Abstract
Aims/Introduction
To determine the efficacy and safety of adding liraglutide to three different insulin regimens in Japanese patients with type 2 diabetes mellitus.
Materials and Methods
In this post‐hoc analysis, results from a 36‐week, randomized, double‐blind, placebo‐controlled, parallel‐group trial are reported. Individuals with type 2 diabetes mellitus were stratified according to their pre‐trial insulin regimen (basal, basal–bolus and premix). The primary objective was to determine whether adding liraglutide (0.9 mg/day) to fixed‐dose insulin therapy was superior vs fixed‐dose insulin monotherapy, assessed by the effect on glycemic control after 16 weeks of treatment.
Results
The treatment effect on glycated hemoglobin reduction was independent of the pre‐trial insulin regimen. Comparing liraglutide with a placebo, liraglutide was associated with glycated hemoglobin reduction in all insulin regimens, with placebo‐corrected reductions at 16 weeks ranging from −1.45 to −1.17%, and maintained at 36 weeks. Liraglutide resulted in a greater reduction in mean plasma glucose obtained from seven‐point self‐monitoring, and greater proportions of patients achieved target glycated hemoglobin. With liraglutide, slightly higher proportions of patients receiving basal and basal–bolus insulin reported confirmed hypoglycemia from 0 to 16 weeks.
Conclusions
The efficacy and safety of adding liraglutide to insulin therapy was confirmed, regardless of pre‐trial insulin regimen.
Keywords: Glycated hemoglobin, Insulin, Liraglutide
Introduction
The global prevalence of diabetes is increasing, with cases recently estimated at 415 million by the International Diabetes Federation1. As with most countries around the world1, the burden of diabetes in Japan is a growing concern2.
Accounting for 90% of cases of diabetes, type 2 diabetes mellitus is a progressive disease, so patients might require treatment intensification over time to achieve and maintain appropriate glycemic control3, 4. For those treated with basal insulin therapy, intensification (beyond dose titration) can include converting to a basal–bolus regimen by the addition of mealtime rapid‐acting insulin, or switching to a premix insulin formulation3, 4, 5. Alternatively, when compared with continued and titrated insulin therapy, the addition of a glucagon‐like peptide 1 receptor agonist (GLP‐1RA) can improve glycated hemoglobin (HbA1c) levels without an elevated risk of hypoglycemia, while at the same time avoiding weight gain6, 7, 8, 9.
A meta‐analysis of 15 studies by Kim et al.10 showed that GLP‐1RAs lowered HbA1c to a greater extent in studies where the cohorts were predominantly Asian than in studies with predominantly non‐Asian cohorts. However, bodyweight changes were comparable between both cohort types. In addition, hypoglycemia tended to be more common in Asian‐dominant studies than in non‐Asian dominant studies10. The aim of the present post‐hoc analysis, which was based on data from the phase 3b Efficacy and Safety of Liraglutide in Combination With Insulin Therapy Compared to Insulin Alone in Japanese Subjects With Type 2 Diabetes (LIRA‐ADD2INSULIN JAPAN) trial (ClinicalTrials.gov NCT01572740)6, was to assess the efficacy and safety of the GLP‐1 analog, liraglutide, in combination with insulin (basal, basal–bolus or premix) compared with insulin monotherapy in Japanese patients with type 2 diabetes mellitus.
Methods
Trial design
LIRA‐ADD2INSULIN JAPAN was a 36‐week, randomized, multicenter, double‐blind, placebo‐controlled, parallel‐group trial involving 257 Japanese patients with type 2 diabetes mellitus6.
The trial design and protocol have been described previously6. In brief, participants were randomized (1:1) to once‐daily subcutaneous liraglutide (0.9 mg) or placebo added to their existing insulin regimen. Participants were stratified according to the type of pre‐trial insulin regimen at randomization. The insulin dose was fixed at the pre‐study dose for the first 16 weeks then subsequently down‐ or uptitrated based on participants’ self‐measured plasma glucose (SMPG; measured using hand‐held OneTouch® UltraVue™ meters; Johnson & Johnson, New Brunswick, NJ, USA) values and an insulin titration algorithm for the remaining study period6.
Eligibility criteria
Eligible participants were men or women, aged ≥20 years, with type 2 diabetes mellitus for ≥6 months, HbA1c 7.5–11.0% (59–97 mmol/mol; inclusive) and body mass index <45.0 kg/m2. In addition to diet and exercise, all participants received a basal, basal–bolus or premix insulin regimen that had to have been stable (maximum daily fluctuation in dose, ±20%) at a total daily dose ≥10 U/day for ≥12 weeks before screening. Participants could not have been taking an oral antidiabetic drug or a GLP‐1RA within the previous 12 weeks before screening.
All participants provided written informed consent before participation, and the trial was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice.
End‐points
The primary end‐point was the change in HbA1c from baseline after 16 weeks. Secondary efficacy end‐points (assessed at 16 and 36 weeks, unless otherwise stated) included change from baseline in: HbA1c (36 weeks); insulin dose (36 weeks); fasting plasma glucose (FPG); seven‐point SMPG profiles (change from baseline in mean plasma glucose [PG] and mean prandial PG increment); bodyweight; participants achieving target HbA1c values <7.0% (<53 mmol/mol) and ≤6.5% (≤48 mmol/mol); participants achieving target HbA1c <7.0 and ≤6.5% without weight gain; and participants achieving target HbA1c <7.0 and ≤6.5% with no confirmed hypoglycemia (recorded PG <3.1 mmol/L [56 mg/dL] or participant unable to treat himself/herself) during the previous 4 weeks. Safety end‐points of the trial included changes in blood pressure and pulse, number of hypoglycemic events during 36 weeks of treatment (confirmed and nocturnal confirmed hypoglycemic events [the period between 00.01 and 05.59 h, with both times included]) and adverse events (AEs; including gastrointestinal disorders) during 36 weeks of treatment. These end‐points were also analyzed by insulin regimen for the post‐hoc analysis.
