Abstract
The present narrative review discusses the role of continuous glucose monitoring (CGM) in glycemic and weight control, and lifestyle behavior adherence in adults with type 2 diabetes. A literature search from January 2001 to November 2017 was carried out (MEDLINE, CINAHL, Web of Science and Scopus). Eligible studies were trials evaluating the use of CGM with the aim of achieving glucose control or lifestyle‐related treatment adherence over a period of ≥8 weeks in adults with type 2 diabetes compared with usual care or another comparison intervention, or observational trials reporting CGM user experience. A total of 5,542 participants were recruited into 11 studies (eight randomized controlled trials [n = 5,346] and three observational studies [n = 196]). The sample size ranged 6–4,678 participants, the mean age was 51.7–60.0 years and diabetes duration was 2.1–19.2 years, with high heterogeneity between studies. Overall, the available evidence showed, compared with traditional self‐monitoring of blood glucose levels, CGM promoted greater reductions in glycated hemoglobin, bodyweight and caloric intake; higher adherence rating to a personal eating plan; and increases in physical activity. High compliance to CGM wear‐time and device calibration was reported (>90%). The addition of lifestyle and/or behavioral counseling to CGM appeared to further potentiate these improvements. Preliminary evidence suggests that CGM use promotes glycemic and weight control, and lifestyle behavior adherence in adults with type 2 diabetes. These benefits might be further enhanced with integration of diet, exercise, and glucose excursion education and counseling. However, specific attributes of effective interventions and the application of CGM information for promoting improved outcomes and healthier choices remain unclear.
Keywords: Glycemic control, Lifestyle, Technology
Introduction
Approximately 422 million adults worldwide have diabetes, with ~90% of cases having type 2 diabetes1. With a disproportionately greater increase in type 2 diabetes in the Asian region, the burden of type 2 diabetes is fast being realized, with <25% reaching good glycemic control2, 3, 4, 5, 6. Hence, effective evidenced‐based strategies are urgently required. Poor blood glucose control underpins diabetes‐related vascular complications, and its increased risk of cardiovascular disease and premature death1, 4, 7. Although diet and lifestyle interventions remain the cornerstones of type 2 diabetes management, alone these are often insufficient to achieve sustained glycemic control and adjunctive therapies are required for effective management.8, 9 Furthermore, current approaches to implementing lifestyle strategies are often laborious, costly and resource intensive, requiring close health professional supervision to provide feedback to blood glucose response changes. This creates challenges for long‐term adherence to lifestyle strategies8, 10, 11, and the need for alternative effective and acceptable treatment strategies for sustained therapy.
Self‐monitoring of blood glucose levels (SMBGL) is widely accepted as being beneficial for long‐term glycemic control in type 2 diabetes, both with or without insulin therapy12. However, limitations and poor adherence to regular SMBGL exist due to inconvenience, costs of disposables, and erroneous and reduced measurement frequency resulting in suboptimal glycemic control13, 14. Alternatively, continuous glucose monitoring systems (CGM) have emerged that utilize sensor technology inserted subcutaneously to measure interstitial glucose levels across the day that could enhance behavior change adherence and glycemic control. CGM enables an individual to observe blood glucose levels, and understand interactions and impact between diet, physical activity and medication choices with greater qualitative and quantitative feedback, providing health practitioners with a unique education tool15, 16. In 2013, a meta‐analysis including four randomized control trials (RCTs) of 116 individuals with type 2 diabetes, reported CGM improves glycated hemoglobin (HbA1c) in type 2 diabetes over 8–12 weeks16. However, this meta‐analysis limited reporting to HbA1c outcomes, and did not assess lifestyle or behavioral change adherence, which are critical for effective diabetes management8, 17. The authors also identified only two of the four RCTs were high quality16. More recently, less intrusive device technology and real‐time CGM (RT‐CGM) technologies have emerged that offer greater potential for combining CGM with lifestyle strategies to enhance type 2 diabetes management. CGM use and sensor wear time have been associated with treatment adherence and improved glycemic control18. The aim of the present article was to provide a narrative review of trials (identified using a systematic search strategy) investigating the effectiveness of CGM interventions to improve HbA1c, bodyweight status and lifestyle behavior adherence in adults with type 2 diabetes. Where possible, a further aim was to understand CGM user acceptance and potential implications for primary care use. A systematic approach with a narrative analysis was used due to insufficient availability of high‐quality published studies and data reporting on the outcomes of interest in the target population to carry out a meta‐analysis.
Methods
Search strategy
Conduct and reporting of the present review was based on guidelines outlined by the Joanna Briggs Institute peer reviewed protocol for scoping reviews19. To identify appropriate key words, an initial limited search of MEDLINE and Cumulative Index to Nursing and Allied Health Literature (CINAHL) was carried out, and the title, abstract and index terms to describe the articles were accepted. Search terms were continuous glucose monitoring OR CGM*, diabetes NOT type 1 diabetes AND obese*/overweight AND lifestyle OR blood glucose control/management OR glycemic control AND/OR physical activity AND/OR nutr* diet*, effectiveness and self‐monitoring OR acceptability.
Key terms were used in the second search phase using MEDLINE, CINAHL, Web of Science and SCOPUS electronic databases to include published and unpublished articles, and limited to adults (aged >18 years) and English language publications. Publication dates were January 2001 (coinciding with Food and Drug Administration approval for commercial CGM use) to November 2017 to capture studies utilizing CGM technology to reflect modern day practice20, 21. Reference lists of retrieved articles and relevant systematic reviews were hand‐searched for other relevant studies16, 21, 22, 23. The present study represents a scoping review underpinned by a systematic process19.
Study selection criteria
Duplications were removed using Endnote (EndNote, Version X7.7; Clarivate Analytics, Boston, Massachusetts, USA). Title and abstracts were manually screened for relevant articles based on selection criteria by the primary reviewer (PT). Full text of potential articles was retrieved for further assessment. Studies were selected if: (i) they were RCTs with a reported intervention utilizing CGM with the aim of achieving glycemic control and/or intervention adherence, or observational studies reporting on CGM user experience; (ii) they were carried out in adults (aged ≥18 years) with clinically diagnosed type 2 diabetes; (iii) they were RCTs that included diet and exercise interventions as one of the intervention groups targeting glycemic control; or (iv) they consisted of a usual care or control group. Studies were excluded if: (i) participants had type 1 diabetes, gestational diabetes or were aged <18 years; (ii) intervention or control was pharmacological/surgical; (iii) participants with pre‐diabetes or type 1 and 2 diabetes were examined, but data for type 2 diabetes could not be separately extracted; or (iv) participants were critically ill or had post‐surgical interventions. Retrieved studies were independently assessed for relevance by all authors and discussed for final selection.
Data extraction
Data required for analysis were extracted from included articles into data extraction tables. Data relating to study methodology/design, CGM protocols, intervention outcomes including CGM wear‐time and use, sample size, attrition rates, age, sex, and outcomes associated with glycemic control were captured. One reviewer (PT) extracted the data from all included articles, while secondary reviewers (CT and GB) independently cross‐checked data extraction reliability. Inconsistencies were resolved through discussion and consensus. Meta‐analysis was not carried out due to high heterogeneity of interventions and outcomes. A narrative analysis summarizing the results was used.
Results
Search outcomes
A total of 277 articles were identified. After removal of duplications, and excluding articles based on title and abstracts, 12 articles, reporting 11 separate studies (one article reporting long‐term follow up24 of a previously reported short‐term intervention25) met the inclusion criteria. Nine articles (eight studies) were RCTs24, 25, 26, 27, 28, 29, 30, 31, 32, and three were observational trials33, 34, 35. Included studies were rated as being of acceptable quality19. The article selection process summary, and the study protocols and intervention specifications are presented in Figure 1 and Table 1, respectively.
