Abstract
Aims/Introduction
Member B of the family with sequence similarity 3 (FAM3B), also known as pancreatic‐derived factor, is mainly synthesized and secreted by islet β‐cells, and plays a role in abnormal metabolism of glucose and lipids. However, the prospective association of FAM3B with metabolic disorders remains unclear. The present study aimed to reveal the predictive relationship between pancreas‐specific cytokine and metabolic syndrome (MetS).
Materials and Methods
A total of 210 adults (88 men and 122 women) without MetS, aged between 40 and 65 years, were recruited and received a comprehensive health examination. Baseline serum FAM3B levels were determined by sandwich enzyme‐linked immunosorbent assay. Subsequently, all participants underwent a follow‐up examination after 5 years. MetS was identified in accordance with the International Diabetes Federation criteria.
Results
During follow up, 35.7% participants developed MetS. In comparison with the non‐MetS group, participants with MetS had an increased serum FAM3B at baseline (21.85 ng/mL [19.38, 24.17 ng/mL] vs 28.56 ng/mL [25.32, 38.10 ng/mL], P < 0.001). Moreover, serum FAM3B was significantly associated with variations in fasting plasma insulin (r = −0.306, P < 0.001), homeostasis model assessment of β‐cell function (r = −0.328, P < 0.001) and homeostasis model assessment of insulin resistance (r = −0.191, P = 0.006). Furthermore, a positive correlation between baseline FAM3B and the incidence of MetS was observed, even after multivariable adjustment (relative risk 1.23 [1.15, 1.31], P < 0.001). Furthermore, the optimal cut‐off values of FAM3B was 23.98 ng/mL for predicting MetS based on the Youden Index.
Conclusions
Elevated circulating FAM3B might be considered as a predictor of newly‐onset MetS and its progression.
Keywords: Member B of the family with sequence similarity 3, Metabolic syndrome, Prospective study
Introduction
Metabolic syndrome (MetS) is a prevalent condition affecting nearly 35% of all adults1. It is defined as a cluster of central obesity, dyslipidemia, hypertension and hyperglycemia. Accumulating evidence has reported the association of MetS with higher risks of cardiovascular morbidity and mortality2, 3. Increasing concerns over MetS and its health consequences have resulted in wide and sustained studies. However, it is difficult to identify a single etiology, as MetS is a result of an interplay between multiple factors. Previously, several studies have confirmed that some cytokines, such as leptin4, adiponectin5, irisin6 and betatrophin7, could play a vital role in the development of MetS. Thus, the study of novel metabolism‐related cytokines could contribute to understanding the mechanisms of metabolic diseases and provide potential therapeutic targets8.
In 2002, the family with sequence similarity 3 (FAM3) gene family was first identified and cloned by Zhu et al.9 Furthermore, the member B of FAM3 (FAM3B) has been proved to be synthesized and secreted by pancreatic islets specifically10, and FAM3B thereby is known as a pancreatic‐derived factor. Growing studies have confirmed that FAM3B has an effect on islet function by inducing apoptosis of insulin‐secreting β‐cells11 and insulin resistance of peripheral tissue12. This evidence suggests that circulating FAM3B could be considered as a regulator of glucose and lipid metabolism. Recently, a cross‐sectional study by Cao et al.13 reported that the serum FAM3B level is associated with MetS in humans. However, the sequence between circulating FAM3B and MetS remains uncertain.
Therefore, the present prospective study was carried out to identify the ability of FAM3B in predicting MetS, based on the hypothesis that serum FAM3B might be associated with higher risks of incident MetS and its components.
Materials and methods
Study participants
In June 2010, a community‐based cohort of 750 individuals aged 40–65 years was established in the urban area of Shenyang, China. After 5 years, in 2015, the population was followed up. According to the baseline characteristics, individuals who met the following criteria were excluded: (i) the presence of metabolic syndrome at baseline; (ii) a history of chronic hepatic and renal diseases, or any previous malignancy; (iii) being pregnant; (iv) a history of drug or surgery for obesity, diabetes mellitus, dyslipidemia or hypertension at baseline; (v) lack of primary data; and (vi) missing communication or unwillingness to participate in the follow up. Finally, 210 individuals (88 men and 122 women) were included for the present study, and average follow‐up duration was 5 years (Figure 1).
