Abstract
Aims/Introduction
Remnant lipoproteins are thought to be atherogenic. Remnant‐like particle cholesterol (RLP‐C), which reflects the levels of various kinds of remnant lipoproteins in the blood, has a significant correlation with insulin resistance.
Materials and Methods
In the present study, we measured the effect of empagliflozin (EMPA) on the levels of RLP‐C, and investigated whether EMPA‐mediated change in RLP‐C is associated with a change in insulin resistance in type 2 diabetes patients who have insulin resistance.
Results
Patients were allocated to receive a placebo (n = 51) or EMPA (n = 58) as an add‐on treatment. Fasting blood samples were collected before and 12 weeks after this intervention. EMPA significantly decreased glycated hemoglobin, bodyweight, systolic blood pressure, plasma triglycerides, liver transaminases and estimated glomerular filtration rate, and increased high‐density lipoprotein cholesterol. Furthermore, EMPA decreased RLP‐C and homeostatic model assessment of insulin resistance. In the placebo group, there were no significant changes in these factors except for slight increases in liver transaminases. Multiple regression analysis showed that the change in homeostatic model assessment of insulin resistance (P = 0.0102) and the change in alanine aminotransferase (P = 0.0301) were significantly associated with the change in RLP‐C in the EMPA group. The change in RLP‐C significantly correlated with the change in homeostatic model assessment of insulin resistance (Pearson correlation coefficient 0.503, 95% confidence interval 0.199–0.719; P = 0.00241).
Conclusion
EMPA decreases RLP‐C levels, which is closely associated with amelioration of insulin sensitivity in diabetes patients who have insulin resistance.
Keywords: Insulin resistance, Remnant lipoprotein, Sodium–glucose cotransporter‐2 inhibitor
Introduction
Type 2 diabetes is characterized by chronic hyperglycemia caused by a combination of insulin resistance and insufficient insulin secretion. Sodium–glucose cotransporter‐2 (SGLT2) inhibitors have recently been developed as novel therapeutic agents for the treatment of type 2 diabetes1, 2. These drugs inhibit the reabsorption of glucose in the proximal tubules of the kidney, leading to increased urinary excretion of glucose and decreased levels of blood glucose in diabetes patients1, 2. In addition, SGLT2 inhibitors reportedly improve muscle insulin resistance and accelerate lipolysis in adipose tissue. Thus, use of SGLT2 inhibitors results in decreased bodyweight and visceral fat mass, and reduced blood pressure.
Empagliflozin (EMPA) is a potent and highly selective inhibitor of SGLT2 used in the treatment of type 2 diabetes. In a secondary prevention study (the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA‐REG OUTCOME®] trial), EMPA successfully suppressed composite adverse cardiovascular (CV) outcomes3. In EMPA‐REG OUTCOME® study, the reductions in the risk of CV/mortality outcomes with EMPA occurred early, suggesting that the benefits were not primarily driven by an effect on atherosclerosis. The mechanisms behind the CV benefits of EMPA are believed to be multifactorial and might involve its beneficial effects on insulin sensitivity, because the failing heart appears to be insulin resistant4.
A method for measuring remnant‐like particle cholesterol (RLP‐C), which reflects the levels of various kinds of the remnant lipoproteins in the blood5, has been established, and several studies have shown that RLP‐C has a significant correlation with insulin resistance. In the present study, we measured the effect of EMPA on RLP‐C levels and investigated whether RLP‐C is associated with insulin resistance in type 2 diabetes patients who have insulin resistance.
Methods
Study design
The present study was an open‐label, single‐center, prospective study. Empagliflozin (10 mg) or a placebo was administered once daily for 12 weeks as add‐on therapy without any other concurrent changes in medication. In both groups, established oral hypoglycemic drugs (sulfonylureas, metformin or an α‐glucosidase inhibitor), antihypertensive drugs (angiotensin II receptor blockers or calcium channel blockers) and antihyperlipidemic drugs (statins or fibrates) were continued. This protocol was approved by the ethics committees of Tohto Clinic, Tokyo, Japan, and designed in compliance with the principles embodied by the Declaration of Helsinki (2013). All patients provided written informed consent before all procedures related to this study. This study is registered with UMIN‐CTR (UMIN000021552).