Statistical analysis
The analysis of efficacy end‐points was based on the full analysis set, defined as all randomized participants who received at least one dose of the trial product, with each participant contributing as randomized. Continuous efficacy end‐points were analyzed using an analysis of variance model, with treatment, insulin regimen, and the interaction between treatment and insulin regimen as fixed effects, and the corresponding baseline value as a covariate. The treatment difference for each type of insulin regimen was estimated with the corresponding 95% confidence interval (CI). The proportions of participants achieving target HbA1c <7.0% (<53 mmol/mol), target HbA1c ≤6.5% (≤48 mmol/mol) and composite end‐points were summarized descriptively by the insulin regimen subgroup. For all analyses, missing data were imputed using the last observation carried forward method.
The analysis of safety end‐points was based on the safety analysis set, defined as all participants who received at least one dose of the trial product, with each participant contributing as treated. For safety end‐points, the number and proportion of participants with at least one event, the number of events, and the event rate per 100 patient years of exposure (PYE) were assessed and presented by insulin regimen subgroup.
Results
Demographics
Of 296 Japanese patients screened, 257 were randomized (liraglutide: 127 patients, placebo: 130 patients). Patients were distributed in an approximate 2:1:2 ratio between basal insulin (n = 100), basal–bolus insulin (n = 55) and premix insulin (n = 102) regimens. Participant disposition stratified by insulin regimen subgroup is summarized in Figure S1.
Baseline characteristics are summarized in Table 1. Minor differences in duration of diabetes, baseline HbA1c, sex distribution and total daily insulin dose were observed among the three insulin regimen subgroups (Table 1).
Table 1.
Baseline characteristics
| Insulin therapy | Basal | Basal–bolus | Premix | |||
|---|---|---|---|---|---|---|
| Add‐on therapy | Liraglutide | Placebo | Liraglutide | Placebo | Liraglutide | Placebo |
| FAS (n) | 50 | 50 | 27 | 28 | 50 | 52 |
| Age (years) | 58.6 ± 11.5 | 60.1 ± 10.7 | 59.2 ± 11.8 | 57.2 ± 13.1 | 65.1 ± 9.0 | 60.9 ± 10.8 |
| Duration of diabetes (years) | 12.24 ± 7.07 | 11.83 ± 7.13 | 14.34 ± 7.08 | 17.19 ± 7.40 | 16.39 ± 10.86 | 16.08 ± 9.80 |
| Female (%) | 36.0 | 36.0 | 51.9 | 53.6 | 52.0 | 42.3 |
| Male (%) | 64.0 | 64.0 | 48.1 | 46.4 | 48.0 | 57.7 |
| Bodyweight (kg) | 67.6 ± 12.6 | 65.2 ± 12.5 | 70.0 ± 21.2 | 66.1 ± 13.5 | 66.5 ± 13.8 | 66.4 ± 13.5 |
| BMI (kg/m2) | 25.9 ± 3.8 | 24.7 ± 3.6 | 26.6 ± 6.5 | 26.1 ± 4.7 | 26.2 ± 4.8 | 25.1 ± 4.1 |
| FPG (mg/dL) | 144 ± 45 | 147 ± 39 | 174 ± 49 | 163 ± 49 | 152 ± 36 | 167 ± 47 |
| HbA1c (%) | 9.0 ± 0.9 | 9.0 ± 0.9 | 8.9 ± 0.9 | 8.6 ± 0.8 | 8.5 ± 1.0 | 8.8 ± 0.9 |
| C‐peptide (ng/mL) | 0.98 ± 0.68 | 0.98 ± 0.67 | 1.06 ± 0.86 | 0.94 ± 0.73 | 1.06 ± 0.60 | 1.14 ± 0.91 |
| Total daily insulin dose (units) | 23 ± 12 | 20 ± 11 | 41 ± 15 | 43 ± 21 | 29 ± 12 | 29 ± 14 |
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin. Full analysis set (FAS) values are mean ± standard deviation unless otherwise indicated.
Efficacy end‐points
Primary end‐point: change in HbA1c from baseline at 16 weeks
Across all insulin subgroup regimens, reductions in HbA1c from baseline to week 16 were greater with liraglutide compared with the placebo (−1.81% vs −0.35%, −1.63% vs −0.37% and −1.71% vs −0.54% in the basal, basal–bolus and premix subgroups, respectively; Figure 1). When comparing estimated treatment differences (ETDs) between liraglutide and the placebo in change in HbA1c at 16 weeks by insulin subgroup regimen, the effect of liraglutide appeared to be larger in the basal insulin subgroup compared with the basal–bolus and premix insulin subgroups, with respective ETDs of −1.45% (95% CI −1.73 to −1.18, P < 0.0001), −1.26% (95% CI −1.62 to −0.89, P < 0.0001) and −1.17% (95% CI −1.44 to −0.90, P < 0.0001; Figure 1). However, the test for interaction did not find any evidence of difference in the treatment effect (the difference between liraglutide and placebo) across insulin regimens (P = 0.3353).
