Abstract
In Indo-Gangetic plains (IGP) of India, natural resources (soil, water, and environment) are degrading under the conventional–till (CT)-based management practices in rice–wheat cropping system. A long-term field experiment was conducted to understand the soil bacterial diversity and abundance under different sets of management scenarios (Sc). The study comprised of four scenarios, namely, -Sc.I CT-based rice–wheat system (farmers’ practice); Sc.II, partial conservation agriculture (CA) based in which rice is under CT—wheat and mungbean under zero-tillage (ZT); Sc.III, full CA-based in which rice–wheat–mungbean are under ZT and Sc.IV, where maize–wheat–mungbean are under ZT. These scenarios varied in cropping system, tillage, and crop residue management practices. Using Illumina MiSeq sequencing technology, the variable regions V3–V4 of 16S rRNA were sequenced and the obtained reads were analyzed to study the diversity patterns in the scenarios. Results showed the presence of 53 bacterial phyla across scenarios. The predominant phyla in all scenarios were Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes which accounted for more than 70% of the identified phyla. However, the rice-based systems (Sc.I, Sc.II, and Sc.III) were dominated by phylum Proteobacteria; however, maize-based system (Sc.IV) was dominated by Acidobacteria. The class DA052 and Acidobacteriia of Acidobacteria and Bacteroidetes of Bacteroidia were exceptionally higher in Sc.IV. Shannon diversity index was 8.8% higher in Sc.I, 7.5% in Sc.II, and 2.7% in Sc.III compared to Sc.IV. The findings revealed that soil bacterial diversity and abundance are influenced by agricultural management practices as bacterial diversity under full CA-based management systems (Sc.III and Sc.IV) was lower when compared to farmer’s practice (Sc.I) and partial CA (Sc.II) scenarios.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1317-9) contains supplementary material, which is available to authorized users.
Keywords: Acidobacteria, Bacterial diversity, Conservation agriculture, Metagenome, Proteobacteria
Introduction
Agricultural practices have major role in deciding diversity of soil microorganisms in any agro-ecosystem. Soil quality (physical, chemical, and biological) parameters are significantly influenced by the crop management practices which, in turn, affect the abundance, diversity, and activity of microorganisms (Ceja-Navarro et al. 2010; Pascault et al. 2010). Not only soil, crop rotation, plant diversity, and residue management also affect soil microorganisms in different ways (Silva et al. 2013). Intensive-till/conventional agriculture and zero-till/conservation agriculture-based management practices have implications on soil moisture, thermal regime, and nutrient dynamics (Gathala et al. 2013; Indoria et al. 2017) that, in turn, influence distribution and abundance of microorganisms (Smith et al. 2016).
In the conventional–till (CT)-based rice–wheat cropping systems of North-West Indo-Gangetic Plains (IGP) of India, farmers preferred clean cultivation by repeated tillage operations and in-situ burning of residues (Lohan et al. 2018). The conventional agriculture management practices of cultivation has resulted in natural resource degradation (soil, water, and energy), low factor productivity, nutrient deficiencies, ground water depletion, and greenhouse gas emission through crop reside burning (Gathala et al. 2013; Jat et al. 2014). Tillage causes soil physical disturbance which, in turn, changes soil moisture, soil temperature, soil aeration, and placement of the crop residues within soil profile which influences biological properties of soil to a great extent (Kladivko 2001; Six et al. 2006). Intensive tillage operations are responsible for soil degradation through erosion (water and wind) and volatilization of fertilizer nutrients. Influence of tillage on soil properties and crop yield is controlled by soil type, climatic conditions, and cropping history (Halvorson et al. 2002). Adoption of CA-based cropping system management practices under different agro-ecologies can address the challenges of natural resource degradation, crises (energy, water, and labor), instability in crop yields, high production costs, low input (water and nutrient) use efficiency, and adverse effects of climate change (Hobbs et al. 2008; FAO 2014). CA embodies three inter-linked principles—minimum mechanical disturbance of soil (reduced or zero-tillage), maintaining a permanent cover on soil surface, diversified, and efficient crop rotations. CA practices in IGP are helpful in improving soil properties and nutrient availability (Jat et al. 2017). CA helps to build soil organic matter, conserves soil moisture and buffers against drought as well as temperature extremes by retaining the crop residues (Gathala et al. 2011) and, at the same time, arresting soil degradation, and provides environmental benefits through avoiding in-situ burning (Sharma et al. 2014).
In agro-systems, it is well known that bacterial diversity varies with wide range of indicators agro-system. The variation across crop production management practices, viz., tillage, crop establishment, water, nutrient, and residue management, influences the soil environment which alters the microbial diversity (Ashworth et al. 2017; Ceja-Navarro et al. 2010; Smith et al. 2016). In CA, impact of systems management practices on soil microbes is poorly known for taxonomic composition. In-depth understanding of the microbial communities in agricultural soils provides the path to study their roles in agro-ecosystem which was not possible by the traditional methods like culture-based methods and microscopic study. These traditional methods can only reveal a fraction of microbial diversity. Efforts were made to study the indigenous soil microbial taxonomic diversity and composition by high-throughput sequencing technologies using Illumina sequencing of 16S rRNA genes. These technologies helped in better assessing the vast diversity of soil microbial communities by generating thousands of sequences from a single-soil DNA sample (Roesch et al. 2007).
