was used; Table 3). The placebo‐corrected PPG change from baseline was greater with SGLT‐2i/INS than with DPP‐4i/INS (P < 0.05). The placebo‐corrected PPG change from baseline between DPP‐4i/INS and AGI/INS and GLP‐1RA/INS treatments was not significantly different (P > 0.05).
Table 3.
Comparisons of postprandial plasma glucose change from baseline in different treatment groups
| No. studies | No. participants (active agents vs PBO) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| PPG change from baseline (mmol/L) | |||||
| DPP‐4i/INS | 2 | 626/470 | −1.65 | −2.34, −0.96 | <0.01 |
| AGI/INS | 3 | 208/207 | −1.76 | −4.19, 0.66 | 0.15 |
| GLP‐1RA/INS | 3 | 705/547 | −2.87 | −8.98, 3.23 | 0.36 |
| SGLT‐2i/INS | 2 | 146/83 | −2.62 | −2.86, −2.37 | <0.01 |
AGI, alpha‐glucosidase inhibitors; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; INS, insulin; PBO, placebo; PPG, postprandial plasma glucose; SGLT‐2i, sodium–glucose cotransporter 2 inhibitor; WMD, weighted mean difference.
Changes in bodyweight
When DPP4i/INS treatment was compared with PBO/INS treatment, the change in bodyweight was not significant (WMD 0.18 kg, 95% CI: −0.08, 0.44, P = 0.17; Table 4). When placebo‐corrected, bodyweight was significantly decreased with SGLT‐2i/INS and GLP‐1RA/INS compared with DPP‐4i/INS (P < 0.05), and was significantly increased with TZD/INS compared with DPP‐4i/INS (P < 0.05). There was no significant difference in placebo‐corrected bodyweight change from baseline between DPP‐4i/INS and AGI/INS treatments (P > 0.05).
Table 4.
Comparisons of bodyweight change from baseline in different treatment groups
| No. studies | No. participants (active agents vs PBO) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Bodyweight change from baseline (kg) | |||||
| DPP‐4i/INS | 7 | 1,848/1,692 | 0.18 | −0.08, 0.44 | 0.17 |
| MET/INS | 3 | 127/135 | −2.66 | −3.91, −1.41 | <0.01a |
| AGI/INS | 4 | 268/267 | −0.70 | −2.15, 0.75 | 0.34 |
| TZD/INS | 6 | 655/656 | 1.88 | 0.29, 3.46 | 0.02a |
| SGLT‐2i/INS | 7 | 994/946 | −1.89 | −2.31, −1.48 | <0.01a |
| GLP‐1RA/INS | 7 | 1,393/1,223 | −1.70 | −2.53, −0.88 | <0.01a |
P‐value < 0.05. AGI, alpha‐glucosidase inhibitors; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; INS, insulin; MET, metformin; PBO, placebo; SGLT‐2i, sodium–glucose cotransporter 2 inhibitor; TZD, thiazolidinediones; WMD, weighted mean difference.
Changes in the dosage of insulin use
The change in daily insulin doses was significantly greater with DPP‐4i/INS than with PBO/INS (WMD −2.17 units/day, 95% CI: −3.18, −1.15, P < 0.01; I 2 = 99%, random effects model was used; Table 5). The placebo‐corrected daily insulin dosage was significantly decreased with TZD/INS compared with DPP‐4i/INS (P < 0.05). Comparisons of the placebo‐corrected insulin dosage change from baseline between DPP‐4i/INS and AGI/INS, GLP‐1RA/INS and SGLT‐2i/INS treatments showed that the difference was not significant (P > 0.05).
Table 5.
Comparisons of daily insulin dosage change from baseline in different treatment groups
| No. studies | No. participants (active agents vs PBO) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Daily insulin dosage change from baseline (U/day) | |||||
| DPP‐4i/INS | 4 | 1,307/1,154 | −2.17 | −3.18, −1.15 | <0.01a |
| AGI/INS | 2 | 142/141 | 0.28 | −2.85, 3.40 | 0.86 |
| TZD/INS | 5 | 518/524 | −16.15 | −25.89, −6.42 | <0.01a |
| SGLT‐2i/INS | 3 | 545/490 | −6.00 | −12.28, 0.27 | 0.06 |
| GLP‐1RA/INS | 7 | 1,393/1,223 | −3.39 | −4.74, −2.04 | <0.01a |
P‐value < 0.05. AGI, alpha‐glucosidase inhibitors; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; INS, insulin; PBO, placebo; SGLT‐2i, sodium–glucose cotransporter 2 inhibitor; TZD, thiazolidinediones; U, unit; WMD, weighted mean difference.