Figure 1.
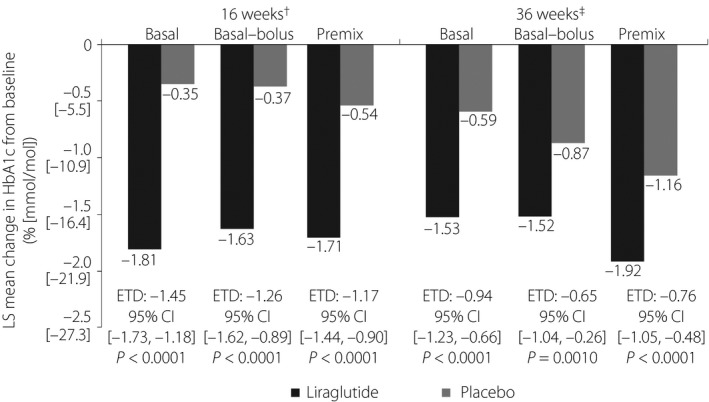
Change in glycated hemoglobin (HbA1c; % [mmol/mol]) from baseline in participants receiving liraglutide or a placebo in addition to basal insulin (n = 100), basal–bolus insulin (n = 55) or premix insulin therapy (n = 102), after 16 and 36 weeks of treatment. The analysis of efficacy end‐points was based on the full analysis set, defined as all randomized participants who received at least one dose of trial product, with each participant contributing as randomized. †Test for interaction P = 0.3353 between treatment and pre‐trial insulin at 16 weeks. ‡Test for interaction P = 0.4511 between treatment and pre‐trial insulin at 36 weeks. CI, confidence interval; ETD, estimated treatment difference; LS, least squares.
Change in HbA1c from baseline at 36 weeks
Across all insulin regimens, reductions in HbA1c from baseline to week 36 were also greater with liraglutide than with the placebo (Figure 1). As at week 16, the ETD between liraglutide and the placebo for the change in HbA1c after 36 weeks appeared to be largest in the basal insulin subgroup (Figure 1), but the test for interaction did not find any evidence of difference in treatment effect across insulin regimens (P = 0.4511).
Change in actual daily insulin dose from baseline at 36 weeks
There was an attenuated increase from baseline in actual daily insulin dose comparing liraglutide with the placebo in the basal (3.6 vs 7.4 units, respectively), basal–bolus (4.1 vs 9.0 units, respectively) and premix insulin subgroups (9.5 vs 15.8 units, respectively) at 36 weeks. The test for interaction in actual daily insulin dose at 36 weeks did not find any evidence of difference in the treatment effect across insulin regimens (P = 0.6317; Table 2).
Table 2.
Efficacy results
| Insulin therapy | Basal | Basal–bolus | Premix | |||
|---|---|---|---|---|---|---|
| Add‐on therapy | Liraglutide | Placebo | Liraglutide | Placebo | Liraglutide | Placebo |
| FAS (n) | 50 | 50 | 27 | 28 | 50 | 52 |
| Change in HbA1c (%) from baseline | ||||||
| 16 weeks | −1.81 | −0.35 | −1.63 | −0.37 | −1.71 | −0.54 |
| 36 weeks | −1.53 | −0.59 | −1.52 | −0.87 | −1.92 | −1.16 |
| ETD [95% CI], P‐value | ||||||
| 16 weeks | −1.45 [−1.73; −1.18], <0.0001 | −1.26 [−1.62; −0.89], <0.0001 | −1.17 [−1.44; −0.90], <0.0001 | |||
| 36 weeks | −0.94 [−1.23; −0.66], <0.0001 | −0.65 [−1.04; −0.26], 0.0010 | −0.76 [−1.05; −0.48], <0.0001 | |||
| Interaction between treatment and pre‐trial insulin at 16 weeks (P‐value) | 0.3353 | |||||
| Interaction between treatment and pre‐trial insulin at 36 weeks (P‐value) | 0.4511 | |||||
| Actual daily insulin dose, log‐transformed† (units) | 26.34 | 33.46 | 27.10 | 30.84 | 31.65 | 37.97 |
| Observed mean change from baseline in actual daily insulin dose at 36 weeks (units) | 3.6 | 7.4 | 4.1 | 9.0 | 9.5 | 15.8 |
| ETR [95% CI], P‐value | 0.79 [0.69; 0.90], 0.0008 | 0.88 [0.73; 1.06], 0.1713 | 0.83 [0.73; 0.96], 0.0094 | |||
| Interaction between treatment and pre‐trial insulin at 36 weeks (P‐value) | 0.6317 | |||||
| Change in FPG (mg/dL) | ||||||
| 16 weeks | −27.0 | −8.9 | −22.6 | 3.1 | −20.7 | −15.0 |
| 36 weeks | −26.8 | −22.9 | −27.7 | −22.4 | −29.1 | −24.2 |
| ETD [95% CI], P‐value | ||||||
| 16 weeks | −18.1 [−31.7; −4.6], 0.0089 | −25.7 [−44.0; −7.4], 0.0061 | −5.8 [−19.3; 7.8], 0.4039 | |||
| 36 weeks | −3.9 [−16.6; 8.8], 0.5451 | −5.3 [−22.4; 11.9], 0.5458 | −5.0 [−17.7; 7.7], 0.4407 | |||
| Interaction between treatment and pre‐trial insulin at 16 weeks (P‐value) | 0.1907 | |||||
| Interaction between treatment and pre‐trial insulin at 36 weeks (P‐value) | 0.9896 | |||||
| Change in mean PG, from 7‐point SMPG (mg/dL) | ||||||