Patients
Type 2 diabetes patients without any history of taking SGLT2 inhibitors whose glycated hemoglobin (HbA1c) was > 6.5%, despite receiving instruction regarding diet, exercise and medical treatment except SGLT2 inhibitors for at least 12 weeks at our clinic, and who might have insulin resistance (body mass index [BMI] > 28 or homeostatic model assessment of insulin resistance [HOMA‐IR] > 1.73 or fasting immunoreactive insulin [IRI] > 10 and fasting blood glucose < 180) were enrolled in the present study. A total of 60 patients were enrolled each in the placebo group and in the EMPA group. At the start of the study, the criteria for participating in this study applied to each patient again. As a consequence, five patients in the placebo group and two patients in the EMPA group were excluded because of the history of taking of SGLT2. Four patients in the placebo group were excluded because they were poorly controlled. Finally, the present study included 109 participants (70 men and 39 women, aged 38–74 years) who were allocated to receive EMPA (n = 58) or a placebo (n = 51).
Measurements
Overnight fasting blood and urine samples were obtained before and 12 weeks after the administration of EMPA or a placebo. All biochemical data were obtained by measurement in our laboratory, except IRI, RLP‐C, brain natriuretic peptide, high‐sensitivity C‐reactive protein and urine albumin, which were measured by LSI Medicine Corporation (Tokyo, Japan). HOMA‐IR in the EMPA group at 12 weeks was calculated as follows: 12 weeks after the administration of EMPA, EMPA administration was stopped temporarily for 72 h until no urine glucose was detected. Fasting blood samples were then obtained, and blood glucose and IRI were measured.
Statistical analysis
Data were expressed as mean ± standard deviation or percentage change after treatment. Statistical analyses were carried out using statistical software (EZR version 1.21; Saitama Medical Center, Jichi Medical University, Saitama, Japan)6. Comparison of parameters before and after treatment was carried out using the paired t‐test, and for intergroup comparisons the unpaired t‐test was used. To determine the factors associated with the change in RLP‐C, multiple linear regression analysis was carried out. The Pearson correlation coefficient test was carried out between the change in RLP‐C and HOMA‐IR. Differences were considered statistically significant at values of P < 0.05.
Results
The study included 109 participants who were randomly allocated to receive EMPA (n = 58) or a placebo (n = 51). Table 1 lists the general characteristics and blood biochemical measurements at baseline and at 12 weeks after initiating treatment with EMPA (EMPA group) or a placebo (placebo group). In the EMPA group, EMPA treatment for 12 weeks significantly reduced bodyweight (from 83.55 ± 17.9 kg to 80.68 ± 18.4 kg) and BMI (from 30.71 ± 4.72 to 29.58 ± 4.85). Reductions in fasting blood glucose (from 134.7 ± 27.3 to 124.0 ± 20.8 mg/dL) and HbA1c (from 7.37 ± 1.34 to 6.97 ± 0.94%) were observed, and reductions in fasting IRI (from 10.59 ± 5.83 to 9.13 ± 5.57 μU/mL) and in HOMA‐IR (from 3.03 ± 1.97 to 2.33 ± 1.95) were also observed at 12 weeks. The level of low‐density lipoprotein cholesterol (LDL‐C) was not altered, whereas HDL cholesterol (HDL‐C) significantly increased and triglyceride was significantly reduced. Importantly, RLP‐C was also significantly decreased (from 8.06 ± 4.84 mg/dL to 5.49 ± 3.39 mg/dL). Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma‐glutamyl transpeptidase were significantly decreased by EMPA. The estimated glomerular filtration rate (from 74.5 ± 16.5 to 71.0 ± 16.2 mL/min/1.73 m2) and urinary albumin excretion (measured as the urinary albumin‐to‐creatinine ratio (from 33.3 ± 51.8 to 23.7 ± 35.6 mg/gCr) significantly decreased. However, high‐sensitivity C‐reactive protein and brain natriuretic peptide were not significantly changed at 12 weeks. EMPA treatment for 12 weeks significantly lowered systolic blood pressure, but not diastolic blood pressure. In contrast, in the placebo group, there were no significant differences in these clinical parameters between at baseline and at 12 weeks, except for small but significant increases in AST and ALT.