Safety outcomes
In the subgroup analysis of safety outcomes, we analyzed the incidence of hypoglycemia and severe hypoglycemia. The risk of hypoglycemia or severe hypoglycemia between treatment with DPP4i/INS and PBO/INS was similar (I 2 = 48% for the incidence of hypoglycemia, I 2 = 41% for the incidence of severe hypoglycemia, fixed effects model was used). In the AGI/INS treatment group, the risk of hypoglycemia significantly increased compared with the PBO/INS treatment group (RR 1.39, 95% CI: 1.07, 1.81, P = 0.01). There was no significant difference in the risk of hypoglycemia or severe hypoglycemia in the other treatment groups compared with the PBO/INS group (Table 6). The placebo‐corrected risk of hypoglycemia or severe hypoglycemia between DPP‐4i/INS and MET/INS, TZD/INS, GLP‐1RA/INS and SGLT‐2i/INS treatments showed no significant difference (P > 0.05).
Table 6.
Comparisons of hypoglycemia or severe hypoglycemia risk in different treatment groups
| No. studies | No. participants (active agents vs PBO) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Incidence of hypoglycemia (%) | |||||
| DPP‐4i/INS | 7 | 1,848/1,692 | 1.02 | 0.91, 1.16 | 0.71 |
| MET/INS | 3 | 127/135 | 0.96 | 0.82, 1.12 | 0.59 |
| AGI/INS | 5 | 375/367 | 1.39 | 1.07, 1.81 | 0.01a |
| TZD/INS | 3 | 236/194 | 1.17 | 0.90, 1.51 | 0.24 |
| SGLT‐2i/INS | 8 | 820/718 | 1.09 | 0.96, 1.25 | 0.18 |
| GLP‐1RA/INS | 7 | 1,367/1,197 | 1.24 | 0.99, 1.56 | 0.06 |
| Incidence of severe hypoglycemia (%) | |||||
| DPP‐4i/INS | 6 | 1,686/1,682 | 0.94 | 0.45, 1.95 | 0.87 |
| AGI/INS | 2 | 154/154 | 1.00 | 0.14, 6.99 | 1.00 |
| SGLT‐2i/INS | 3 | 1,008/997 | 1.28 | 0.81, 2.03 | 0.28 |
| GLP‐1RA/INS | 7 | 1,393/1,223 | 1.86 | 0.45, 7.57 | 0.39 |
P‐value < 0.05. AGI, alpha‐glucosidase inhibitors; CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated hemoglobin; INS, insulin; MET, metformin; PBO, placebo; SGLT‐2i, sodium–glucose cotransporter 2 inhibitor; TZD, thiazolidinediones; WMD, weighted mean difference.
Discussion
In the present meta‐analysis, we found that treatment with DPP‐4 inhibitors in combination with insulin improved glycemic control without an increased risk of hypoglycemia and weight gain compared with a placebo in combination with insulin. When compared with AGI/INS, TZD/INS and GLP‐1RA/INS treatments, DPP‐4i/INS treatment had equivalent placebo‐corrected effects on HbA1c, FPG and PPG control.
DPP‐4 inhibitors prevent the degradation of the gastrointestinal incretins GLP‐1 and GIP5. With this insulin‐independent mode of action, the combination of DPP‐4 inhibitors and insulin showed superior glycemic control compared with insulin alone. It is recommended that patients begin insulin therapy when they cannot achieve glycemic control with oral antihyperglycemic agents alone1, 2. These patients usually suffer from poorer islet cell function and have a higher risk of hypoglycemic episodes with insulin alone. The present meta‐analysis showed that the combined use of DPP‐4 inhibitors and insulin results in a significant decrease in daily insulin dosage, which further lowers the risk of hypoglycemia. The results regarding the efficacy and safety of DPP‐4i/INS treatment in our meta‐analysis were generally consistent with those from previous systematic reviews and meta‐analyses6, 7, and we further updated the included studies. Taken together, the data supports that DPP‐4 inhibitors represent a good option as adjunctive agents to insulin therapy.