| 16 weeks | −46.0 | −1.8 | −47.1 | −18.7 | −38.4 | −12.1 |
| 36 weeks | −42.5 | −11.6 | −50.5 | −32.1 | −51.2 | −33.4 |
| ETD [95% CI], P‐value | ||||||
| 16 weeks | −44.1 [−58.3; −30.0], <0.0001 | −28.4 [−47.4; −9.3], 0.0037 | −26.3 [−40.6; −12.0], 0.0004 | |||
| 36 weeks | −30.9 [−43.9; −17.9], <0.0001 | −18.4 [−35.9; −0.8], 0.0403 | −17.8 [−30.9; −4.7], 0.0079 | |||
| Interaction between treatment and pre‐trial insulin at 16 weeks (P‐value) | 0.1787 | |||||
| Interaction between treatment and pre‐trial insulin at 36 weeks (P‐value) | 0.3150 | |||||
| Change in mean prandial glucose increment, all meals (mg/dL) | ||||||
| 16 weeks | −21.8 | −0.0 | −36.3 | −25.6 | −19.6 | −13.7 |
| 36 weeks | −15.4 | −6.2 | −31.1 | −30.6 | −29.0 | −20.3 |
| ETD [95% CI], P‐value | ||||||
| 16 weeks | −21.8 [−35.2; −8.3], 0.0016 | −10.7 [−28.9; 7.5], 0.2472 | −5.9 [−19.5; 7.7], 0.3954 | |||
| 36 weeks | −9.1 [−25.6; 7.3], 0.2760 | −0.6 [−22.7; 21.6], 0.9599 | −8.8 [−25.6; 8.1], 0.3058 | |||
| Interaction between treatment and pre‐trial insulin at 16 weeks (P‐value) | 0.2505 | |||||
| Interaction between treatment and pre‐trial insulin at 36 weeks (P‐value) | 0.8061 | |||||
| Change in bodyweight (kg) | ||||||
| 16 weeks | 0.02 | −0.29 | −0.31 | −0.09 | −0.93 | −0.38 |
| 36 weeks | 0.32 | 0.32 | 0.47 | 0.35 | −0.15 | 0.81 |
| ETD [95% CI], P‐value | ||||||
| 16 weeks | 0.31 [−0.32; 0.94], 0.3328 | −0.21 [−1.06; 0.63], 0.6199 | −0.55 [−1.17; 0.08], 0.0858 | |||
| 36 weeks | −0.00 [−0.88; 0.88], 0.9982 | 0.12 [−1.07; 1.31], 0.8412 | −0.96 [−1.83; −0.08], 0.0318 | |||
| Interaction between treatment and pre‐trial insulin at 16 weeks (P‐value) | 0.1621 | |||||
| Interaction between treatment and pre‐trial insulin at 36 weeks (P‐value) | 0.2148 | |||||
CI, confidence interval; ETD, estimated treatment difference; ETR, estimated treatment ratio; FAS, full analysis set; FPG, fasting plasma glucose; PG, plasma glucose; SMPG, self‐measured plasma glucose. Values are least squares mean changes from baseline unless otherwise indicated. †The log‐transformed actual daily insulin dose after 36 weeks of treatment was analyzed using an ancova method with treatment, pre‐trial insulin at screening and interaction between treatment and pre‐trial insulin at screening as fixed effects, and log‐transformed actual daily insulin dose at baseline as a covariate.
Change in FPG from baseline at 16 and 36 weeks
At 16 weeks, there were greater reductions in FPG in participants treated with liraglutide compared with the placebo in the basal (ETD −18.1 mg/dL, 95% CI −31.7 to −4.6, P = 0.0089) and basal–bolus insulin subgroups (ETD −25.7 mg/dL, 95% CI −44.0 to −7.4, P = 0.0061), but this was not observed in the premix insulin subgroup (ETD −5.8 mg/dL, 95% CI −19.3 to 7.8, P = 0.4039]). At 36 weeks, the ETDs were comparable (ETDs from −5.3 to −3.9 mg/dL) across insulin subgroups (Table 2). The test for interaction did not find any evidence of difference in the treatment effect across insulin regimens (P = 0.1907 and P = 0.9896 at 16 and 36 weeks, respectively).
Change in mean seven‐point SMPG and mean prandial glucose increment from baseline at 16 and 36 weeks
Decreases in mean PG derived from the seven‐point SMPG profiles from baseline to weeks 16 and 36 were greater with liraglutide than placebo in all insulin subgroups (Table 2; Figure S2). Although the reduction in mean PG from the seven‐point SMPG appeared largest with liraglutide compared with the placebo in the basal insulin subgroup, the test for interaction did not find any evidence of difference in the treatment effect across insulin regimens (P = 0.1787 and P = 0.3150, respectively).