Figure 1.
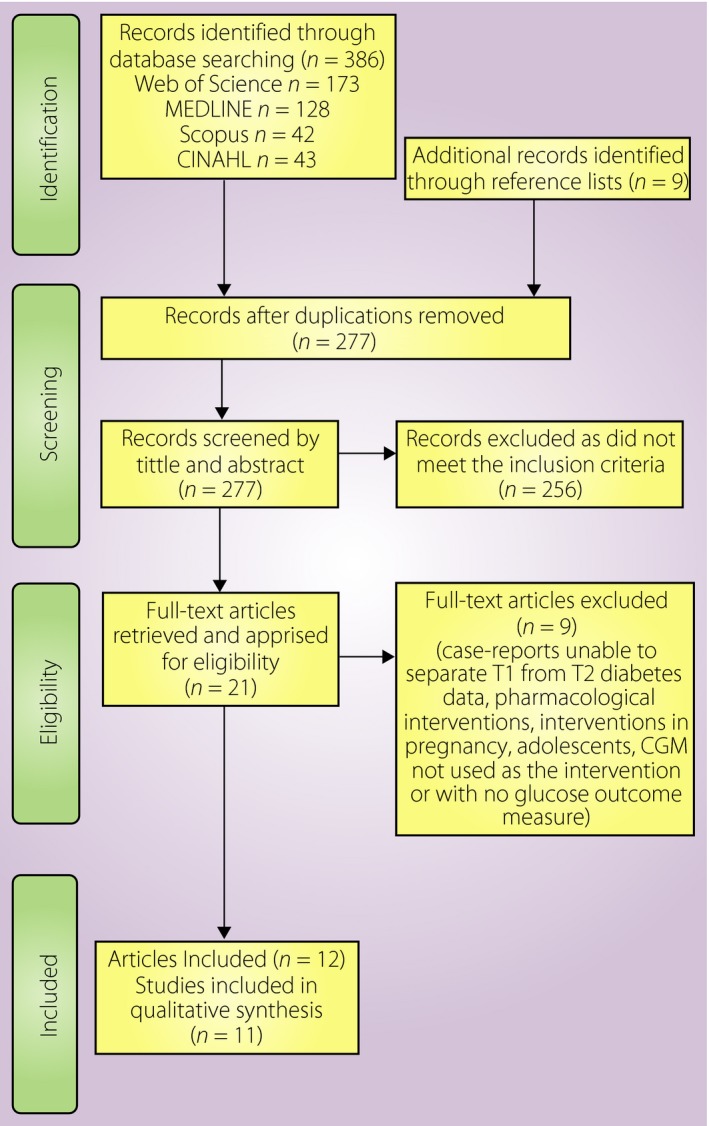
Article selection process. CGM, continuous glucose monitoring; T1, type 1; T2, type 2.
Table 1.
Protocol summary of continuous glucose monitoring intervention studies in adults with type 2 diabetes
| Author, year, reference | Study population | Mean age (years) | Duration of type 2 diabetes (years) | Study duration (weeks) | Intervention | Control | CGM system type | CGM wear time protocol | Calibration protocol | Total CGM Wear | Reported CGM acceptance/ satisfaction/ usability | Compliance to CGM protocol (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational studies | ||||||||||||
| Allen et al. (2009)34 | Nine adults community health clinic (USA) | 57.0 ± 15 (SD) | 8.6 ± 6 (SD) | 3 days |
Phase 1: Retrospective CGM + education Phase 2: Focus group interview |
NA | Medtronic Minimed. | 3 days | NR | 3 days |
|
NR |
| Cox et al. (2016)33 | Six adults (Canada) | 55.3 (mean) | 2.1 years (mean) | 12 | RT‐CGM + lifestyle program | NA | DexCom™ G4 Platinum | NR | Unclear | NR | × | NR |
| Mohan et al. (2016)35 |
181 Adults 11 Health clinics (India) |
54.1 ± 10 (SD) | 14.6 ± 8.1 (SD) | 12 | Retrospective CGM + education and profession support | NA | Medtronic IPro™ | Unclear | NR | Unclear |
|
NR |
| RCT – RT‐CGM vs SMBGL | ||||||||||||
| Beck et al. (2017)30 |
158 Adults receiving multi‐dose insulin 25 Endocrine clinics. (USA and Canada) |
SMBGL 60 ± 9 (SD) RT‐CGM 60 ± 11 (SD) |
SMBGL 18 (range 12–23) RT‐CGM 17 (range 11–23) |
24 | RT‐CGM + health usual care† | SMBGL + usual care† | DexCom™ G4 Platinum | Daily wear (168 days) | Calibrate 2× daily and glucometer testing 2× daily | 159.5 of 168 days (Mean) | NR | 95% |
| Ehrhardt et al. (2011)25 & Vigersky et al. (2012)24 | 100 Adults military healthcare beneficiaries (USA) |
SMBGL 60 ± 11.9 (SD) RT‐CGM 55.5 ± 9.6 (SD) SMBGL 60 ± 11.9 (SD) RT‐CGM 55.5 ± 9.6 (SD) |
NR NR |
12 52 |
RT‐CGM + usual care†
40‐week follow up with SMBGL |
SMBGL + usual care†
40‐week follow up with SMBGL |
DexCom™ Seven© | 12 weeks of intermittent CGM followed by SMBGL only 40 weeks | As per manufacturer's instructions | 56 days per protocol; 48 days accepted minimum |
|
68% ≥48 days 32% ≤48 days |
| Tang et al. (2014)26 |
40 Adults Endocrinology clinic (Canada) |
RT‐CGM 59.13 ± 8.70 (SD) IBGM 60.65 ± 10.19 (SD) |
RT‐CGM 19.2 ± 7.4 (SD) IBGM 17.24 ± 5.96 (SD) |
26 | RT‐CGM + BGL + fortnightly health professional feedback | IBGM + BGL + fortnightly health professional feedback | Guardian RT‐CGM Medtronic Minimed | Unclear | Unclear | Unclear |
|
NR |
| Yoo et al. (2008)27 |
65 adults Hospital based Korea (Seoul) |
RT‐CGM 54.6 ± 6.8 (SD) SMBGL 57.5 ± 9 (SD) |
RT‐CGM 11.7 ± 5.8 (SD) SMBGL 13.3 ± 4.9 (SD) |
12 | RT‐CGM + usual care† | SMBGL + usual care† | Guardian RT‐CGM Medtronic Minimed | 3 days per month for study duration | 3× daily | 9 days | × | NR |
| RCT – Retrospective CGMS feedback vs control | ||||||||||||
| Allen et al. (2008)28 |
52 adults Community health Service (USA) |
Intervention 57.0 ± 12.47 (SD) Control 57.0 ± 14.56 (SD) |
Intervention 8.3 ± 6.31 (SD) Control 8.5 ± 6.23 (SD) |
8 | Retrospective CGM + usual care† + self‐efficacy counseling | Usual care† | Medtronic Minimed. | 3 days continuous at baseline and post | NR | 6 days | × | NR |
| Anjana et al. (2017)31 |
4,678 Adults (61% Male) Seven public health diabetes clinics (India) |
Intervention 57.3 ± 12.1 (SD) Control 57.1 ± 12/2 (SD) |
Intervention 15.7 ± 8.5 (SD) Control 13.6 ± 8.1 (SD) |
12 | Retrospective flash‐CGM + visual charts used by clinician to adjust diabetes medication | Usual care† | Abbotts FreeStyle LibrePro™ Flash Glucose Monitoring | 14‐day continuous wear time | NR | 14 days continuous wear time | NR‡ | NR |
| Haak et al. (2017)32 |
224 Adults 140 Intervention 75 Control receiving multi‐dose insulin 26 public diabetes clinics (European) |
Intervention 59.0 ± 9.9 (SD) Control 59.5 ± 9.9 (SD) |
Intervention 17 ± 8 (SD) Control 18 ± 8 (SD) |
24 | Retrospective flash‐CGM + clinician to adjust insulin | SMBGL + usual care† | Abbotts FreeStyle LibrePro™ Flash Glucose Monitoring | 14‐day continuous wear time |
Intervention: Scan the flash sensor every 8 h Both groups: Recorded blood glucose levels daily |
14 days |
|
NR |
| RCT – CGM with counseling vs CGM without counseling | ||||||||||||
| Allen et al. (2011)29 |
29 Women Community health service (USA) |
Retrospective CGM + problem‐solving counseling 52.2 ± 6.5 (SD) Retrospective CGM + usual care† 51.7 ± 8.0 (SD) |
Retrospective CGM + problem‐solving counseling 6.7 ± 6.0 (SD) Retrospective CGM + diabetes education 6.7 ± 4.6 (SD) |
12 | Retrospective CGM + problem‐solving counseling | Retrospective CGM + usual care† | NR | 3 days continuous at baseline and post‐intervention | NR | 6 days | × | 96% compliance (28/29) completed |
†Usual care consisting of diabetes education/physical activity is defined as per the diabetes management guidelines of the country where the study was carried out. ‡Unable to separate type 1 diabetes data from type 2 diabetes data for this outcome. CGM, continuous glucose monitoring; IBGM, internet blood glucose monitoring; NA, not applicable; NR, not reported; RCT, randomized control trial; RT‐CGM, real‐time continuous glucose monitoring; SD, standard deviation; SMBGL, Self‐Monitoring Blood Glucose Levels.