Figure 1.
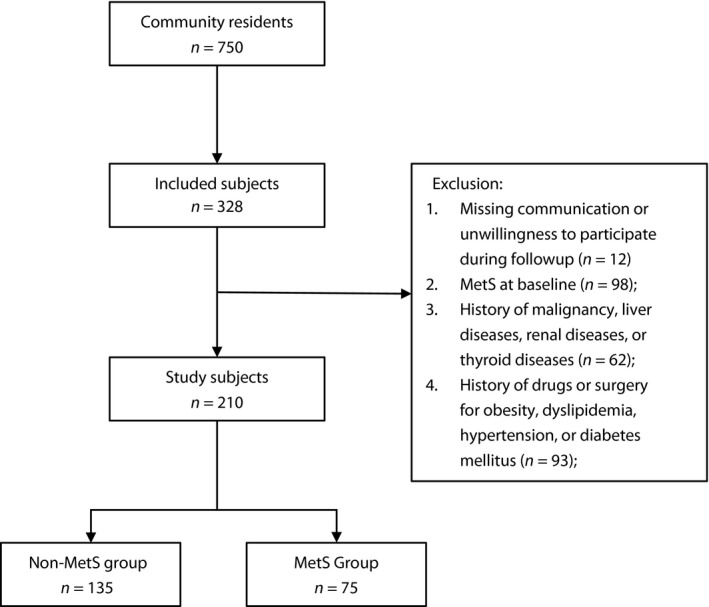
Flow graph of recruitment. MetS, metabolic syndrome.
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of China Medical University, and was carried out in accordance with related guidelines and regulations. Written consent was provided by all the participants from the cohort.
Data collection
At baseline and end‐point, all the individuals underwent comprehensive medical examinations. A standardized questionnaire, including demographic information, lifestyle characteristics, health status and medical history, was completed by each participant with trained staff. Measurements of height, weight, waist circumference (WC) and blood pressure were also carried out. Body mass index is equal to the weight (kg) divided by the square of height (m2).
After an overnight fast, all the participants underwent a 75‐g oral glucose tolerance test. Fasting plasma glucose (FPG), 2‐h plasma glucose, fasting plasma insulin (FINS), glycated hemoglobin, blood urea nitrogen, serum creatinine (Cr), uric acid, alanine aminotransferase, aspartate aminotransferase (AST) and gamma glutamyl transpeptidase (GGT), as well as lipid profile including triglyceride (TG), total cholesterol, high‐density lipoprotein cholesterol (HDL‐c) and low‐density lipoprotein cholesterol were examined in the center laboratory of the First Affiliated Hospital of China Medical University. Homeostasis model assessment of insulin resistance (HOMA‐IR) was estimated as FINS (IU/mL) × FPG (mmol/L) / 22.5, and homeostasis model assessment of β‐cell function (HOMA‐β) was evaluated as FINS (IU/mL) × 20 / (FPG [mmol/L] − 3.5).
Measurement of FAM3B
After centrifugation (1,500 g for 15 min at 4°C), the supernatant of the fasting blood sample was preserved under −80°C until FAM3B analysis. The baseline serum levels of FAM3B in the fasting serum were assessed using a sandwich enzyme‐linked immunosorbent assay (FAM3B Quantitative ELISA kit; Cloud‐Clone Corp., Katy, Texas, USA).
MetS definition
Based on the International Diabetes Federation criteria 200914 and Chinese‐specific abdominal obesity standard15 in adults, participants who met at least three of the following were diagnosed as MetS: (i) central obesity, namely a WC ≥ 90 cm for men or ≥85 cm for women; (ii) high TG, namely serum TG ≥ 1.70 mmol/L; (iii) low HDL‐c, namely serum HDL‐c < 1.0 mmol/L for men and <1.3 mmol/L for women; (iv) hypertension, namely systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg, or a history of hypertension; and (v) hyperglycemia, namely FPG ≥5.6 mmol/L, or a diagnosis of type 2 diabetes mellitus.