The decrease in mean prandial glucose increments from baseline to week 16 was greater with liraglutide than the placebo in the basal insulin subgroup (ETD −21.8 mg/dL, 95% CI −35.2 to −8.3, P = 0.0016]), whereas non‐statistically significant reductions were observed in the basal–bolus (ETD −10.7 mg/dL, 95% CI −28.9 to 7.5, P = 0.2472) and premix insulin subgroups (ETD −5.9 mg/dL, 95% CI −19.5 to 7.7, P = 0.3954; Table 2). At 36 weeks, the decrease in mean prandial glucose increments from baseline was not significantly different with liraglutide as compared with the placebo in any of the three insulin subgroups (basal insulin ETD −9.1 mg/dL, 95% CI −25.6 to 7.3, P = 0.2760]; basal–bolus ETD −0.6 mg/dL, 95% CI −22.7 to 21.6, P = 0.9599; premix insulin ETD −8.8 mg/dL, 95% CI −25.6 to 8.1, P = 0.3058). In addition, the test for interaction did not find any evidence of difference in the treatment effect across insulin regimens (P = 0.2505 and P = 0.8061 at 16 and 36 weeks, respectively).
Change in bodyweight from baseline at 16 and 36 weeks
A modest reduction in bodyweight from baseline at week 36 was observed with liraglutide compared with the placebo in the premixed insulin subgroup (ETD −0.96 kg, −1.83 to −0.08, P = 0.0318; Table 2). The test for interaction did not find any evidence of difference in the treatment effect across insulin regimens for either week 16 or 36 (P = 0.1621, P = 0.2148, respectively; Table 2).
Achievement of target HbA1c values and composite end‐points at 16 and 36 weeks
A greater proportion of participants reached target HbA1c levels with liraglutide compared with the placebo at 16 and 36 weeks, irrespective of insulin subgroup (Figure 2; Table S1).
Figure 2.
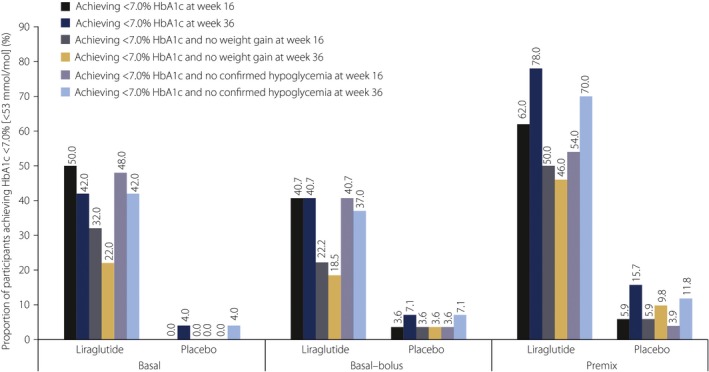
Proportion of participants who achieved glycated hemoglobin (HbA1c) <7.0% (<53 mmol/mol), HbA1c <7.0% with no weight gain and HbA1c <7.0% with no confirmed hypoglycemia (after 16 and 36 weeks of treatment) with addition of liraglutide or placebo to basal insulin (n = 100), basal–bolus insulin (n = 55) or premix insulin therapy (n = 102). The analysis of efficacy end‐points was based on the full analysis set defined as all randomized participants who received at least one dose of the trial product, with each participant contributing as randomized.
The proportion of participants achieving target HbA1c <7.0% (<53 mmol/mol) after 16 weeks appeared to be greatest in the premix insulin subgroup (62.0% vs 5.9% with liraglutide and the placebo, respectively) than in the basal (50.0% vs 0.0%) and basal–bolus insulin subgroups (40.7% vs 3.6%; Figure 2). Similar trends were observed for target HbA1c ≤6.5% (≤48 mmol/mol; Table S1).
With regard to the composite end‐points, greater proportions of participants randomized to liraglutide achieved target HbA1c (<7.0 and ≤6.5%) without weight gain or confirmed hypoglycemia compared with the placebo, irrespective of insulin subgroup at 16 and 36 weeks (Figure 2; Table S1).
Safety end‐points
Vital signs
At week 36, there was an observed decrease in systolic blood pressure with liraglutide across all insulin subgroups, and a small increase with placebo. There was an increase in diastolic blood pressure with liraglutide in the basal and basal–bolus insulin subgroups, a small decrease with liraglutide in the premix subgroup, and a small increase with the placebo across all subgroups. Increases in pulse rate were noted with liraglutide compared with the placebo across all insulin subgroups (Table S2).
Hypoglycemia
Hypoglycemia data are summarized in Table 3. In the basal and basal–bolus subgroups, the event rate (expressed as events per 100 PYE) of confirmed hypoglycemia appeared higher at 0–36 weeks in participants randomized to liraglutide compared with those receiving the placebo. In the premix subgroup, however, the event rate was notably lower with liraglutide compared with the placebo. In the premix subgroup, the proportion of participants with confirmed hypoglycemic events was similar with liraglutide and the placebo at 0–36 weeks, but in the basal and basal–bolus subgroups, a larger proportion of participants had confirmed hypoglycemic events with liraglutide compared with the placebo (Table 3). No severe hypoglycemic events occurred during the trial. In all insulin subgroups, the occurrence of nocturnal confirmed hypoglycemic events was low with both liraglutide and the placebo.
Table 3.