Participants
A total of 5,542 participants were recruited into eight RCTs (n = 5,342) and three observational trials (n = 196), with 60% men26, 34, and ~9% (n = 487) receiving multiple daily insulin injections30, 32. Nine studies assessed baseline body mass index (BMI)24, 25, 26, 27, 28, 30, 31, 32, 33, 35, with the majority of participants classified as overweight or obese (BMI >25 kg/m2; n = 5,432). The RCT sample size ranged between 29 and 4,678 participants, with a mean age range of 52–60 years and diabetes duration of 6.2–19.2 years24, 25, 26, 27, 28, 29, 30, 31, 32, 35; one RCT (n = 40) did not report diabetes duration29. The observational trials examined 6 to 181 participants with a mean age range of 54–57 years and diabetes duration of 2.1–14.6 years33, 34, 35. Of the 11 studies, a total of 159 of 5,542 participants (~3%) were reported as dropouts; one trial (n = 6) did not report attrition rates33.
All studies, except Cox et al.33, reported participant recruitment strategies including military healthcare beneficiaries (n = 100)24, 25, public endocrinology clinic and/or cardiac unit (n = 5,100)26, 30, 31, 32, community health services (n = 271)29, 34, 35 and general hospitals (n = 65)27. Studies were carried out in the USA24, 25, 28, 29, 33, 34, Canada26, India31, 35, Europe32, South Korea27, and across multiple sites throughout the USA and Canada30.
Study aims and Intervention description
The aims of the studies were to determine whether CGM use elicits changes in glycemic control24, 25, 26, 27, 30, 31, 32, 33, 35, weight24, 25, 27, 29, 32, 33 and physical activity28, 29 to investigate CGM in relation to self‐efficacy and self‐monitoring behavior26, 28, 32, and acceptability, satisfaction and tolerance to CGM wear and use26, 29, 34, 35. Across the studies, different intervention protocols were used and five distinct research themes identified (Table 1). These research themes included: (i) four RCTs determining the effects of RT‐CGM (user can view visual feedback of blood glucose responses instantaneously) compared with SMBGL (control)24, 25, 26, 27, 30; (ii) one RCT determining the effects of using retrospective CGM data with diabetes education combined with self‐efficacy counseling compared with diabetes education with SMBGL (control)28; (iii) three RCTs determining the effects of retrospective CGM with problem‐solving counseling compared with retrospective CGM with standard diabetes education (control)29, 31, 32; (iv) two observational studies describing the effect of retrospective CGM data (requiring researchers/health professionals to download ‘blinded’ CGM data into a graphical format to share with participants for counseling purposes)34, 35, with one including accelerometer data34; and (v) one observational study describing the effects of RT‐CGM combined with a lifestyle program to improve patient outcomes33. Intervention durations for observational studies were 3 days34 and 12 weeks33, 35, and for RCTs the range was 8–52 weeks24, 25, 26, 27, 28, 29, 30, 31, 32.
Effectiveness of CGM interventions on outcomes
HbA1c changes
All RCTs reported HbA1c changes (Table 2)24, 25, 26, 27, 28, 29, 30, 31, 32, 33. Of the four studies comparing RT‐CGM with conventional SMBGL combined with standard diabetes education over 26 weeks (n = 363) where 43% (n = 158) were on multiple daily insulin injections, three studies reported RT‐CGM achieved significant greater HbA1c reductions by a magnitude of 1% (absolute)25, 27, 30. A 40‐week follow up from one of these studies assessing the residual effects without wearing RT‐CGM showed the greater HbA1c lowering with RT‐CGM use was maintained compared with SMBGL control after 52 weeks (0.8 vs 0.2%; P < 0.001)24, 25. RT‐CGM compared with an internet‐based SMBGL protocol after 26 weeks showed no difference between treatments (−0.9 vs −1.07%; P = 0.312)26.
Table 2.