Statistical analysis
At baseline, all the participants included were free from MetS. They were then divided into the newly‐MetS group and non‐MetS group according to whether MetS was developing or not during follow up. The data were summarized as the mean ± standard deviation, medians (interquartile range) or counts (percentages) based on the different variable types. For estimating the relative elements of MetS and its components, univariate analyses were carried out by t‐test, Mann–Whitney U‐test or χ2‐test depending on the data characteristics. Variation in the number of MetS components was defined as the number of MetS components at the end subtracted by that at baseline. Partial correlation analysis was carried out to assess the association between variations in metabolic risk parameters and baseline FAM3B levels, with adjustment for age and sex. Logistic regression was also carried out to analyze the relative risks for the incident metabolic syndrome and each component after adjustment for multiple variables. Receiver operating characteristic (ROC) curve analyses were used to determine the appropriate cut‐off of FAM3B in predicting incident MetS, and the optimal value was obtained according to the Youden Index. All the statistical analyses were carried out with SPSS (version 22.0; SPSS Inc., Chicago, Illinois, USA), and two‐sided P‐values <0.05 were considered significant.
Results
Baseline characteristics of study individuals
Table 1 shows the baseline characteristics of the cohort by occurrence of MetS during follow up. After 5‐years follow up, a proportion of 35.7% of the participants developed MetS, and 41 of them were men (54.7% of all MetS). Compared with the non‐MetS group, baseline body mass index, WC, FINS, HOMA‐IR, TG, low‐density lipoprotein cholesterol, systolic blood pressure, diastolic blood pressure, Cr, uric acid, alanine aminotransferase, AST and GGT in the newly‐MetS group were significantly increased (all P < 0.05), whereas the percentage of men, HDL and the smoking rate were decreased significantly (all P < 0.05). Significantly, participants who developed MetS had higher serum FAM3B levels at baseline (21.85 [19.38, 24.17] vs 28.56 [25.32,38.10] ng/mL, P < 0.001). However, no significant difference was observed in the other variables.
Table 1.
Baseline characteristics
| Non‐MetS group n = 135 | Newly‐MetS group n = 75 | P | |
|---|---|---|---|
| Male, n (%) | 47 (34.8) | 41 (54.7) | 0.005 |
| Age (years) | 49.52 ± 6.6 | 50.9 ± 6.4 | 0.140 |
| BMI (kg/m2) | 23.2 ± 2.8 | 25.7 ± 2.5 | <0.001 |
| WC (cm) | 80.2 ± 8.3 | 87.9 ± 7.5 | <0.001 |
| FPG (mmol/L) | 5.2 (4.9–5.7) | 5.3 (5.1–5.6) | 0.084 |
| 2hPG (mmol/L) | 6.7 (5.7–7.8) | 7.1 (6.0–8.7) | 0.061 |
| FINS (IU/L) | 14.2 (11.1–18.5) | 15.9 (12.5–21.4) | 0.014 |
| HOMA‐β | 165.00 (110.31–213.90) | 161.56 (128.55–243.56) | 0.197 |
| HOMA‐IR | 3.48 (2.53–4.59) | 3.91 (3.09–5.16) | 0.017 |
| HbA1c (%) | 5.8 (5.4–6.1) | 6.0 (5.5–6.2) | 0.334 |