Table 1.
Clinical parameters before and after administration of placebo or empagliflozin
| Placebo (n = 51) | EMPA (n = 58) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre Tx | Post Tx | Change (%) | P‐value | Pre Tx | Post Tx | Change (%) | P‐value | |
| BW (kg) | 80.7 ± 10.1 | 82.55 ± 12.4 | 2.2 | 0.146 | 83.55 ± 17.9 | 80.68 ± 18.4 | −3.4 | 0.001748* |
| BMI | 28.8 ± 3.2 | 29.07 ± 3.24 | 0.7 | 0.0522 | 30.7 ± 4.7 | 29.5 ± 4.8 | −3.6 | < 0.001* |
| FBG (mg/dL) | 130 ± 26.5 | 127.3 ± 23.9 | −2.1 | 0.326 | 134.7 ± 27.3 | 124 ± 20.7 | −7.9 | < 0.001* |
| HbA1c (%) | 6.91 ± 0.61 | 6.95 ± 0.58 | 0.5 | 0.479 | 7.37 ± 1.34 | 6.97 ± 0.94 | −5.4 | 0.00334* |
| IRI (mU/mL) | 8.81 ± 3.59 | 9.15 ± 3.54 | 3.8 | 0.432 | 10.59 ± 5.83 | 9.13 ± 5.5 | −13.7 | 0.00128* |
| HOMA‐R | 2.86 ± 0.59 | 2.98 ± 0.76 | 4.1 | 0.486 | 3.03 ± 1.97 | 2.33 ± 1.95 | −23.1 | < 0.001* |
| LDL‐C (mg/dL) | 119.6 ± 23.3 | 121.9 ± 21.4 | 1.9 | 0.353 | 114.4 ± 29.9 | 116 ± 27.5 | 1.8 | 0.262 |
| HDL‐C (mg/dL) | 54.9 ± 12.8 | 54.8 ± 10.8 | −0.1 | 0.939 | 55.9 ± 13.8 | 57.7 ± 14.6 | 3.2 | 0.0338* |
| TG (mg/dL) | 120.6 ± 47.7 | 123.0 ± 43.2 | 1.9 | 0.672 | 144.8 ± 60.8 | 126.8 ± 43.1 | −12.4 | 0.0104* |
| RLP‐C (mg/dL) | 7.77 ± 4.93 | 7.08 ± 4.06 | −8.8 | 0.36 | 8.06 ± 4.84 | 5.49 ± 3.39 | −31.8 | 0.001302* |
| AST (IU/L) | 25.9 ± 9.8 | 28.4 ± 12.1 | 9.6 | 0.01* | 28.6 ± 13.9 | 26.1 ± 11.5 | −8.7 | 0.0382* |
| ALT (IU/L) | 31.8 ± 19.5 | 38.4 ± 28.1 | 20.7 | 0.0214* | 35.3 ± 20.4 | 30.9 ± 19.4 | −12.4 | 0.00958* |
| γGTP (IU/L) | 48.9 ± 36.8 | 49.5 ± 34.2 | 1.2 | 0.857 | 39.4 ± 23.4 | 35.5 ± 21.5 | −9.8 | 0.00755* |
| eGFR (mL/min/1.73 m2) | 73.4 ± 18.7 | 73.7 ± 18.6 | 0.4 | 0.755 | 74.5 ± 16.5 | 71 ± 16.2 | −4.6 | < 0.001* |
| ACR (mg/gCr) | 33.2 ± 66.1 | 54.7 ± 126 | 64.7 | 0.121 | 33.3 ± 51.8 | 23.7 ± 35.6 | −28.8 | < 0.001* |
| BNP (pg/mL) | 12 ± 8.4 | 14 ± 7 | 16 | 0.236 | 13.4 ± 12.2 | 12.6 ± 12.2 | −5.9 | 0.541 |
| hsCRP (mg/L) | 0.13 ± 0.14 | 0.12 ± 0.15 | −7.1 | 0.61 | 0.12 ± 0.11 | 0.12 ± 0.09 | 3.3 | 0.082 |
| SBP (mmHg) | 126.2 ± 15.2 | 125.7 ± 17.6 | −0.3 | 0.777 | 134.8 ± 15.8 | 126 ± 15.3 | −5.9 | < 0.001* |
| DBP (mmHg) | 76.6 ± 10.2 | 75.5 ± 9.6 | −1.4 | 0.37 | 77.7 ± 10.5 | 75.5 ± 11.9 | −2.8 | 0.0942 |