Changes in glycemic control, bodyweight, physical activity, diet and behavioral outcomes from continuous glucose monitoring studies in adults with type 2 diabetes
| Author, year reference | Study population | Mean age (years) | Duration of type 2 diabetes (years) | Study duration (weeks) | Intervention | Control | Glycemic control: intervention vs control (P‐value) | Weight (kg) or BMI (kg/m2): Intervention vs Control (P‐value) | Physical activity: intervention vs control (P‐value) | Diet: intervention vs control (P‐value) | Behavioral: intervention vs control (P‐value) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational studies | |||||||||||
| Allen et al. (2009)34 | Nine adults community health clinic (USA) | 57.0 ± 15 (SD) | 8.6 ± 6 (SD) | 3 days |
Phase 1: retrospective CGM + education Phase 2: Focus group interview |
NA | NR | NA |
Accelerometer Activity counts 313,726 vs no control (NA) Activity level 1,403 min per day (light/sedentary) |
NR | NR |
| Cox et al. (2016)33 | Six adults (Canada) | 55.3 (mean) | 2.1 years (mean) | 12 | RT‐CGM + lifestyle program | NA |
HbA1c (%) −1.1 (change) (P‐value ‐ NR) |
Weight (kg) −7.3 (change) (P‐value ‐ NR) |
Pedometer (step counts) Pre: 8,400 Post: 13,000 |
Total energy intake −884 Kcal (change) Total carbohydrate −92.8 g (change) |
PAID score Pre: 6.5 Post: 3.3 |
| Mohan et al. (2016)35 |
181 Adults 11 Health clinics (India) |
54.1 ± 10 (SD) | 14.6 ± 8.1 (SD) | 12 | Retrospective CGM + education and professional support | NA |
HbA1c (%) −0.6 ± 1.11 (SD) (P < 0.001) |
NR | NR | NR | NR |
| RCT –RT‐CGM vs SMBGL | |||||||||||
| Beck et al. (2017)30 |
158 Adults receiving multi‐dose insulin 25 Endocrine clinics. (USA and Canada) |
SMBGL 60 ± 9 (SD) RT‐CGM 60 ± 11 (SD) |
SMBGL 18 (range 12–23) RT‐CGM 17 (range 11–23) |
24 | RT‐CGM + health professional support (0, 4,12 and 24 weeks) | SMBGL + usual care† (0, 4, 12 and 24 weeks) |
HbA1c (%): 12 weeks−1.0 vs −0.6 (P = 0.005) 24 weeks−0.8 vs −0.5 (P = 0.022) |
Change weight (kg) 24 weeks 1.3 vs −0.2 (P‐value ‐ NR) | NR | NR | NR |
| Ehrhardt et al. (201125 )& Vigersky et al. (2012)24 | 100 Adults military healthcare beneficiaries (USA) |
SMBGL 60 ± 11.9 (SD) RT‐CGM 55.5 ± 9.6 (SD) SMBGL 60 ± 11.9 (SD) RT‐CGM55.5 ± 9.6 (SD) |
NR NR |
12 52 |
RT‐CGM + usual care†
40‐week follow up with SMBGL |
SMBGL + usual care†
40‐week follow up with SMBGL |
12 weeks Change HbA1c (%)−1.0 vs −0.5 (P = 0.006) 52 weeks Change HbA1c (%):−0.8 vs −0.2 (P < 0.001) |
12 weeks Change weight (kg) −1.8 vs −0.4 (P = 0.42) 52 weeks Change weight (kg):−1.9 vs −0.9 (P = 0.2) |
NR | NR |
12 weeks Change PAID score−6.2 vs −6.8 (P‐value ‐ NR) 52 weeks Change PAID score (week 12–52) −0.3 vs +1.3 (P‐value ‐ NR) Change PAID score (week 0–52) (P = 0.96) |
| Tang et al. (2014)26 | 40 Adults endocrinology clinic (Canada) |
RT‐CGM 59.13 ± 8.70 (SD) IBGM 60.65 ± 10.19 (SD) |
RT‐CGM 19.2 ± 7.4 (SD) IBGM 17.24 ± 5.96 (SD) |
26 | RT‐CGM + BGL + fortnightly health professional feedback | IBGM + BGL + fortnightly health professional feedback | HbA1c (%): −0.9 vs −1.07 (P = 0.312) | BMI (kg/m2): 1.44 vs 0.35 (P = 0.48) | NR | NR |
Diabetes treatment satisfaction Questionnaire scores (arbitrary units) Overall rating 24.8 vs 33.4 (P < 0.001) Convenience 3.8 vs 5.25 (P = 0.004) Flexibility 4.0 vs 5.41 (P = 0.004) Likelihood to recommend treatment to others 3.4 vs 5.94 (P = 0.001) Willingness to continue treatment 3.4 vs 5.71 (P = 0.000) |
| Yoo et al. (2008)27 |
65 Adults With poorly controlled type 2 diabetes (HbA1c 8–10%) Hospital based Korea (Seoul) |
RT‐CGM 54.6 ± 6.8 (SD) SMBGL 57.5 ± 9 (SD) |
RT‐CGM 11.7 ± 5.8 (SD) SMBGL 13.3 ± 4.9 (SD) |
12 | RT‐CGM ‐ with hyperglycemia counseling | SMBGL + usual care† | HbA1c (%):−1.1 vs −0.4 (P = 0.004) | Change Weight (kg): −2.2 vs −1.4 (P = 0.43) | Exercise time (total min/week) 158.4 vs 43.5 (P = 0.02) |
Change in total energy intake (kcal/day) −168.7 vs −114.0 (P = 0.05) Post 12 weeks 1,859 vs 1,690 (P = 0.002) |
NR |
| RCT – Retrospective CGM feedback vs control | |||||||||||
| Allen et al. (2008)28 |
52 Adults Community health service (USA) |
Intervention 57.0 ± 12.47 (SD) Control 57.0 ± 14.56 (SD) |
Intervention 8.3 ± 6.31 (SD) Control 8.5 ± 6.23 (SD) |
8 | Usual care† + self‐efficacy counseling | Usual care† | HbA1c (%):−1.2 vs −0.3 (P < 0.05) | BMI (kg/m2): −0.53 vs −0.12 (P = 0.05) | Accelerometer (step counts) 31,144 vs −9,281 (P < 0.05) | NR | Self‐Efficacy for Exercise Behaviour Survey (‘sticking to it’ domain) 0.52 vs −0.11 (P < 0.05) |
| Anjana et al. (2017)31 |
4,678 Adults (61% male) Seven public diabetes clinics (India) |
Intervention 57.3 ± 12.1 (SD) Control 57.1 ± 12.2 (SD) |
Intervention 15.7 ± 8.5 (SD) Control 13.6 ± 8.1 (SD) |
12 | Retrospective flash‐CGM + visual charts used by clinician to adjust diabetes medication | SMBGL and usual care† | HbA1c (%)−0.9 vs −0.7 (P < 0.001) | NR | NR | NR | NR |
| Haak et al. (2017)32 |
224 Adults 149 Intervention75 Control multi‐dose insulin therapy 26 public diabetes clinics (European) |
Intervention 59.0 ± 9.9 (SD) Control 59.5 ± 9.9 (SD) |
Intervention 17 ± 8 (SD) Control 18 ± 8 (SD) |
24 | Retrospective flash‐CGM + clinician to adjust insulin | SMBGL + usual care† | HbA1c (%)−0.28 vs −0.21 (P = 0.822) |
Change weight (kg) (change values ‐ NR) (P = 0.250) Change BMI (kg/m2): (change values ‐ NR) (P = 0.267) |
NR | NR |
Diabetes Treatment Satisfaction Questionnaire scores (arbitrary units) Overall rating 13.1 vs 9.0 (P < 0.001) |
| RCT – CGM with counseling vs CGM without counseling | |||||||||||
| Allen et al. (2011)29 |
29 Women Community health service (USA) |
Retrospective CGM + problem‐solving counseling 52.2 ± 6.5 (SD) Retrospective CGM + usual care† 51.7 ± 8.0 (SD) |
Retrospective CGM + problem‐solving counseling 6.7 ± 6.0 (SD) Retrospective CGM + usual care† 6.7 ± 4.6 (SD) |
12 | Retrospective CGM + problem‐Solving counseling | Retrospective CGM + usual care† | HbA1c (%):−0.7 vs −0.5 (P = 0.69) | Weight (kg):−6.2 vs +2.4 (P = 0.09) |
Accelerometer Activity counts 1,500 vs −400 (P = 0.48) Level of activity (mins/day) 40 vs 2 (sedentary) (P = 0.43) 1 vs 6 (light) (P = 0.78) 5 vs −3 (moderate) (P = 0.11) |
Subscale of diabetes self‐care score Healthy eating plan 2.7 vs 0.7 (P = 0.01) Healthful eating plan 2.7 vs 1.2 (P = 0.17) Fruits and vegetables 1.5 vs 1.2 (P = NR) High‐fat foods −1.6 vs −1.2 (P = 0.55) |
Diabetes Problem‐Solving Inventory score 1.06 vs 0.43 (P = 0.02) |
†Usual care consisting of diabetes education/physical activity is defined as per the diabetes management guidelines of the country where the study was carried out. Significant between group difference identified as P < 0.05. CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin; IBGM, internet blood glucose monitoring; NA, not applicable; NR, not reported; PAID, Problem Areas in Diabetes; RCT, randomized control trial; RT‐CGM, real‐time continuous glucose monitoring; SD, standard deviation; SMBGL, Self‐Monitoring Blood Glucose Levels.
Greater HbA1c reduction was observed after 8 weeks of retrospective CGM with self‐efficacy counseling compared with SMBGL with standard education (−1.16 vs 0.32%, P < 0.05)28. Two studies compared retrospective CGM using flash CGM with visual charts and counseling to SMBGL31, 32. Greater HbA1c reductions were observed following CGM after 12 weeks (−0.9 vs −0.7%, P < 0.001) in 4,678 individuals31, but not after 24 weeks (−0.28 vs −0.21%, P = 0.822) of 224 individuals taking multiple daily insulin injections32. After 12 weeks, HbA1c reduction with retrospective CGM was similar when combined with either problem‐solving counseling or standard diabetes education (no counseling; −0.7 vs −0.5; P = 0.69)29.