DPP‐4 inhibitors showed a level of hypoglycemic efficacy and safety equivalent to AGI or TZD when added to insulin therapy. This gives patients an alternate therapeutic option, especially those who cannot tolerate the side‐effects of metformin, AGI or TZD. In the comparison between the DPP‐4i/INS and MET/INS groups, MET/INS treatment showed a better placebo‐corrected effect in HbA1c control with less weight gain. However, the numbers of participants of these two groups differed greatly, which might limit the statistical power.
GLP‐1 receptor agonist, another incretin‐based therapy, had a significant HbA1c‐lowering effect in combination with insulin compared with PBO/INS.
In a previous meta‐analysis that included 89 RCTs, GLP‐1 receptor agonist had an effect on weight loss45. This effect was reproduced when GLP‐1 receptor agonists and DPP‐4 inhibitors were used with insulin therapy. The placebo‐corrected risk of hypoglycemia or severe hypoglycemia between DPP‐4i/INS and GLP‐1RA/INS treatments was not significantly different (P > 0.05). Therefore, patients with a weight problem could consider using a GLP‐1 receptor agonist in combination with insulin for the weight loss effect.
SGLT‐2is induce urinary glucose excretion through inhibition of renal glucose reabsorption, improve glycemic control and reduce bodyweight46, 47, 48. Significant HbA1c‐ and PPG‐lowering effects were seen with SGLT‐2i/INS compared with PBO/INS. There was also significant weight reduction that was not accompanied by a higher risk of hypoglycemia or severe hypoglycemia in the present meta‐analysis.
In the comparison between the DPP‐4i/INS and the SGLT‐2i/INS groups, SGLT‐2i/INS treatment exerted a better placebo‐corrected effect in PPG control, with less weight gain and no higher risk of hypoglycemia. This result is generally consistent with a previous study49. However, in the systematic review and meta‐analysis by Min et al., the HbA1c reduction was significantly greater in the SGLT2i/INS group than in the DPP4i/INS group after adjusting for age, sex, BMI and baseline insulin dose50. Several previous studies have reported the weight‐neutral effect of DPP‐4 inhibitors and the weight reduction effect of SGLT‐2 inhibitors. Results from the present meta‐analysis suggested that these effects were preserved with the addition of insulin therapy. It is well known that obesity is associated with insulin resistance, and weight loss improves insulin resistance and glycemic control. Taken together, these results support that SGLT‐2i is a better option for glycemic control, especially for those patients with a higher BMI.
The present meta‐analysis systematically evaluated the efficacy and safety of DPP‐4i/INS treatment compared with a placebo or other antihyperglycemic agents in combination with insulin therapy. However, there are several potential limitations. First, the present meta‐analysis comprised studies with different baseline characteristics and therapeutic regimens, which might lead to bias of the results. Second, the numbers of participants in some treatment groups were different greatly, such as DPP‐4i/INS vs MET/INS treatment, which might limit the statistical power. Additionally, hypoglycemia and severe hypoglycemia were not clearly defined in several studies, leading to heterogeneity across studies. More studies with a larger sample size, longer duration of follow up and more head‐to‐head comparisons are required to substantiate the present results.
According to this meta‐analysis, treatment with DPP‐4 inhibitors combined with insulin improves glycemic control without increasing the risk of hypoglycemia and weight gain compared with insulin treatment alone. DPP‐4i/INS treatment was equally effective in placebo‐corrected glycemic control compared with AGI/INS, TZD/INS and GLP‐1RA/INS treatments.
Disclosure
LNJ has received fees for lecture presentations and for consulting from AstraZeneca, Merk, Novartis, Lilly, Roche, Sanofi‐Aventis and Takeda. The other authors declare no conflict of interest.
Supporting information
Figure S1 ¦ Summary of bias on the included studies.
Table S1 ¦ Characteristics of included randomized controlled trials.
Acknowledgments
This meta‐analysis was supported by AstraZeneca Ltd. (China). The funding agencies had no role in the study design, data collection or analysis, decision to publish or preparation of the manuscript. We are grateful to our colleagues during the study at Peking University People's Hospital Endocrinology and Metabolism Department. We also thank Xia Zhang and Xia Li at AstraZeneca China for their kind help.