Data are expressed as mean ± standard deviation or percent change after treatment. *P < 0.05 (P‐values for the intragroup comparison). γGTP, gamma‐glutamyl transpeptidase; ACR, albumin‐to‐creatinine ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI body mass index; BNP, brain natriuretic peptide; BW, bodyweight; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EMPA, empagliflozin; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; RLP‐C, remnant‐like particle cholesterol; SBP, systolic blood pressure; TG, triglyceride.
Changes from baseline to at 12 weeks in these clinical parameters between the placebo group and the EMPA group were compared (Table 2). The mean change was significantly greater for bodyweight, BMI, fasting blood glucose, HbA1c, IRI, HOMA‐IR, RLP‐C, AST, ALT, gamma‐glutamyl transpeptidase, albumin‐to‐creatinine ratio and brain natriuretic peptide in the EMPA group than in the placebo group. However, the mean change was not significantly different for LDL‐C, HDL‐C, triglyceride, estimated glomerular filtration rate and high‐sensitivity C‐reactive protein between the placebo group and the EMPA group. The change in systolic blood pressure, but not in diastolic blood pressure, was significantly different between the two groups.
Table 2.
Comparison of change in clinical parameters before and after administration of placebo or empagliflozin
| Placebo | EMPA | P‐value | |
|---|---|---|---|
| ΔBW (kg) | 0.57 ± 1.28 | −3.10 ± 2.02 | 0.001176* |
| ΔBMI | 0.32 ± 0.61 | −1.11 ± 0.94 | 0.004555* |
| ΔFBG (mg/dL) | −2.6 ± 18.8 | −10.9 ± 23.0 | 0.0448* |
| ΔHbA1c (%) | −0.07 ± 0.37 | −0.49 ± 1.1 | 0.0295* |
| ΔIRI (μU/mL) | 0.17 ± 0.62 | −1.06 ± 0.84 | < 0.001* |
| ΔHOMA‐R | 0.1 ± 0.437 | −0.54 ± 1.11 | 0.0105* |
| ΔLDL‐C (mg/dL) | 4.13 ± 16.0 | 2.44 ± 15.8 | 0.695 |
| ΔHDL‐C (mg/dL) | 0.40 ± 6.3 | 1.4 ± 5.4 | 0.504 |
| ΔTG (mg/dL) | −2.0 ± 30.6 | −9.2 ± 47.2 | 0.486 |
| ΔRLP‐C mg/dL) | 0.51 ± 5.5 | −0.51 ± 5.5 | 0.00988* |
| ΔAST (IU/L) | 2.0 ± 5.9 | −2.2 ± 9.7 | 0.0324* |
| ΔALT (IU/L) | 3.4 ± 11 | −4.3 ± 13 | 0.0213* |
| ΔγGTP (IU/L) | 6.0 ± 15 | −5.2 ± 17 | 0.0116* |
| ΔeGFR (mL/min/1.73 m2) | −1.5 ± 7.1 | −3.5 ± 6.1 | 0.00585* |
| ΔACR (mg/gCr) | −0.07 ± 9.6 | −12.02 ± 8.4 | 0.0216* |
| ΔBNP (pg/mL) | 1.6 ± 7.8 | −3.2 ± 9.0 | 0.0421* |
| ΔhsCRP (mg/L) | 0.017 ± 0.16 | −0.013 ± 0.83 | 0.432 |
| ΔSBP (mmHg) | −1.7 ± 8.2 | −9.2 ± 12 | 0.0115* |
| ΔDBP (mmHg) | −2.90 ± 6.9 | −1.05 ± 10.5 | 0.409 |