| TC (mmol/L) | 4.9 (4.3–5.5) | 5.2 (4.5–5.8) | 0.098 |
| TG (mmol/L) | 1.2 (0.9–1.5) | 1.5 (1.1–1.9) | <0.001 |
| HDL (mmol/L) | 1.5 (1.3–1.7) | 1.3 (1.1–1.5) | 0.003 |
| LDL (mmol/L) | 3.0 (2.5–3.6) | 3.3 (2.7– 3.9) | 0.009 |
| SBP (mmHg) | 117 ± 13 | 124 ± 11 | <0.001 |
| DBP (mmHg) | 75 ± 8 | 80 ± 8 | <0.001 |
| HR (/min) | 79 ± 9 | 78 ± 10 | 0.840 |
| BUN (mmol/L) | 5.16 ± 1.25 | 5.39 ± 1.38 | 0.232 |
| Cr (μmol/L) | 59.96 ± 11.89 | 66.12 ± 14.31 | 0.002 |
| UA (μmol/L) | 271 ± 81 | 332 ± 79 | <0.001 |
| ALT (IU/L) | 16 (11–20) | 22 (15–32) | <0.001 |
| AST (IU/L) | 19 (17–22) | 23 (18–28) | <0.001 |
| GGT (IU/L) | 18 (14–25) | 28 (19–57) | <0.001 |
| Smoke, n (%) | 33 (24.4) | 31 (41.3) | 0.011 |
| Drink, n (%) | 20 (14.8) | 14 (18.7) | 0.468 |
| FAM3B (ng/mL) | 21.85 (19.38–24.17) | 28.56 (25.32–38.10) | <0.001 |
Data presented as mean ± standard deviation or median (quartile), unless otherwise stated. 2hPG, 2‐h plasma glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; Cr, serum creatinine; DBP, diastolic blood pressure; FAM3B, member B of the family with sequence similarity 3; FINS, fasting plasma insulin; FPG, fasting plasma glucose; GGT, gamma glutamyl transpeptidase; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein cholesterol; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; HR, heart rate; LDL, low‐density lipoprotein cholesterol; MetS, metabolic syndrome; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Furthermore, all the participants were stratified for the variation in number of MetS components between the baseline and end. In Figure 2, the median serum FAM3B concentration was 18.39, 21.84, 24.31, 27.53 and 35.29 ng/mL for participants whose variation numbers were <0, 0, 1, 2 and ≥3, respectively. Serum FAM3B levels were raised with an increasing number of MetS components variation (P for trend <0.001).
Figure 2.
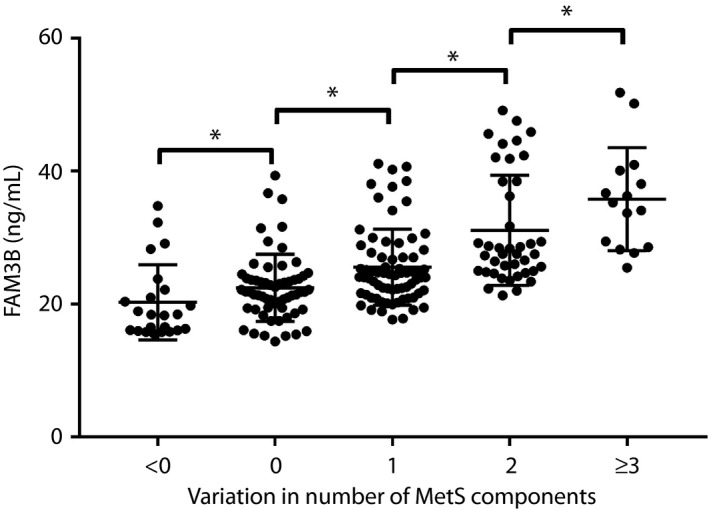
Association of member B of the family with sequence similarity 3 (FAM3B) with variation in the number of metabolic syndrome (MetS) components, *P < 0.05.
Variations in metabolic parameters and their correlation with baseline FAM3B
Table 2 presents the variations in metabolic risk parameters and the association of them with baseline FAM3B levels. Compared with the baseline, variations in body mass index (0.8 kg/m2 [−0.1, 1.8 kg/m2]), WC (3.0 cm [−1.0, −6.5 cm]), systolic blood pressure (10 mmHg [1, 18 mmHg]), diastolic blood pressure (8 mmHg [1, 15 mmHg]), FPG (0.4¸ mmol/L [0.1, 0.7 mmol/L]), HDL (0.1 mmol/L [−0.1, 0.2 mmol/L]), low‐density lipoprotein cholesterol (0.2 mmol/L [−0.2, 0.6 mmol/L]) and Cr (4.42 μmol/L [0.30, 8.88 μmol/L]) were significantly increased (all P < 0.05) after 5‐years follow up, whereas that in FINS (−6.1 IU/L [−10.2, −1.9 IU/L]), HOMA‐β (−87.76 [−132.38, −37.55]), HOMA‐IR (−1.33 [−2.42, −0.16]), glycated hemoglobin (−0.2% [−0.5, −0.1%]), AST (−2 IU/L [−4, 2 IU/L]) and GGT (‐1 IU/L [−7, 3 IU/L]) were decreased significantly (all P < 0.05).