Hypoglycemia analysis†
| Insulin therapy | Basal | Basal–bolus | Premix | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Add‐on therapy | Liraglutide | Placebo | Liraglutide | Placebo | Liraglutide | Placebo | ||||||||||||||||||
| Safety analysis set (n) | 50 | 50 | 27 | 28 | 50 | 52 | ||||||||||||||||||
| n | % | E | R | n | % | E | R | n | % | E | R | n | % | E | R | n | % | E | R | n | % | E | R | |
| Hypoglycemic events by classification and time (0 to 36 weeks) | ||||||||||||||||||||||||
| Confirmed | 8 | 16.0 | 19 | 57 | 4 | 8.0 | 11 | 32 | 13 | 48.1 | 52 | 290 | 9 | 32.1 | 45 | 242 | 21 | 42.0 | 52 | 157 | 23 | 44.2 | 105 | 311 |
| Nocturnal confirmed | 2 | 4.0 | 2 | 6 | 2 | 4.0 | 3 | 9 | 1 | 3.7 | 3 | 17 | 2 | 7.1 | 8 | 43 | 3 | 6.0 | 5 | 15 | 7 | 13.5 | 17 | 50 |
| Hypoglycemic events by classification and time (0 to ≤16 weeks) | ||||||||||||||||||||||||
| Confirmed | 8 | 16.0 | 18 | 119 | 3 | 6.0 | 5 | 33 | 9 | 33.3 | 24 | 300 | 5 | 17.9 | 22 | 264 | 12 | 24.0 | 18 | 120 | 11 | 21.2 | 35 | 232 |
| Nocturnal confirmed | 1 | 2.0 | 1 | 7 | 2 | 4.0 | 2 | 13 | 1 | 3.7 | 1 | 12 | 2 | 7.1 | 4 | 48 | 3 | 6.0 | 4 | 27 | 1 | 1.9 | 4 | 27 |
| Hypoglycemic events by classification and time (>16 to 36 weeks) | ||||||||||||||||||||||||
| Confirmed | 1 | 2.0 | 1 | 5 | 3 | 6.1 | 6 | 32 | 7 | 26.9 | 28 | 281 | 8 | 29.6 | 23 | 224 | 17 | 35.4 | 34 | 188 | 22 | 44.9 | 70 | 376 |
| Nocturnal confirmed | 1 | 2.0 | 1 | 5 | 1 | 2.0 | 1 | 5 | 1 | 3.8 | 2 | 20 | 1 | 3.7 | 4 | 39 | 1 | 2.1 | 1 | 6 | 7 | 14.3 | 13 | 70 |
†No severe hypoglycemia occurred during the trial. Confirmed hypoglycemia: participant unable to treat himself/herself and/or have a recorded plasma glucose <3.1 mmol/L (56 mg/dL). Nocturnal period: the period between 00.01 and 05.59 h (both included). %, Percentage of participants; E, number of events; R, event rate per 100 patient exposure years.
Within the first 16 weeks, when insulin doses were stable, a greater proportion of participants reported confirmed hypoglycemic events with liraglutide than the placebo in the basal and basal–bolus subgroups. In the premix subgroup, the proportions of participants who reported hypoglycemic events were largely similar (Table 3). During the same time‐period, the event rate for confirmed hypoglycemic events was also higher with liraglutide than with the placebo in the basal and basal–bolus subgroups, but was lower in the premix subgroup.
At >16–36 weeks, similar proportions of participants reported confirmed hypoglycemic events with liraglutide and the placebo in the basal and basal–bolus subgroups. In the premix subgroup, a lower proportion of participants randomized to liraglutide reported confirmed hypoglycemic events compared with the placebo. During the same time‐period, the confirmed hypoglycemic event rates were lower with liraglutide than the placebo in the basal and premix subgroups, but not in the basal–bolus subgroup.
AEs
The proportion of participants with AEs was generally comparable among insulin subgroups (Table S3), with AE rates per 100 PYE appearing to be slightly higher with liraglutide compared with the placebo in the basal and premix subgroups. The majority of AEs were mild, and the most frequently reported AE overall was nasopharyngitis. The proportion of participants with serious AEs and the event rates per 100 PYE appeared to be similar with liraglutide and the placebo in the basal and basal–bolus insulin subgroups, and higher with liraglutide compared with the placebo in the premix subgroup. However, the incidence of serious AEs was low overall.
The proportion of participants with gastrointestinal AEs was numerically higher in the liraglutide groups, independent of the insulin regimen. In the premix subgroup, there were two withdrawals as a result of AEs (one each in the liraglutide and placebo groups, respectively). No deaths were reported during the trial.
Discussion
The present post‐hoc analysis shows that the superior efficacy of liraglutide compared with the placebo observed in the overall cohort6 was not dependent on the type of pre‐trial insulin regimen, and that insulin‐treated Japanese patients with type 2 diabetes mellitus not achieving glycemic targets can be considered for liraglutide treatment, irrespective of their current insulin regimen.
Patients receiving liraglutide experienced greater reductions in HbA1c and mean seven‐point SMPG than those receiving the placebo across all three insulin subgroups, whereas FPG was reduced more with liraglutide in the basal and basal–bolus insulin subgroups at week 16. Furthermore, the proportions of participants achieving target HbA1c of <7.0% (<53 mmol/mol) and ≤6.5% (≤48 mmol/mol) were higher with liraglutide compared with the placebo. These findings are in line with results from other trials investigating liraglutide added to basal and basal–bolus insulin, and with previous meta‐analyses of trials investigating the addition of GLP‐1RAs to insulin8, 9, 11, 12, 13, 14. Liraglutide showed greater placebo‐adjusted HbA1c reductions when added to basal insulin than when added to basal–bolus or premix; however, no statistical significance at either week 16 or 36 was shown in the interaction tests between the treatment and insulin regimens. Consequently, there was no evidence of any apparent differences in the treatment effect of liraglutide by insulin regimen.