A 12‐week lifestyle program combined with RT‐CGM produced an average 1.1% HbA1c reduction in six participants33. The authors compared these data with findings from a separate, independent 12‐week intervention (n = 47) that used the same lifestyle program with SMBGL and reported similar HbA1c reductions (1%)23, 33. However, population size heterogeneity between the two datasets carried out over different time‐periods limits the ability to draw effective comparisons.
Bodyweight changes
For bodyweight changes, BMI (kg/m2) is reported in the instance where weight (kg) data is not reported. Eight studies reported bodyweight changes (Table 2)24, 25, 26, 27, 28, 29, 30, 32, 33. Three intervention studies compared RT‐CGM combined with education including RT‐CGM output interpretation to SMBGL combined with standard diabetes education (control)24, 25, 27, 30. Two studies showed a trend for greater weight loss with RT‐CGM after 12 weeks (range −1.8 to −2.2 kg RT‐CGM vs −1.4 to −0.4 kg control)24, 25, 27. A further study reported 1.3‐kg weight gain with RT‐CGM vs −0.2 kg control (P‐value not reported), and was the only trial to include individuals taking insulin (Table 2)30. Another intervention showed a non‐significant greater BMI reduction in the RT‐CGM group compared with control participants over 26 weeks (−1.44 vs −0.35 kg/m2)26. A 40‐week follow up from a previous 12‐week CGM study showed a sustained, non‐significant greater weight reduction in the RT‐CGM group compared with control participants (−1.9 vs −0.9 kg; P = 0.2)24. The specific details of counseling and education provided were unclear, although contact frequency was reduced from every 3 weeks during the initial 12‐week intervention to 3 monthly during 40‐week follow up24.
A 12‐week intervention using retrospective CGM with problem‐solving counseling produced a non‐significant greater weight loss compared with retrospective CGM with standard diabetes education (−6.2 vs +2.4 kg; P = 0.09)29. Furthermore, retrospective CGM plus behavior change counseling for 8 weeks produced significantly greater BMI reduction compared with SMBGL and standard diabetes education (−0.53 vs −0.12 kg/m2; P < 0.05)28.
A 12‐week lifestyle program combined with counseling and RT‐CGM achieved a 7.3‐kg weight loss33. This was greater than the weight loss reported in a previous study using the same lifestyle program combined with counseling and SMBGL (−2.5 kg)33.
Change in behavior, physical activity and diet
Eight of the 11 studies reported at least one of the three lifestyle behavior modification domains of physical activity, dietary and behavioral outcomes (Table 2).
Behavioral change
Six studies – one observational33 and five RCTs24, 25, 26, 28, 29, 32 – reported behavioral changes with CGM, noting a generally minimal effect. Two studies comparing RT‐CGM vs SMBGL with standard diabetes education over 12 weeks25 and a 40‐week follow up without RT‐CGM24 reported no differences between groups for changes in diabetes distress24, 25. Meanwhile, greater diabetes treatment satisfaction was observed in a control group utilizing SMBGL combined with an internet‐based monitoring protocol and standard diabetes education compared with a RT‐CGM intervention group26. Conversely, greater improvements in diabetes treatment satisfaction were observed after an intervention using retrospective, flash CGM with clinician interaction to adjust insulin compared with SMBGL combined with usual care control32. A further study reported retrospective CGM with problem‐solving counseling achieved greater improvements in diabetes problem‐solving compared with retrospective CGM with standard education (1.06 vs 0.43 arbitrary units; P = 0.02)29.
An 8‐week intervention of retrospective CGM achieved greater increases in the ‘sticking to it’ domain of the Self‐Efficacy for Exercise Behavior Survey compared to SMBGL with standard diabetes education (0.52 vs −0.11; P = 0.05)28. A 12‐week lifestyle program combined with RT‐CGM lowered the diabetes distress score33. However, similar improvements were achieved with the same lifestyle program combined with SMBGL23.
Physical activity outcomes
Four studies (one observational trial,33 three RCTs27, 28, 29) reported physical activity outcomes after CGM intervention with differing assessment methodologies and results. RT‐CGM with standard diabetes education produced greater increases in total exercise minutes compared with SMBGL with standard diabetes education (+158.4 vs +43.5 min/week; P = 0.02)27. Similarly, 8 weeks after retrospective CGM with education relating to exercise‐associated blood glucose response produced greater increases in mean activity counts/day compared with SMBGL with standard exercise guideline education (31,144 vs −9,281 counts; P ≤ 0.05)28. Another study investigating retrospective CGM with counseling and problem‐solving or with standard education showed no differences in changes in physical activity levels after 12 weeks; although the group that received retrospective CGM with problem‐solving skills showed a non‐significant greater increase in activity counts (+15,000/day vs −4,000/day; P = 0.48) and levels of moderate activity (5 min/day vs −3 min/day; P = 0.11)29. An increase in absolute step counts (8,400–13,000 steps/per day) occurred after a 12‐week lifestyle intervention combined with RT‐CGM33. A similar magnitude of change was reported in a separate study carried out in a different cohort following the same lifestyle program combined with SMBGL23.
Dietary outcomes
Three studies (one observational33 and two RCTs27, 29) assessed dietary outcomes. There were greater reductions in caloric intake after 12 weeks of RT‐CGM compared with SMBGL and standard diabetes education (−168.7 vs −113.9 kcal/day; P = 0.05)27. It was found that 12 weeks of retrospective CGM with problem‐solving counseling achieved higher ratings of adherence to a personal eating plan compared with retrospective CGM and standard diabetes education (2.7 vs 0.7 arbitrary units; P = 0.01), with no difference between the groups for domains of healthful eating, fruits and vegetables, and high‐fat foods29.
A 12‐week observational study showed a lifestyle intervention combined with RT‐CGM reduced total energy intake (pre: 2,680 Kcal, post: 1,796 Kcal [difference −884 Kcal]) and total carbohydrate intake (pre: 243.3 g, post: 150.5 g (difference −92.8 g)33.
Device acceptability, useability and wear time
Total CGM sensor wear time and compliance is described in Table 1. One study showed no change in CGM wear acceptance using a system useability scale score before and after 12 weeks of RT‐CGM wear, indicating moderate‐to‐good useability25. Another study (n = 181) reported a high CGM wear comfort (6.1/7) and improved diabetes awareness (6.2/7)35. Three studies (n = 287) recorded compliance with CGM protocols defined as attendance at CGM counseling sessions and compliance with CGM wear protocol24, 25, 29, 30. One study (n = 29) comparing CGM with problem‐solving counseling with CGM without counseling showed high levels of compliance to the intervention protocol based on session attendance (>90%)29. Two studies compared RT‐CGM with SMBGL without counseling; a 12‐week intervention used a wear time protocol of 2 weeks of continuous device wear separated by 1 week of no wear (i.e., 56 days total wear time required), and showed 32% of participants (n = 16) used the CGM device <48 days, and 68% (n = 34) for >48 days24, 25; whereas a 24‐week intervention using a continuous daily wear protocol for 168 days reported 95% compliance with mean wear time of 159 days30.
A follow‐up interview of participants prescribed to wear a blinded CGM device for 72 h with limited support reported just 57% of participants remembered to use event buttons on the monitor to enter medication, and meal and physical activity times, and reported many other problems in contrast to using manual logs34. Issues with wearing appropriate clothes to attach the monitor at night were also reported34. Positive emerging themes included visual CGM graphs reinforcing the need for behavior change, and the role of diet, exercise and stress on glucose levels34.