Table 2.
Partial correlation analysis between member B of the family with sequence similarity 3 and selected variables
| Variables | Variation | Partial correlation analysisa | |
|---|---|---|---|
| r | P | ||
| BMI (kg/m2) | 0.8 (−0.1, 1.8) | 0.064 | 0.356 |
| WC (cm) | 3.0 (−1.0, 6.5) | 0.106 | 0.128 |
| SBP (mmHg) | 10 (1, 18) | 0.064 | 0.359 |
| DBP (mmHg) | 8 (1, 15) | 0.087 | 0.209 |
| FPG (mmol/L) | 0.4 (0.1, 0.7) | 0.063 | 0.367 |
| 2hPG (mmol/L) | −0.1 (−1.3, 1.4) | 0.018 | 0.801 |
| FINS (IU/L) | −6.1 (−10.2, −1.9) | −0.306 | <0.001 |
| HOMA‐β | −87.76 (−132.38, −37.55) | −0.328 | <0.001 |
| HOMA‐IR | −1.33 (−2.42, −0.16) | −0.191 | 0.006 |
| HbA1c (%) | −0.2 (−0.5, 0.1) | 0.017 | 0.804 |
| TC (mmol/L) | −0.1 (−0.6, 0.4) | −0.050 | 0.470 |
| TG (mmol/L) | −0.1 (−0.3, 0.2) | −0.084 | 0.228 |
| HDL (mmol/L) | 0.1 (−0.1, 0.2) | −0.126 | 0.070 |
| LDL (mmol/L) | 0.2 (−0.2, 0.6) | −0.025 | 0.722 |
| UA (μmol/L) | 0 (−27, 41) | 0.037 | 0.599 |
| Cr (μmol/L) | 4.42 (0.30, 8.88) | −0.079 | 0.256 |
| BUN (mmol/L) | −0.20 (−0.94, 0.66) | 0.020 | 0.774 |
| ALT (IU/L) | 0 (−5, 4) | −0.036 | 0.609 |
| AST (IU/L) | −2 (−4, 2) | −0.089 | 0.200 |
| GGT (IU/L) | −1 (−7, 3) | 0.003 | 0.969 |
Adjustment for age and sex. 2hPG, 2‐h plasma glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; Cr, serum creatinine; DBP, diastolic blood pressure; FAM3B, member B of the family with sequence similarity 3; FINS, fasting plasma insulin; GGT, gamma glutamyl transpeptidase; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein cholesterol; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Furthermore, a partial correlation analysis between variations in metabolic risk parameters and FAM3B was carried out, with adjustment for age and sex. These results showed that variations in FINS (r = −0.306, P < 0.001), HOMA‐β (r = −0.328, P < 0.001) and HOMA‐IR (r = −0.191, P = 0.006) were negatively correlated with baseline FAM3B.
Association of serum FAM3B level with the incident risk of MetS and its components
As shown in Table 3, the incidences of MetS, central obesity, high TG, low HDL, hypertension and hyperglycemia were 35.7%, 32.3%, 14.6%, 4.5%, 51.5% and 48.0%, respectively. After multivariable adjustment for age, sex, Cr, uric acid, alanine aminotransferase, AST, GGT, current smoke and alcohol intake, the significant association of FAM3B with incident risks of MetS and its components remained.
Table 3.