Data are expressed as mean ± standard deviation. *P < 0.05. γGTP, gamma‐glutamyl transpeptidase; ACR, albumin‐to‐creatinine ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI body mass index; BNP, brain natriuretic peptide; BW, bodyweight; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EMPA, empagliflozin; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; RLP‐C, remnant‐like particle cholesterol; SBP, systolic blood pressure; TG, triglyceride. (pvalues for intergroup comparison).
To determine the factors associated with the change in RLP‐C, multiple linear regression analysis was carried out for the EMPA group. In this model, the change in RLP‐C was used as an objective variable, and changes in BMI, HbA1c, HOMA‐IR, AST, ALT, gamma‐glutamyl transpeptidase, LDL‐C, HDL‐C, estimated glomerular filtration rate, systolic blood pressure and treatment or not with statins or fibrates were used as explanation variables. Explanation variables were selected among those that the change from baseline to at 12 weeks was significantly greater than that in the placebo group, except for treatment or not with statins or fibrates. When several variables had a direct relationship to each other, one was selected among them. This analysis (R 2 = 0.7421, P = 0.0006316) showed that the change in HOMA‐IR (t‐value = 2.823, P = 0.0102) and the change in ALT (t‐value = −2.325, P = 0.0301) were significantly associated with the change in RLP‐C. The other factors, including treatment or not with statins or fibrates, were not significantly associated with the RLP‐C change (Table 3).
Table 3.
Contributors to the change in remnant‐like particle cholesterol through multiple linear regression coefficients
| Estimate | Std. error | t‐value | P‐value | |
|---|---|---|---|---|
| ΔBMI | 0.238806 | 0.369723 | 0.643 | 0.5253 |
| ΔeGFR (mL/min/1.73 m2) | 0.031203 | 0.029639 | 1.053 | 0.3044 |
| ΔγGTP (IU/L) | −0.013788 | 0.019971 | −0.69 | 0.4975 |
| ΔAST (IU/L) | 0.052287 | 0.055369 | 0.944 | 0.3557 |
| ΔALT (IU/L) | −0.068618 | 0.029507 | −2.325 | 0.0301* |
| ΔHbA1c (%) | −0.19907 | 0.283503 | −0.702 | 0.4903 |
| ΔHDL‐C (mg/dL) | −0.022251 | 0.017289 | −1.287 | 0.2121 |
| ΔHOMA‐IR | 0.515829 | 0.182713 | 2.823 | 0.0102* |
| ΔSBP (mmHg) | 0.015423 | 0.014045 | 1.098 | 0.2846 |
| ΔTG (mg/dL) | 0.00841 | 0.006638 | 1.267 | 0.2191 |
| Fibrates (+/−) | 1.055769 | 0.703878 | 1.5 | 0.1485 |
| Statins (+/−) | ‐0.552454 | 0.421693 | ‐1.31 | 0.2043 |
R 2 = 0.7421, P‐value = 0.0006316. *P < 0.05. γGTP, gamma‐glutamyl transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment of insulin resistance; SBP, systolic blood pressure; Std., standard; TG, triglyceride.