Discussion
Continuous glucose monitoring has a potential role in limiting the frequency and/or onset of hypoglycemia in type 2 diabetes patients, particularly for those receiving insulin therapy, who have a history of severe hypoglycemia or with irregular routines (skipping meals, vigorous exercise and poor sleep patterns)8, 21, 36, 37, 38. The present narrative review evaluated the evidence of the acceptance and effectiveness of CGM use on improving glycemic control, weight status and behavior change in adults with type 2 diabetes. Published observational and randomized controlled studies over the past decade were included, and revealed a relatively small number of studies with high heterogeneity in intervention design and outcomes reported. Of the studies available, a high level of CGM technology acceptance was reported and, compared with standard SMBGL combined with diabetes education, CGM use promoted greater HbA1c lowering and weight loss. The addition of lifestyle and/or behavioral counseling to CGM promoted higher diabetes treatment satisfaction and reduced diabetes‐related distress. However, specific counseling or lifestyle attributes that were most effective could not be ascertained. Although visual glucose outputs from CGMs were repeatedly reported as beneficial for educational purposes, communication specifics surrounding effective delivery strategies were not provided26, 29, 31, 32, 35.
Compared with standard SMBGL with standard education, RT‐CGM achieved a 0.4–0.7% (absolute) greater HbA1c reduction over 12–24 weeks in non‐insulin‐ (n = 165) and insulin‐dependent (n = 158) individuals with type 2 diabetes, respectively25, 27, 30. In non‐insulin‐dependent individuals, a 40‐week follow‐up period without continued RT‐CGM use showed these differential changes between the groups were maintained24. Similarly, provision of retrospective CGM feedback delivered with diabetes education plus self‐efficacy counseling achieved a 0.9% (absolute) greater reduction in HbA1c compared with standard diabetes education alone over 8 weeks28. More recently, a 12‐week study of 4,678 individuals showed the use of retrospective flash CGM, delivered with professional support at weeks 0, 4 and 12 using visual glucose charts to address medication management, achieved greater HbA1c reduction compared with SMBGL with standard education (−0.2% absolute; P < 0.001)31. These data suggest that access to CGM information can promote greater HbA1c reductions compared with standard care, and that these effects are sustained over the longer term. Furthermore, the addition of self‐efficacy counseling and not problem‐solving counseling has been shown to further magnify the CGM treatment effects without medication intensification28, 29. The exact reason for the variation of HbA1c change observed between the studies (0.4–0.9%) is not clear, but might arise because the study showing the greatest absolute change provided a walking and exercise plan as part of the standard education28, compared with exercise information and monitoring25, 27. A meta‐analysis has shown that supervised walking achieved greater HbA1c reduction compared with non‐walking controls (−0.5%)39, 40. This suggests an additive benefit of prescriptive exercise instruction in conjunction with CGM for intensifying glycemic control.
The current study results concur with a previous meta‐analysis that reported a grouped mean −0.31% greater HbA1c reduction over an 8–12‐week period of CGM use compared with SMBGL in adults with type 2 diabetes16. The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study for the use of CGM in type 1 diabetes management in children and adults also showed a −0.53% greater reduction in HbA1c compared with SMBGL over 26 weeks for individuals aged ≥25 years41. This smaller HbA1c change in the Juvenile Diabetes Research Foundation cohort compared with studies reported in the current analysis could be explained by the tighter glycemic control at baseline (HbA1c <8 vs >8%). By way of comparison of magnitude, lifestyle‐based weight loss interventions lasting between 16 weeks and 9 years produced a smaller non‐significant greater reduction in HbA1c of −0.29% compared with usual diabetes care and education in type 2 diabetes patients42. A 1% HbA1c reduction is estimated to reduce the risk of diabetes‐related death by 21%, myocardial infarction by 14% and microvascular complications by 37%43. Hence, the additional ~0.5% reduction achieved by RT‐CGM use could translate to significant further reductions in diabetes complication risk.
Interestingly, no differences in HbA1c changes were observed between RT‐CGM and SMBGL when delivered through an internet protocol with endocrinologist feedback. The reason for this lack of additional effect of RT‐CGM is difficult to explain. However, these data suggest using an internet platform to monitor blood glucose readings throughout the day and/or close professional support might engender similar effects to RT‐CGM.
Excess weight is a strong predictor of type 2 diabetes and HbA1c44, 45, 46. Approximately 80% of people with type 2 diabetes are overweight or obese1, 46. Studies comparing SMBGL with RT‐CGM showed no statistically significant differences in weight loss or BMI changes between treatments24, 25, 26, 27, 30. Nevertheless, three of the four studies using RT‐CGM consistently achieved ~1 kg greater weight loss reduction compared with standard SMBGL over 12–26 weeks24, 26, 27; an effect that was sustained 40 weeks after the cessation of RT‐CGM treatment24. It is possible the relatively small sample sizes used in previous studies precluded realization of the relatively modest effect sizes as statistically significant. Previous studies have shown as little as a 1‐kg or 1% weight loss can have a substantial benefit for glycemic control, morbidity and mortality47, 48. It is therefore possible that RT‐CGM could offer modest, yet clinically relevant, weight loss benefits24.
Interestingly, a 24‐week RT‐CGM intervention in patients with type 2 diabetes on multiple daily injections of insulin showed a 1.3‐kg weight gain compared with a −0.2‐kg weight loss for the control30. This was despite significant greater HbA1c reductions with RT‐CGM, suggesting that, although HbA1c and weight are closely linked, for those requiring insulin, weight gain might be acceptable30. However, more research documenting insulin titration strategies for weight management using RT‐CGM is required.
The studies identified in the present review further suggest the possibility that CGM data could be used to potentiate the weight loss effects of other intervention initiatives to provide a comprehensive weight loss strategy. CGM information combined with self‐efficacy and/or problem‐solving counseling (the latter which incorporates individualized counseling based on blood glucose responses) provided an additional ~2‐kg greater loss compared with CGM with standard diabetes education28, 29. However, only few, small studies have examined the effects of diet and lifestyle advice combined with CGM use on bodyweight, and more research is required to establish these effects. Nevertheless, personalization achieved through feedback or tailored information received during counseling support has been established as a critical component of successful weight loss interventions49, 50. The Action for Health in Diabetes study (Look AHEAD) showed a 5–10% weight loss in individuals with type 2 diabetes effectively reduces HbA1c, and diabetes and lipid‐lowering medication requirements49, 50. Hence, the potential role for CGM technology as an adjunctive therapy for sustainable weight loss management warrants further investigation.
Diet, physical activity and behavioral therapy are the cornerstones of type 2 diabetes management. It is well established that dietary habits, food choices and physical inactivity profoundly affect blood glucose response and control8, 9, 37, 51, 52, and self‐monitoring is a key driver for behavior change53. However, therapeutic strategies are often required for optimal glycemic control5. Recent availability of real‐time and ambulatory CGM technology provides the opportunity to use this monitoring information to educate patients on blood glucose response to lifestyle choices (diet and exercise), with the objective of modifying choices and augmenting adherence to effective behaviors, in the absence of medication intensification. Preliminary data suggest short‐term use of RT‐CGM that incorporates graph interpretation and hyperglycemic alarms without counseling can promote greater physical activity levels and reductions in caloric intake9, 10, 11, 54. Yoo et al.27 successfully instructed participants to increase exercise and reduce food portions in response to RT‐CGM‐generated hyperglycemic event alarms (>300 mg/dL). Similarly, provision of retrospective CGM data with feedback on physical activity responses using self‐efficacy counseling was shown to increase physical activity levels and confidence in engaging with a physical activity routine28. These early data suggest that access to CGM information might promote favorable changes, and adherence to diet and exercise behaviours. However, the ease and significance of data interpretation for the user is an important consideration. Future lifestyle and behavior change studies need to investigate the integrated role between CGM and the quality and quantity of information provided to the user with type 2 diabetes to identify practices that facilitate adherence to lifestyle modification changes.