Relative risk for metabolic syndrome and its components according to baseline serum member B of the family with sequence similarity 3 level
| Incident, n (%) | Model 1, RR (95% CI) | Model 2, RR (95% CI) | Model 3, RR (95% CI) | |
|---|---|---|---|---|
| MetS | 75 (35.7) | 1.24** (1.16–1.32) | 1.23** (1.15–1.31) | 1.23** (1.15–1.31) |
| Central obesity | 49 (32.3) | 1.14** (1.08–1.21) | 1.14** (1.08–1.21) | 1.15** (1.08–1.21) |
| High TG | 24 (14.6) | 1.18** (1.10–1.26) | 1.20** (1.11–1.29) | 1.23** (1.13–1.35) |
| Low HDL | 8 (4.5) | 1.19** (1.09–1.30) | 1.19** (1.09–1.31) | 1.25** (1.09–1.44) |
| Hypertension | 84 (51.5) | 1.07** (1.02–1.11) | 1.06* (1.01–1.11) | 1.06* (1.01–1.11) |
| Hyperglycemia | 71 (48.0) | 1.08** (1.03–1.13) | 1.07** (1.02–1.12) | 1.07** (1.02–1.12) |
Model 1, adjusted no variable. Model 2, adjusted for age and sex. Model 3, adjusted for model 2 plus serum creatinine, uric acid, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transpeptidase, current smoke and alcohol intake. *P < 0.05; **P < 0.01. CI, confidence interval; HDL, high‐density lipoprotein cholesterol; MetS, metabolic syndrome; RR, relative risk; TG, triglyceride.
ROC analysis for FAM3B in predicting risk of MetS and its components
The area under the ROC curve (AUC) was used to estimate the ability of FAM3B in predicting MetS risk.
As shown in Figure 3, FAM3B showed a significance in predicting MetS (AUC 0.816 [0.757, 0.866], P < 0.001). Moreover, AUCs of FAM3B and WC were compared. It was found that FAM3B has a higher AUC than WC in predicting MetS, though the difference was not significant (0.816 [0.757, 0.866] vs 0.778 [0.715, 0.832], P = 0.393). Furthermore, AUCs for FAM3B in predicting central obesity, high TG, low HDL, hypertension and hyperglycemia were 0.742 (0.665, 0.810; P < 0.001), 0.658 (0.580, 0.730; P = 0.027), 0.704 [0.631, 0.770; P = 0.111), 0.615 (0.536, 0.690; P = 0.010) and 0.635 (0.552, 0.712; P = 0.003), respectively.
Figure 3.

Receiver operating characteristic (ROC) analysis of member B of the family with sequence similarity 3 (FAM3B) for predicting metabolic syndrome (MetS). AUC, area under the receiver operating characteristic curve.
In addition, the Youden Index was calculated, indicating that the optimal cut‐offs of FAM3B for predicting MetS, central obesity, high TG, low HDL, hypertension and hyperglycemia were 23.98, 24.96, 25.32, 27.50, 21.39 and 23.87 ng/mL, respectively.
Discussion
The findings of the present prospective study of a community‐based cohort showed that FAM3B is an independent predictor of MetS and its components. The number of MetS components also showed an increasing trend as the circulating FAM3B levels raised, demonstrating that FAM3B is involved in the development of MetS.
It has been reported that FAM3B is secreted by β‐cells along with insulin16. However, the present prospective results showed that increased FAM3B levels at baseline could predict significant reductions of FINS and HOMA‐β during follow up. It is probably because FAM3B, both full‐length and recombinant, can also induce islet β‐cell apoptosis by upregulating caspase‐310, 11, 17, 18. Coincidentally, a cross‐sectional study has proved that FAM3B is negatively associated with FINS in humans13. Furthermore, compared with new‐onset diabetes and healthy controls, circulating FAM3B levels are significantly increased in individuals with long‐standing diabetes mellitus19. These findings suggest that FAM3B might be related to the severity of β‐cell dysfunction. Another possible reason is that the liver could be an additional source of FAM3B based on a recent study20. Thus, increased FAM3B levels might be attributed to β‐cells, the liver or both. In brief, there is sufficient evidence that FAM3B plays a vital role in β‐cell dysfunction and impaired glucose metabolism.
Subsequently, the present data have shown a negative correlation of FAM3B and long‐term variation in HOMA‐IR. One of the reasons might be that severe islet cell dysfunction leads to a significant reduction of FINS, which in turn reduces the value of HOMA‐IR. In fact, we have analyzed the correlation between FAM3B and variation in HOMA‐IR after adjustment for baseline age, sex and follow‐up FINS, but no significant association has been observed (r = −0.045, P = 0.517). Meanwhile, the negative correlation between FAM3B and variation in HOMA‐β remained (r = −0.253, P < 0.001).