There was an attenuated increase from baseline in actual daily insulin dose with the addition of liraglutide regimens after 36 weeks of treatment. This is supported by previous studies that explored liraglutide as an add‐on therapy to insulin8, 9, 14, 15, 16. Furthermore, the reduction in prandial glucose increment was observed to be larger with liraglutide than the placebo in the basal insulin subgroup at 16 weeks (when the insulin dose was capped), and the same trend was observed for the basal–bolus and premix subgroups. The difference between the liraglutide and placebo arms was, however, diminished by week 36 (after insulin titration was allowed). As prandial insulin therapy aims to reduce upward prandial glucose fluctuation, it could be anticipated that liraglutide used as an add‐on therapy would have less of an effect on this parameter when prandial insulins are used. Liraglutide can, however, perform a similar role in enhancing postprandial glucose reduction when added to a basal‐only insulin regimen.
When insulin dose was stable, in weeks 0 to ≤16, the mean prandial glucose increment was still reduced with liraglutide relative to the placebo, by 36.3 and 19.6 mg/dL for the basal–bolus and premix subgroups, respectively, although this benefit was greater in the basal‐only insulin subgroup than in the basal–bolus or premix subgroups. These data are supported by Ogawa et al.13, who observed reduced postprandial glucose and prandial insulin dose when liraglutide was added to basal–bolus therapy, and by Lind et al.14, who observed reduced postprandial glucose and total insulin dose. In this regard, daily prandial and premix insulin doses could potentially also be reduced in a real‐world setting.
Individuals with type 2 diabetes mellitus often do not reach glycemic targets, because they might be reluctant to intensify insulin therapy as a result of concerns over bodyweight gain17. In previous trials investigating liraglutide in combination with insulin therapy, weight loss was observed8, 9, 11, 12, 14. However, in the LIRA‐ADD2INSULIN JAPAN trial, very limited changes in bodyweight were observed with either liraglutide or placebo across all insulin subgroups, despite higher insulin doses with placebo in all subgroups. The difference in glycemic control could imply under‐dosing of insulin by the study participants, perhaps as a result of fear of hypoglycemia or weight gain.
The reductions in systolic blood pressure and increases in pulse rate that were observed with liraglutide in the present post‐hoc analysis have also been observed previously18. In the current analysis, this effect did not appear to differ across insulin regimens. Increases in resting heart rate have been reported with GLP‐1RAs, and although the underlying physiological mechanisms have not yet been defined, the activation of the GLP‐1 receptors in the sinoatrial node could play a role19. Although an increase in heart rate was also seen in the recently reported Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial20, lower rates of cardiovascular events and death from any cause were confirmed with liraglutide compared with the placebo when added to the standard of care.
Gastrointestinal AEs were more common with liraglutide, and overall rates of AEs were also slightly higher, regardless of the insulin regimen used. From baseline to week 16, where the insulin dose was kept stable, a larger proportion of participants reported confirmed hypoglycemic events with liraglutide than the placebo in the basal and basal–bolus subgroups. From >16 to 36 weeks, when insulin titration was allowed, similar proportions of participants reported confirmed hypoglycemic events with liraglutide and the placebo in the basal and basal–bolus subgroups. In the same time‐period, a slightly lower proportion of participants reported confirmed hypoglycemia with liraglutide compared with the placebo in the premix subgroup. This observation might suggest that liraglutide could be added to any insulin regimen without an increased risk of hypoglycemia provided attention is also given to insulin dose adjustment.
In the period of 0–16 weeks, hypoglycemic event rates were similar with liraglutide treatment between the basal and premix subgroups. Overall, however, hypoglycemic event rates were much lower in the basal insulin subgroup (both liraglutide and placebo) than in the basal–bolus and premix subgroups, despite the greater efficacy shown in the basal subgroup with liraglutide. This supports the notion that prandial insulin is responsible for the majority of hypoglycemic events, and that liraglutide can be used as an alternative to prandial insulins.
The limitations of the present study include the fact that the data might not be reflective of routine clinical practice, and the original trial was not powered to perform this post‐hoc analysis in which the comparator groups are small. There were also differences in baseline characteristics; such variation would be expected as a result of inherent distinctions between participants being empirically selected for dissimilar insulin regimens rather than being randomized. There is also likely to have been variation in the efficacy and safety profiles of the different insulin regimens, which might have confounded the results. Nevertheless, these limitations do not invalidate the conclusion that liraglutide can be added to all three insulin regimens with the expectation of achieving clinical benefits.
In summary, the present post‐hoc analysis confirmed the efficacy and safety of adding liraglutide to ongoing insulin therapy in Japanese patients with type 2 diabetes mellitus, regardless of the insulin regimen used. The analysis also suggested there was no difference in the treatment effect of liraglutide across insulin regimens.