J Diabetes Investig 2018;9:813–821
References
- 1. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(Suppl 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- 2. Jianping W, Linong J, Weiping J, et al Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev 2016; 32: 442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 4. Janardhan S, Sastry GN. Dipeptidyl peptidase IV inhibitors: a new paradigm in type 2 diabetes treatment. Curr Drug Targets 2014; 15: 600–621. [DOI] [PubMed] [Google Scholar]
- 5. Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretin‐ based therapies. Int J Gen Med 2013; 6: 877–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai C, Qilin Y, Shu Z, et al Assessing the efficacy and safety of combined DPP‐4 inhibitor and insulin treatment in patients with type 2 diabetes: a meta‐analysis. Int J ClinExpPathol 2015; 8: 14141–14150. [PMC free article] [PubMed] [Google Scholar]
- 7. Yeong GK, Se HM, Seokyung H, et al Efficacy and safety of the addition of a dipeptidyl peptidase‐4 inhibitor to insulin therapy in patients with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2016; 116: 86–95. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, UK: The Cochrane Collaboration, 2011. [Google Scholar]
- 9. Barnett AH, Charbonnel B, Donovan M, et al Effect of saxagliptin as add‐ on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin 2012; 28: 513–523. [DOI] [PubMed] [Google Scholar]
- 10. Fonseca V, Schweizer A, Albrecht D, et al Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007; 50: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 11. Yki‐Jarvinen H, Rosenstock J, Duran‐Garcia S, et al Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥52‐week randomized, double‐blind study. Diabetes Care 2013; 36: 3875–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaku K, Mori M, Kanoo T, et al Efficacy and safety of alogliptin added to insulin in Japanese patients with type 2 diabetes: a randomized, double‐blind, 12‐week, placebo‐controlled trial followed by an open‐label, long‐term extension phase. Expert Opin Pharmacother 2014; 15: 2121–2130. [DOI] [PubMed] [Google Scholar]
- 13. Kothny W, Foley J, Kozlovski P, et al Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15: 252–257. [DOI] [PubMed] [Google Scholar]
- 14. Rosenstock J, Rendell MS, Gross JL, et al Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA1C without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab 2009; 11: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 15. Vilsboll T, Rosenstock J, Yki‐Jarvinen H, et al Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 167–177. [DOI] [PubMed] [Google Scholar]
- 16. Avilés‐Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin‐treated type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 1999; 131: 182–188. [DOI] [PubMed] [Google Scholar]
- 17. Gram J, Henriksen JE, Grodum E, et al Pharmacological treatment of the pathogenetic defects in type 2 diabetes: the randomized multicenter South Danish Diabetes Study. Diabetes Care 2011; 34: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hermann LS, Kalen J, Katzman P, et al Long‐term glycaemic improvement after addition of metformin to insulin in insulin‐treated obese type 2 diabetes patients. Diabetes Obes Metab 2001; 3: 428–434. [DOI] [PubMed] [Google Scholar]
- 19. Henriksen K, Byrjalsen I, Qvist P, et al Efficacy and safety of the PPARgamma partial agonist balaglitazone compared with pioglitazone and placebo: a phase III, randomized, parallel‐group study in patients with type 2 diabetes on stable insulin therapy. Diabetes Metab Res Rev 2011; 27: 392–401. [DOI] [PubMed] [Google Scholar]
- 20. Hollander P, Yu D, Chou HS. Low‐dose rosiglitazone in patients with insulin‐requiring type 2 diabetes. Arch Intern Med 2007; 167: 1284–1290. [DOI] [PubMed] [Google Scholar]
- 21. Mattoo V, Eckland D, Widel M, et al Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: results of a six‐month, randomized, double‐blind, prospective, multicenter, parallel‐group study. ClinTher 2005; 27: 554–567. [DOI] [PubMed] [Google Scholar]
- 22. Raskin P, Rendell M, Riddle MC, et al A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin‐treated type 2 diabetes. Diabetes Care 2001; 24: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 23. Reynolds LR, Konz EC, Frederich RC, et al Rosiglitazone amplifies the benefits of lifestyle intervention measures in long‐standing type 2 diabetes mellitus. Diabetes Obes Metab 2002; 4: 270–275. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz S, Raskin P, Fonseca V, et al Effect of troglitazone in insulin‐treated patients with type II diabetes mellitus. Troglitazone and Exogenous Insulin Study Group. N Eng J Med 1998; 338: 861–866. [DOI] [PubMed] [Google Scholar]
- 25. Kelley DE, Bidot P, Freedman Z, et al Efficacy and safety of acarbose in insulin‐treated patients with type 2 diabetes. Diabetes Care 1998; 21: 2056–2061. [DOI] [PubMed] [Google Scholar]