The change in RLP‐C significantly correlated with the change in HOMA‐IR (Pearson correlation coefficient 0.503, 95% confidence interval 0.199–0.719, P = 0.00241), suggesting the causal relationship between RLP‐C and insulin resistance (Figure 1).
Figure 1.
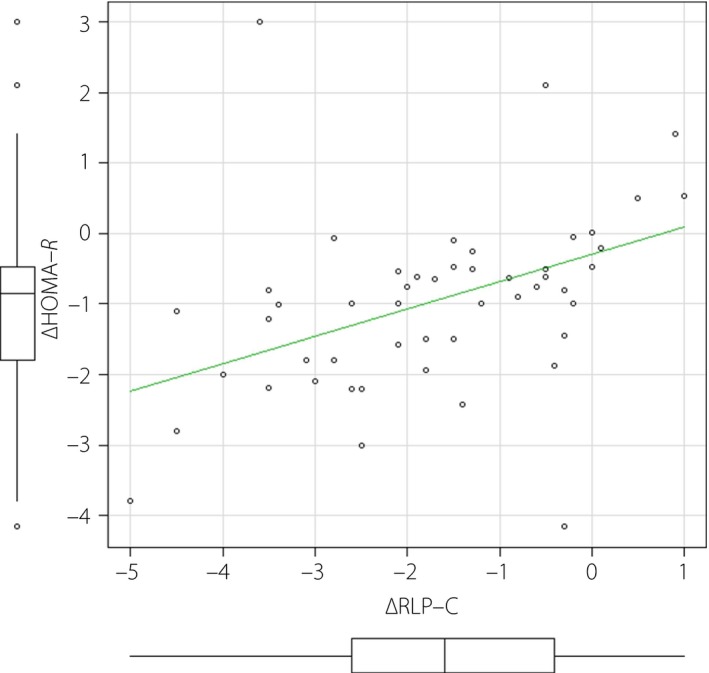
The change in remnant‐like particle cholesterol (RLP‐C) significantly correlated with the change in homeostatic model assessment of insulin resistance (HOMA‐IR; Pearson correlation coefficient 0.503, 95% confidence interval 0.199–0.719; P = 0.00241) in empagliflozin group.
Discussion
The major findings in the present study were that EMPA decreased RLP‐C in fasting serum, which is significantly associated with decreases in HOMA‐IR and ALT. RLP particles have been reported to be elevated in diabetes patients7. RLP‐C is also known to be elevated in metabolic syndrome8. RLP‐C appears to be higher in individuals with insulin resistance, and it is indeed reported that HOMA‐IR is closely related to RLP‐C during medical examination9, 10. In the present study, RLP‐C levels in fasting serum were higher at baseline in both the placebo group and EMPA group, and the enrolled participants in both groups had insulin resistance. RLP‐C levels decreased after the administration of EMPA, but not of a placebo for 12 weeks. In the EMPA group, multiple regression analysis showed that the change in HOMA‐IR and the change in ALT were significantly associated with the change in RLP‐C. Several reports have shown that SGLT2 inhibitors ameliorate peripheral insulin resistance in humans11, 12. Thus, the present findings suggest that the EMPA‐associated decrease in RLP‐C is closely related to the EMPA‐mediated improvement in insulin resistance. Remnant lipoproteins are taken up and metabolized by the liver. The EMPA‐mediated decrease in ALT could reflect a reduction in liver fat content, which might activate the uptake and metabolism of remnant lipoproteins in liver.
Recently, it was reported that dapagliflozin decreases small dense LDL‐C and increases HDL2‐C in patients with type 2 diabetes13. In that study, RLP‐C levels were not significantly changed before and after the administration of dapagliflozin. It is unclear why the present results differ from those reported for dapagliflozin. It is possible that the beneficial effect on RLP‐C observed in the present study could be a unique feature of EMPA, or it could be attributed to differences in study participants.