Disclosure
K Nishijima is a Novo Nordisk Pharma Ltd employee. H Bosch‐Traberg is a Novo Nordisk A/S employee. Y Seino is a medical advisor to Astellas, BD, Boehringer Ingelheim, Eli Lilly, GSK, Johnson & Johnson, Novo Nordisk, Otsuka, Sanofi, Taisho Pharmaceutical Co., Ltd and Takeda. S Kaneko has conflicts of interest in respect to Takeda, Lilly, Novartis, Novo Nordisk, Astellas, Boehringer Ingelheim, AstraZeneca, Kowa, Mitsubishi Tanabe Pharma, Sanofi, Daiichi Sankyo, Sumitomo Dainippon Pharma and Ono. K Kaku is an advisor to Novo Nordisk, Sanwa Kagaku Kogyo, Takeda and Taisho Pharmaceutical Co., Ltd; received honoraria for lectures from MSD, Kowa, Sumitomo Dainippon Pharma, Novartis, Novo Nordisk, Takeda and Mitsubishi Tanabe Pharma; and received scholarship grants from AstraZeneca, Nippon Boehringer Ingelheim Co., Ltd, Chugai, Daiichi Sankyo, MSD, Novartis, Novo Nordisk, Sanofi and Takeda.
Supporting information
Table S1 | Observed proportions of participants achieving glycated hemoglobin ≤6.5% (≤48 mmol/mol) at weeks 16 and 36, and associated composite end‐points.
Table S2 | Mean changes in vital signs from baseline to week 36.
Table S3 | Adverse events.
Figure S1 | Participant disposition.
Figure S2 | Mean seven‐point self‐measured plasma glucose profiles at baseline and at 16 and 36 weeks for participants randomized to (a,c,e) liraglutide or (b,d,f) placebo in addition to (a,b) basal insulin, (c,d) basal–bolus insulin or (e,f) premix insulin therapy.
Acknowledgments
This post‐hoc analysis and the LIRA‐ADD2INSULIN JAPAN trial (ClinicalTrials.gov [NCT01572740]; Japanese Clinical Trials Registry [JapicCTI‐121802]) were sponsored by Novo Nordisk. The authors thank Michael Skjødt and Rubdeep Bindra (Novo Nordisk) for their review and input to the manuscript. We also thank Nathan Ley and Erin Slobodian of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, for writing and editorial assistance, funded by Novo Nordisk.
J Diabetes Investig 2018;9:840–849
References
- 1. International Diabetes Federation . IDF Diabetes, 7th edn Brussels, Belgium: International Diabetes Federation; Available from: http://www.diabetesatlas.org/, 2015. Accessed March 1, 2016. [Google Scholar]
- 2. Neville SE, Boye KS, Montgomery WS, et al Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009; 25: 705–716. [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. Japanese Ministry of Health, Labor and Welfare . Guideline for Clinical Evaluation of Oral Hypoglycemic Agents. Available from: https://www.pmda.go.jp/files/000208194.pdf, 2010. Accessed February 1, 2016.
- 5. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577–1596. [DOI] [PubMed] [Google Scholar]
- 6. Seino Y, Kaneko S, Fukuda S, et al Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Investig 2016; 7: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eng C, Kramer CK, Zinman B, et al Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet 2014; 384: 2228–2234. [DOI] [PubMed] [Google Scholar]
- 8. Ahmann A, Rodbard HW, Rosenstock J, et al Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab 2015; 17: 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathieu C, Rodbard HW, Cariou B, et al A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD–ON). Diabetes Obes Metab 2014; 16: 636–644. [DOI] [PubMed] [Google Scholar]
- 10. Kim YG, Hahn S, Oh TJ, et al Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 900–909. [DOI] [PubMed] [Google Scholar]
- 11. Berlie H, Hurren KM, Pinelli NR. Glucagon‐like peptide‐1 receptor agonists as add‐on therapy to basal insulin in patients with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes 2012; 5: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lane W, Weinrib S, Rappaport J, et al The effect of addition of liraglutide to high‐dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab 2014; 16: 827–832. [DOI] [PubMed] [Google Scholar]
- 13. Ogawa S, Nako K, Okamura M, et al Stabilization of postprandial blood glucose fluctuations by addition of glucagon like polypeptide‐analog administration to intensive insulin therapy. J Diabetes Investig 2015; 6: 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lind M, Hirsch IB, Tuomilehto J, et al Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI Liraglutide trial). BMJ 2015; 351: h5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li CJ, Li J, Zhang QM, et al Efficacy and safety comparison between liraglutide as add‐on therapy to insulin and insulin dose‐increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol 2012; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lind M, Jendle J, Torffvit O, et al Glucagon‐like peptide 1 (GLP‐1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes 2012; 6: 41–46. [DOI] [PubMed] [Google Scholar]
- 17. Davies M. The reality of glycaemic control in insulin treated diabetes: defining the clinical challenges. Int J Obes Relat Metab Disord 2004; 28(Suppl 2): S14–S22. [DOI] [PubMed] [Google Scholar]
- 18. Fonseca VA, Devries JH, Henry RR, et al Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient‐level pooled analysis of six randomized clinical trials. J Diabetes Complications 2014; 28: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pyke C, Heller RS, Kirk RK, et al GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014; 155: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 20. Marso SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and cardiovascular outcomes in Type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Observed proportions of participants achieving glycated hemoglobin ≤6.5% (≤48 mmol/mol) at weeks 16 and 36, and associated composite end‐points.
Table S2 | Mean changes in vital signs from baseline to week 36.
Table S3 | Adverse events.
Figure S1 | Participant disposition.
Figure S2 | Mean seven‐point self‐measured plasma glucose profiles at baseline and at 16 and 36 weeks for participants randomized to (a,c,e) liraglutide or (b,d,f) placebo in addition to (a,b) basal insulin, (c,d) basal–bolus insulin or (e,f) premix insulin therapy.