As previously shown, FAM3B secretion from islets can be stimulated by glucose and insulin, hence it has been recognized as a regulator of metabolic homeostasis. A recent study has fully proved the involvement of FAM3B in the potential progression of type 2 diabetes mellitus21. However, the association of circulating FAM3B with other metabolic disorders is still unknown. In the present study, we observed a significant association of baseline FAM3B levels with all the incident MetS components after multivariable adjustment. Indeed, plasma FAM3B was found to be associated with dyslipidemia in mice12. Similar results were also reported in humans (high TG and low HDL)13. Increased activity of forkhead box protein O1 and hepatic lipase secondary to hyperglycemia might be possible reasons for hepatic increased TG synthesis and degraded HDL22, but the precise mechanism has not yet been clearly elucidated. In addition, it was also reported that apoptosis of SH‐HY5Y, as a neuroblastoma cell line, can be induced by FAM3B, indicating its role in diabetic neuropathy23. In brief, there might be more complicated interactions between FAM3B and metabolic diseases.
Furthermore, in ROC analysis, we confirmed the ability of FAM3B to predict incident MetS and most of its components. We compared the abilities of FAM3B and WC in predicting MetS, and no significant difference was observed. As WC has been considered as an effective and widely used index24, it is suggested that FAM3B has a similar ability as WC in predicting MetS. However, FAM3B has no significant predictive significance for low HDL. This might be because there were fewer patients that suffered from low HDL during the follow up. Accordingly, a study with a larger sample size and longer follow‐up period is required. Overall, the present study has shown that FAM3B has a comprehensive assessment of the risk of metabolic diseases in individuals, and FAM3B could thereby be recognized as a promising biomarker and therapeutic target of MetS.
Several limitations of the present study should be considered. First, this study was confined to middle‐aged and elderly urban residents, and might not be generalized for other populations. Second, we evaluated the serum levels of FAM3B at baseline only, so that its dynamic changes should be assessed in further studies. Furthermore, due to the methods and participants of the present study, we could not confirm if there was any liver‐derived FAM3B involved, though it is generally believed that FAM3B is mainly secreted by β‐cells. Additionally, although the present study identified the involvement of FAM3B in the development of MetS, it remains to be observed whether the targeted intervention can prevent, or even ameliorate, MetS and its components.
In conclusion, the present findings showed that serum FAM3B level is closely associated with long‐term MetS risk and islet β‐cells dysfunction in the population without MetS. Furthermore, FAM3B ≥ 23.98 ng/mL was the optimal cut‐off to identify the increased risk of incident MetS.
Disclosure
The author declares no conflict of interest.
Acknowledgments
We especially thank the patients and staff at the Department of Endocrinology of the First Affiliated Hospital of China Medical University for their support. This work was supported by funding from the Chinese National Natural Science Foundation (Grant: 81300645), the National Science and Technology Support Program (Grant: 2009BAI80B00), and Important Platform of Science and Technology for the Universities in Liaoning Province (Grant: 16010). The funders had no role in study design, data collection or analysis, or in the presentation or publication of the results.