The few studies reporting user acceptance and/or compliance to CGM wear25, 26, 27, 29, 30, 34, 35 suggest a high‐level device wear compliance, confidence with using RT‐CGM devices27, 30 and moderate‐to‐good useability25. Secondary analysis of 7,916 individuals with type 1 diabetes and type 2 diabetes using glucose sensors for >15 days during any 6‐month period over 2 years18 reported that compliance with sensor use in the first month of therapy predicted longer‐term compliance and self‐management adherence18. High satisfaction and perceived value ratings have also been reported in interventions using CGM combined with either problem‐solving and/or self‐efficacy counseling or standard diabetes education29, 34, 35, 54. Some evidence exists suggesting greater CGM satisfaction when combined with problem‐solving and targeted feedback directly linked to diet and exercise behaviors29, 34. These data suggest the relative ease of use and high short‐term acceptance of CGM systems among type 2 diabetes patients. This concurs with reported responses in type 1 diabetes55, 56 that could assist to promote long‐term compliance and acceptance of this device technology.
The reported high acceptance of CGM as a useful therapeutic and educational tool15, 18, 38, 54, 57, 58 should be considered in the context of the relatively limited feedback alternatives provided by standard diabetes self‐management strategies that rely on 3‐ or 6‐monthly HbA1c measures and SMBGL5, 37. The latter only provides a snapshot of current glucose levels and lacks the sensitivity to detect small changes or specific glucose responses to food or activity. The limited ability of these established tools to provide individualized insight into glucose response might therefore contribute to the reported frustration and poor compliance with current diabetes self‐management and attendant poor glycemic control11, 12, 59. Overall, the present review suggests a potential role for CGM to improve awareness of the effectiveness for diet and exercise in diabetes treatment, and enhance adherence to behavioral strategies in primary care11, 54, 57, 59. Therefore, it is disappointing that because of the limited evidence available to date, current consensus on the optimal frequency and duration of CGM use in primary care remains unclear. The role and the type of counseling to accompany CGM, and a clear strategy for use of CGM in insulin management in this milieu is even more unclear. To promote effective use of CGM in clinical practice, research must consider and report on the type of complementary advice provided for medication management and counseling style used in order to determine its full benefits and overall effects8, 60.
The relatively small number of studies available and small sample sizes associated with some of the studies have resulted in the high heterogeneity of outcomes assessed and study design features used. This suggests results should be interpreted with some caution. This also has precluded the ability to carry out a meta‐analysis at this time, and to draw specific and reliable conclusions regarding the effectiveness and acceptability of CGM use for type 2 diabetes. Additionally, the quality, quantity and diversity of adjunctive counseling, education techniques and clinical involvement further confound interpretation of these studies for optimization of clinical type 2 diabetes management. With the view of using CGM in primary care, there is an urgent need to provide evidence to identify individual risk and the frequency of hypoglycaemia for the effective development of risk management strategies for clinical practice. More, larger, longer‐term studies that detail intervention methods and delivery protocols will provide better understanding of the chronic effects and durability of CGM, its acceptance and the appropriate balance of its application with clinical involvement. Additionally, given the greater disproportionate burden of type 2 diabetes in Asians4, future studies should also focus on these populations. As more rigorous evidence emerges from larger studies using homogenous study designs, a meta‐analysis is warranted. The current review was also unable to directly compare and differentiate responses to CGM use between insulin‐dependent and non‐insulin‐dependent individuals with type 2 diabetes, or the effects of medication types and doses in these populations. Individuals requiring insulin for glycemic control might experience greater risk of hypoglycemia using CGM when compared with their non‐insulin‐taking counterparts. Therefore, larger, controlled studies that consider these factors and examine diverse population subgroups are required to better understand the wider benefits and application of CGM use in primary care. The paucity of detail regarding specific education and counseling protocols used within the reported studies also precludes description or the comparison of the type and frequency of education and support counseling provided within the lifestyle interventions. The American Association of Clinical Endocrinologists and American College of Endocrinology37 have identified the necessity for users and administrators in primary care to be well informed of the benefits and potential issues associated with CGM device use, and the appropriate translation of such information into lifestyle strategies to optimize glycemic control and diabetes care. It is imperative therefore, when reporting study protocols in future studies, that counseling, education and feedback strategies are clearly described. If these strategies potentiate the benefits of CGM wear, this will assist the translation of these new clinical practices into established diabetes management strategies. It is long overdue that CGM devices with lifestyle interventions are compared with usual clinical care in both depth and detail.
In conclusion, the present narrative review suggests that CGM promotes improvements in glycemic and weight control, and lifestyle behaviour change in adults with type 2 diabetes, and that these benefits might be enhanced when CGM is integrated with diet, exercise, and glucose excursion education and counseling. However, the limited number of highly heterogeneous studies available makes it difficult to identify specific attributes of effective interventions and to draw cohesive robust evidence for multidisciplinary clinical practice that promotes healthier choices for the type 2 diabetes patient population. As new evidence begins to emerge, there is a considerable need for a meta‐analysis to inform a greater understanding of the application of CGM for practice. In particular, its integration with pre‐existing and established monitoring and education tools should facilitate user engagement and device acceptability in type 2 diabetes and lifestyle management.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Darren Jones, Information Officer in Information Management & Technology for the Commonwealth Scientific and Industrial Organisation (CSIRO), for assistance in determining the search protocol for the review.
J Diabetes Investig 2018;9:713–725
References
- 1. World Health Organisation (WHO) . Global Report on Diabetes. Geneva: World Health Organisation, 2016. Available from http://www.who.int. Accessed June 7, 2017. [Google Scholar]
- 2. Herman WH, Zimmet P. Type 2 diabetes: an epidemic requiring global attention and urgent action. Diabetes Care 2012; 35: 943–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nanditha A, Ma RC, Ramachandran A, et al Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care 2016; 39: 472–485. [DOI] [PubMed] [Google Scholar]
- 4. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus[mdash]present and future perspectives. Nat Rev Endocrinol 2012; 8: 228–236. [DOI] [PubMed] [Google Scholar]
- 5. Ahmad NS, Islahudin F, Paraidathathu T. Factors associated with good glycemic control among patients with type 2 diabetes mellitus. J Diabetes Investig 2014; 5: 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anoop M, Lokesh K, Sumit I, et al South Asian diets and insulin resistance. Br J Nutr 2009; 101: 465–473. [DOI] [PubMed] [Google Scholar]
- 7. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128: 40–50. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association (ADA) . Standards of Medical Care in Diabetes ‐ 2017. J Clin Appl Res Educat 2017; 41(Suppl. 1): 1–156. [Google Scholar]
- 9. Hu FB, Manson J, Stampfer M, et al Diet, lifestyle and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345: 790–797. [DOI] [PubMed] [Google Scholar]
- 10. Franz MJ, Boucher JL, Rutten‐Ramos S, et al Lifestyle weight‐loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta‐analysis of randomized clinical trials. J Acad Nutr Diet 2015; 115: 1447–1463. [DOI] [PubMed] [Google Scholar]
- 11. Wens J, Vermeire E, Royen PV, et al GPs’ perspectives of type 2 diabetes patients’ adherence to treatment: a qualitative analysis of barriers and solutions. BMC Fam Pract 2005; 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schnell OAH, Battelini T, Ceriello A, et al Self‐monitoring of blood glucose in type 2 diabetes: recent studies. J Diabetes Sci Technol 2013; 7: 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erbach M, Freckmann G, Hinzmann R, et al Interferences and limitations in blood glucose self‐testing: an overview of the current knowledge. J Diabetes Sci Technol 2016; 10: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Czupryniak L, Barkai L, Bolgarska S, et al Self‐monitoring of blood glucose in diabetes: from evidence to clinical reality in Central and Eastern Europe–recommendations from the international Central‐Eastern European expert group. Diabetes Technol Ther 2014; 16: 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen NA, Fain JA, Braun B, et al Continuous glucose monitoring in non‐insulin‐using individuals with type 2 diabetes: acceptability, feasibility, and teaching opportunities. Diabetes Technol Ther 2009; 11: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta‐analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 2013; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dunkley AJ, Charles K, Gray LJ, et al Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta‐analysis. Diabetes Obes Metab 2012; 14: 616–625. [DOI] [PubMed] [Google Scholar]
- 18. Battelino T, Liabat S, Veeze HJ, et al Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diab Med 2015; 32: 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joanna Briggs Institute (JBI) . “The Joanna Briggs Institute Reviewer’ Manual: 2015 edition / Supplement.” Available from: http://joannabriggs.org/assets/docs/sumari/Reviewers-Manual_Methodology-for-JBI-Scoping-Reviews_2015_v2.pdf Accessed February 12, 2017.