Finally, remnant lipoproteins are thought to be atherogenic, and higher levels of remnant lipoproteins are indeed reported to predict future coronary events in patients with coronary artery disease, independent of other risk factors14. The enigma from the EMPA‐REG OUTCOME® study is that the CV benefits occur as early as 3 months after initiation of the administration of EMPA, and that there is no reduction in traditional atherothrombolic CV events15. However, EMPA‐mediated improvement of RLP‐C might contribute to the diminishment of future coronary events in diabetes patients.
The present study had several limitations. EMPA is reported to improve insulin sensitivity, as determined using tracer glucose11. Dapagliflozin has also been shown to improve muscle insulin sensitivity, as determined by euglycemic insulin clamp studies12. We chose HOMA‐IR to evaluate insulin resistance. However, HOMA‐IR is not a good indicator when the patients examined are poorly controlled, whose fasting glucose levels are relatively high and/or fasting IRI might be already exhausted. Another problem is that as SGLT2 inhibitors inhibit the reabsorption of glucose in the proximal tubules of the kidney, leading to increased urinary excretion of glucose and decreased levels of blood glucose in diabetes patients, HOMA‐IR measurements just after the use of EMPA might not always reflect insulin resistance. Thus, we excluded poorly controlled patients from the present study, and at 12 weeks after initiating the administration of EMPA, the administration of EMPA was stopped temporally for 72 h until SGLT2 inhibition was totally ineffective, then blood glucose and IRI in fasting blood samples were measured to calculate HOMA‐IR as at 12 weeks in the EMPA group. As this is a small‐sized study, further studies, including direct evaluation of insulin sensitivity, are required to establish the relationship between RLP‐C and insulin resistance.
Disclosure
The author declares no conflict of interest.
J Diabetes Investig 2018;9:870–874
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000021552
References
- 1. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015; 75: 33–59. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Zinman B, Wanner C, et al SGLT‐2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015; 12: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 4. Iozzo P, Chareonthaitawee P, Dutka D, et al Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes 2002; 51: 3020–3024. [DOI] [PubMed] [Google Scholar]
- 5. Okazaki M, Usui S, Tada N, et al Relation between RLP‐triglyceride to RLP‐cholesterol ratio and particle size distribution in RLP‐cholesterol profiles by HPLC. Clin Chim Acta 2000; 296: 135–149. [DOI] [PubMed] [Google Scholar]
- 6. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaefer EJ, McNamara JR, Shah PK, et al Elevated remnant‐like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care 2002; 25: 989–994. [DOI] [PubMed] [Google Scholar]
- 8. Chan DC, Watts GF, Barrett PH, et al Relationships between cholesterol homoeostasis and triacylglycerol‐rich lipoprotein remnant metabolism in the metabolic syndrome. Clin Sci (Lond) 2003; 104: 383–388. [DOI] [PubMed] [Google Scholar]
- 9. Ohnishi H, Saitoh S, Takagi S, et al Relationship between insulin‐resistance and remnant‐like particle cholesterol. Atherosclerosis 2002; 164: 167–170. [DOI] [PubMed] [Google Scholar]
- 10. Abbasi F, McLaughlin T, Lamendola C, et al Fasting remnant lipoprotein cholesterol and triglyceride concentrations are elevated in nondiabetic, insulin‐resistant, female volunteers. J Clin Endocrinol Metab 1999; 84: 3903–3906. [DOI] [PubMed] [Google Scholar]
- 11. Ferrannini E, Muscelli E, Frascerra S, et al Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merovci A, Solis‐Herrera C, Daniele G, et al Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto S, Tomoyasu M, Osamura A, et al Dapagliflozin decreases small dense low‐density lipoprotein‐cholesterol and increases high‐density lipoprotein 2‐cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol 2017; 16: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kugiyama K, Doi H, Takazoe K, et al Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation 1999; 99: 2858–2860. [DOI] [PubMed] [Google Scholar]
- 15. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME Study? A unifying hypothesis Diabetes Care 2016; 39: 1115–1122. [DOI] [PubMed] [Google Scholar]