J Diabetes Investig 2018;9:782–788
References
- 1. Aguilar M, Bhuket T, Torres S, et al Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015; 313: 1973. [DOI] [PubMed] [Google Scholar]
- 2. Cornier M‐A, Dabelea D, Hernandez TL, et al The metabolic syndrome. Endocr Rev 2008; 29: 777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014; 2014: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Pérez‐Pérez A, Vilariño‐García T, Fernández‐Riejos P, et al Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev 2017; 35: 71–84. [DOI] [PubMed] [Google Scholar]
- 5. Song Y‐M, Lee K, Sung J. Adiponectin levels and longitudinal changes in metabolic syndrome: the healthy twin study. Metab Syndr Relat Disord 2015; 13: 312–318. [DOI] [PubMed] [Google Scholar]
- 6. Hee Park K, Zaichenko L, Brinkoetter M, et al Circulating Irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 2013; 98: 4899–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Lai Y, Han C, et al The effects of serum ANGPTL8/betatrophin on the risk of developing the metabolic syndrome – A prospective study. Sci Rep 2016; 6: 28431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mechanick JI, Zhao S, Garvey WT. The adipokine‐cardiovascular‐lifestyle network. J Am Coll Cardiol 2016; 68: 1785–1803. [DOI] [PubMed] [Google Scholar]
- 9. Zhu Y, Xu G, Patel A, et al Cloning, expression, and initial characterization of a novel cytokine‐like gene family. Genomics 2002; 80: 144–150. [DOI] [PubMed] [Google Scholar]
- 10. Cao X, Gao Z, Robert CE, et al Pancreatic‐derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin‐secreting‐cells. Diabetes 2003; 52: 2296–2303. [DOI] [PubMed] [Google Scholar]
- 11. Yang J, Gao Z, Robert CE, et al Structure–function studies of PANDER, an Islet specific cytokine inducing cell death of insulin‐secreting β cells† . Biochemistry 2005; 44: 11342–11352. [DOI] [PubMed] [Google Scholar]
- 12. Li J, Chi Y, Wang C, et al Pancreatic‐derived factor promotes lipogenesis in the mouse liver: role of the Forkhead box 1 signaling pathway. Hepatology 2011; 53: 1906–1916. [DOI] [PubMed] [Google Scholar]
- 13. Cao X, Yang C, Lai F, et al Elevated circulating level of a cytokine, pancreatic‐derived factor, is associated with metabolic syndrome components in a Chinese population. J Diabetes Investig 2016; 7: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alberti KGMM, Eckel RH, Grundy SM, et al Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 15. Bao Y, Lu J, Wang C, et al Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis 2008; 201: 378–384. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Robert CE, Burkhardt BR, et al Mechanisms of glucose‐induced secretion of pancreatic‐derived factor (PANDER or FAM3B) in pancreatic ‐cells. Diabetes 2005; 54: 3217–3228. [DOI] [PubMed] [Google Scholar]
- 17. Burkhardt BR, Greene SR, White P, et al PANDER‐induced cell‐death genetic networks in islets reveal central role for caspase‐3 and cyclin‐dependent kinase inhibitor 1A (p21). Gene 2006; 369: 134–141. [DOI] [PubMed] [Google Scholar]
- 18. Cao X. Effects of overexpression of pancreatic derived factor (FAM3B) in isolated mouse islets and insulin‐secreting TC3 cells. AJP Endocrinol Metab 2005; 289: E543–E550. [DOI] [PubMed] [Google Scholar]
- 19. Shehata MM, Kamal MM, El‐Hefnawy MH, et al Association of serum pancreatic derived factor (PANDER) with beta‐cell dysfunction in type 2 diabetes mellitus. J Diabetes Complications 2017; 31: 748–752. [DOI] [PubMed] [Google Scholar]
- 20. Ratliff WA, Athanason MG, Chechele AC, et al Hepatic nutrient and hormonal regulation of the PANcreatic‐DERived factor (PANDER) promoter. Mol Cell Endocrinol 2015; 413: 101–112. [DOI] [PubMed] [Google Scholar]
- 21. Wilson CG, Robert‐Cooperman CE, Burkhardt BR. PANcreatic‐DERived factor: novel hormone PANDERing to glucose regulation. FEBS Lett 2011; 585: 2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rashid S, Watanabe T, Sakaue T, et al Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem 2003; 36: 421–429. [DOI] [PubMed] [Google Scholar]
- 23. Narayan SB, Rakheja D, Pastor JV, et al Over‐expression of CLN3P, the Batten disease protein, inhibits PANDER‐induced apoptosis in neuroblastoma cells: further evidence that CLN3P has anti‐apoptotic properties. Mol Genet Metab 2006; 88: 178–183. [DOI] [PubMed] [Google Scholar]
- 24. Wang H, Liu A, Zhao T, et al Comparison of anthropometric indices for predicting the risk of metabolic syndrome and its components in Chinese adults: a prospective, longitudinal study. BMJ Open 2017; 7: e016062. [DOI] [PMC free article] [PubMed] [Google Scholar]