- 26. Hwu C, Ho L, Fuh M, et al Acarbose improves glycemic control in insulin‐treated Asian type 2 diabetic patients: results from a multinational, placebo‐controlled study. Diabetes Res Clin Pract 2003; 60: 111–118. [DOI] [PubMed] [Google Scholar]
- 27. Mitrakou A, Tountas N, Raptis AE, et al Long‐term effectiveness of a new alpha‐glucosidase inhibitor (BAY m1099‐miglitol) in insulin‐treated type 2 diabetes mellitus. Diabet Med 1998; 15: 657–660. [DOI] [PubMed] [Google Scholar]
- 28. Nemoto M, Tajima N, Kawamori R. Efficacy of combined use of miglitol in type 2 diabetes patients receiving insulin therapy‐placebo‐controlled double‐blind comparative study. Acta Diabetol 2011; 48: 15–20. [DOI] [PubMed] [Google Scholar]
- 29. Schnell O, Mertes G, Standl E. Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy. Diabetes Obes Metab 2007; 9: 853–858. [DOI] [PubMed] [Google Scholar]
- 30. Ahmann A, Rodbard HW, Rosenstock J, et al Efficacy and safety of liraglutide versus placebo added to basal insulin agonists (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab 2015; 17: 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riddle MC, Aronson R, Home P, et al Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care 2013; 36: 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riddle MC, Forst T, Aronson R, et al Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care 2013; 36: 2497–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buse JB, Bergenstal RM, Glass LC, et al Use of twice‐daily exenatide in Basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011; 154: 103–112. [DOI] [PubMed] [Google Scholar]
- 34. Buse JB, Vilsbøll T, Thurman J, et al Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014; 37: 2926–2933. [DOI] [PubMed] [Google Scholar]
- 35. Seino Y, Min KW, Niemoeller E, et al Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab 2012; 14: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seino Y, Kaneko S, Fukuda S, et al Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Investig 2016; 7: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Araki E, Onishi Y, Asano M, et al Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: results of the interim analysis of 16‐week double‐blind treatment period. J Diabets Investig 2016; 7: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cefalu WT, Leiter LA, de Bruin TW, et al Dapagliflozin's effects on glycemia and cardiovascular risk factors in high‐risk patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. Diabetes Care 2015; 38: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inagaki N, Harashima S, Maruyama N, et al Efficacy and safety of canagliflozin in combination with insulin: a double‐blind, randomized, placebo‐controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2016; 18: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neal B, Perkovic V, De Zeeuw D, et al Efficacy and safety of canagliflozin, an inhibitor of sodium‐glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 2015; 38: 403–411. [DOI] [PubMed] [Google Scholar]
- 41. Rosenstock J, Jelaska A, Frappin G, et al Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately con‐ trolled type 2 diabetes. Diabetes Care 2014; 37: 1815–1823. [DOI] [PubMed] [Google Scholar]
- 42. Rosenstock J, Jelaska A, Zeller C, et al Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2015; 17: 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wilding JP, Norwood P, T'joen C, et al A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care 2009; 32: 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilding JP, Woo V, Soler NG, et al Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 2012; 156: 405–415. [DOI] [PubMed] [Google Scholar]
- 45. Aroda VR, Henry RR, Han J, et al Efficacy of GLP‐1 receptor agonists and DPP‐4 inhibitors: meta‐analysis and systematic review. Clin Ther 2012; 34: 1247–1258. [DOI] [PubMed] [Google Scholar]
- 46. Kasichayanula S, Liu X, LaCreta F, et al Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium‐glucose co‐transporter type 2. Clin Pharmacokinet 2014; 53: 17–27. [DOI] [PubMed] [Google Scholar]
- 47. Devineni D, Curtin CR, Polidori D, et al Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 2013; 53: 601–610. [DOI] [PubMed] [Google Scholar]
- 48. Kadokura T, Zhang W, Krauwinkel W, et al Clinical pharmacokinetics and pharmacodynamics of the novel SGLT2 inhibitor ipragliflozin. Clin Pharmacokinet 2014; 53: 975–988. [DOI] [PubMed] [Google Scholar]
- 49. Nair S, Wilding JP. Sodium glucose co‐transporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 34–42. [DOI] [PubMed] [Google Scholar]
- 50. Min SH, Yoon JH, Hahn S, et al Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta‐analysis. Diabetes Metab Res Rev 2017; 33: e2818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Summary of bias on the included studies.
Table S1 ¦ Characteristics of included randomized controlled trials.