- 20. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self‐monitoring of diabetes mellitus. Diabetes Metab Syndr: Clin Res Rev 2017. https://doi.org/10.1016/j.dsx.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 21. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther 2017; 19: S25–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoeks L, Greven WL, de Valk HW. Real‐time continuous glucose monitoring system for treatment of diabetes: a systematic review. Diab Med 2011; 28: 386–394. [DOI] [PubMed] [Google Scholar]
- 23. Cox DJ, Taylor AG, Singh H, et al Glycemic load, exercise, and monitoring blood glucose (GEM): a paradigm shift in the treatment of type 2 diabetes mellitus. Diabetes Res Clin Pract 2016; 111: 28–35. [DOI] [PubMed] [Google Scholar]
- 24. Vigersky RA, Fonda SJ, Chellappa M, et al Short‐ and long‐term effects of real‐time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012; 35: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ehrhardt NM, Chellappa M, Walker MS, et al The effect of real‐time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011; 5: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang TS, Digby EM, Wright AM, et al Real‐time continuous glucose monitoring versus internet‐based blood glucose monitoring in adults with type 2 diabetes: a study of treatment satisfaction. Diabetes Res Clin Pract 2014; 106: 481–486. [DOI] [PubMed] [Google Scholar]
- 27. Yoo HJ, An HG, Park SY, et al Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 2008; 82: 73–79. [DOI] [PubMed] [Google Scholar]
- 28. Allen NA, Fain JA, Braun B, et al Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 2008; 80: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen N, Whittemore R, Melkus G. A continuous glucose monitoring and problem‐solving intervention to change physical activity behavior in women with type 2 diabetes: a pilot study. Diabetes Technol Ther 2011; 13: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 30. Beck RW, Riddlesworth TD, Ruedy K, et al Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Annals Intern Med 2017; 167: 365–374. [DOI] [PubMed] [Google Scholar]
- 31. Anjana RM, Kesavadev J, Neeta D, et al A multicenter real‐life study on the effect of flash glucose monitoring on glycemic control in patients with type 1 and type 2 diabetes. Diabetes Technol Ther 2017; 19: 533–540. [PubMed] [Google Scholar]
- 32. Haak T, Hanaire H, Ajjan R, et al Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter, open‐label randomized controlled trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cox DJ, Taylor AG, Moncrief M, et al Continuous glucose monitoring in the self‐management of type 2 diabetes: a paradigm shift. Diabetes Care 2016; 39: E71–E73. [DOI] [PubMed] [Google Scholar]
- 34. Allen NA, Jacelon CS, Chipkin SR. Feasibility and acceptability of continuous glucose monitoring and accelerometer technology in exercising individuals with type 2 diabetes. J Clin Nurs 2009; 18: 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohan V, Jain S, Kesavadev J, et al Use of retrospective continuous glucose monitoring for optimizing management of type 2 diabetes in India. J Assoc Phys India 2016; 64: 16–21. [PubMed] [Google Scholar]
- 36. Klonoff DC, Buckingham B, Christiansen JS, et al Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011; 96: 2968–2979. [DOI] [PubMed] [Google Scholar]
- 37. Bailey TS, Grunberger G, Bode BW, et al American Association of Clinical Endocrinologists and American College of Endocrinology 2016 Outpatient Glucose Monitoring Consensus Statement. Endocr Pract 2016; 22: 231–261. [DOI] [PubMed] [Google Scholar]
- 38. Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther 2017; 19: S4–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qiu S, Cai X, Schumann U, et al Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta‐analysis. PLoS One 2014; 9: e109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Figueira FR, Umpierre D, Casali KR, et al Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: crossover randomized trial. PLoS One 2013; 8: e57733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. The Juvenile Diabetes Research Foundation CGM monitoring Study Group . Continuous glucose monitoring and intenstive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 42. Terranova CO, Brakenridge CL, Lawler SP, et al Effectivenss of lifestyle‐based weight loss interventions for adults with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab 2015; 17: 371–378. [DOI] [PubMed] [Google Scholar]
- 43. Stratton IMA, Neil HA, Matthews DR, et al Association of glycaemia with macrovascular and mictrovasular complications of type 2 diabetes (UKPD 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lorenzo C, Okoloise M, Williams K, et al The metabolic syndrome as predictor of type 2 diabetes ‐ The San Antonio Heart Study. Diabetes Care 2003; 26: 3153–3159. [DOI] [PubMed] [Google Scholar]
- 45. Wander PL, Boyko EJ, Leonetti DL, et al Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care 2013; 36: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gomez‐Ambrosi J, Silva C, Galofre JC, et al Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring) 2011; 19: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 47. Ross SA, Dzida G, Vora J, et al Impact of weight gain on outcomes in type 2 diabetes. Curr Med Res Opin 2011; 27: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 48. DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010; 53: 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wing RR, Look Ahead Research Group . Long‐term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four‐year results of the Look AHEAD trial. Arch Intern Med 2010; 170: 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wing RR, Lang W, Wadden TA, et al The Look AHEAD research group benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011; 34: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ardisson Korat AV, Willett WC, Hu FB. Diet, lifestyle, and genetic risk factors for type 2 diabetes: a review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow‐up Study. Curr Nutr Rep 2014; 3: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karstoft K, Winding K, Knudsen SH, et al The effects of free‐living interval‐walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2013; 36: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baker RC, Kirshenbaum DS. Self‐monitoring may be necessary for successful weight control. Behav Ther 1993; 24: 377–394. [Google Scholar]
- 54. Bailey KJ, Little JP, Jung ME. Self‐monitoring using continuous glucose monitors with real‐time feedback improves exercise adherence in individuals with impaired blood glucose: a pilot study. Diabetes Technol Ther 2016; 18: 185–193. [DOI] [PubMed] [Google Scholar]
- 55. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther 2016; 18(Suppl 2): S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Borges U Jr, Kubiak T. Continuous glucose monitoring in type 1 diabetes. J Diabetes Sci Technol 2016; 10: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joubert M, Reznik Y. Personal continuous glucose monitoring (CGM) in diabetes management: review of the literature and implementation for practical use. Diabetes Res Clin Pract 2012; 96: 294–305. [DOI] [PubMed] [Google Scholar]
- 58. Blumer I. The contemporary role of masked continuous glucose monitoring in a real‐time world. J Diabetes Sci Technol 2016; 10: 790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner J, Tennen H, Wolpert H. Continuous glucose monitoring: a review for behavioral researchers. Psychosom Med 2012; 74: 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rodbard D. Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgrad Med 2011; 123: 107–118. [DOI] [PubMed] [Google Scholar]