In cereal-based systems, overall diversity of microorganisms is affected by wide range of indicators like cropping system, tillage, crop residue, water, and nutrient management practices. Some researchers (Dong et al. 2017; Souza et al. 2016) studied the impact of tillage on soil bacterial diversity and found bacterial communities differed significantly between the different tillage treatments. However, information on bacterial diversity using metagenomics under CA-based systems with varied indicators is not available in IGP of India where 1.5 Mha (million hectares) area is under CA-based rice–wheat cropping system. Henceforth, need was realized to assess the impact of CA management practices on soil bacterial diversity in IGP to understand whether CA-based management may or may not have any significant effect on bacterial diversity. Under different climatic conditions, there have been reports that bacterial diversity increases with CA practices (Ceja-Navarro et al. 2010) and a few reports also shown its decrease (Lienhard et al. 2014) when compared to the conventional systems. Therefore, study was carried out after 6 years of continuous cultivation with the hypothesis that different management practices (CT vs CA) will differently influence soil bacterial diversity and abundance in cereal-based cropping system of IGP.
Materials and methods
Description of site and treatments
A field experiment was started at Indian Council of Agricultural Research-Central Soil Salinity Research Institute (ICAR-CSSRI) (29.42°N latitude, 76.57°E longitude, 243 msl elevation), Karnal, Haryana, India, in 2009. Climate of the region is semi-arid and sub-tropical with average annual rainfall of 670 mm, of which 75–80% received between June and September. The mean annual maximum temperature is 34 °C and minimum is 18 °C. However, in June, daily temperatures rise from 41 to 44 °C, and in January, temperatures go to a minimum of 0 to 4 °C. The relative humidity varies from 55 to 90% throughout the year. The treatments comprised of four cereal-based scenarios varying in cropping system, tillage, crop establishment methods, and residue management practices. Treatments were replicated thrice and organized in a randomized complete block design in production-scale plots, each measuring of 2000 m2 (20 m × 100 m). The scenarios were designed keeping in view present as well as future drivers of agricultural changes in the region (Table 1) and their details can be obtained from earlier publication (Gathala et al. 2013).
Table 1.
Different scenarios of conservation agriculture management practices
| Scenario | Practices | Cropping system | Management system |
|---|---|---|---|
| Sc.I | Farmers’ practice | Rice–wheat | Rice-puddling, wheat-conventional till; all residues removed |
| Sc.II | Integrated crop and resource management | Rice–wheat–mungbean | Rice-puddling, Wheat-zero-till, Mungbean-zero-till; full (100%) rice residue retained; partial (30%) wheat residue (anchored); and full (100%) mungbean residues incorporated during puddling in rice season |
| Sc.III | Conservation agriculture-based systems | Rice–wheat–mungbean | Rice-zero-till, Wheat-zero-till, Mungbean-zero-till; full rice and mungbean, whereas partial (anchored) wheat residue retained on soil surface |
| Sc.IV | Futuristic conservation agriculture-based systems | Maize–wheat–mungbean | Maize-zero-till, Wheat-zero-till, Mungbean-zero-till; full maize and mungbean, whereas partial (anchored) wheat residue retained on soil surface |
Soil sampling and processing
Soil samples were taken after harvesting of wheat after 6 years (May 2015) of continuous experiment with the same management practices. Crop residues were removed from surface and soil samples were taken from replicated plots. Four samples were randomly taken from each plot at 0–15 cm soil depth aseptically using an auger and thoroughly mixed to make a composite sample. After sampling, soil samples were immediately transferred to laboratory for chemical analysis and DNA extraction.
Physical and chemical analyses of soil samples
Soil pH and electrical conductivity (EC) in soil: water ratio of 1:2 was determined by following standard methods (Jackson 1973). Soil bulk density (BD) was measured by core method collecting soil cores at 0–15 cm depth (Blake and Hartge 1986) after harvest of wheat in May 2015. The oxidizable organic carbon (OC) content of the soils was determined using method of Walkley and Black (1934). The available nitrogen (N) in soil was determined by alkaline permanganate method (Subbiah and Asija 1956).
DNA extraction and sequencing analysis
DNA was extracted from soil samples using PowerSoil® DNA isolation kit (MO BIO Laboratories Inc., Carlsbad, California, USA) following the manufacturer’s protocol. Purity and quantity of DNA were verified using electrophoresis in 1% agarose gels and its quantity were adjusted to 50 ng/µL. The purity of the DNA was assessed using ND1000 nanodrop spectrophotometer (Thermo Scientific, MA, USA) at 260 and 280 nm. DNA of replicated samples of each treatment was pooled before sequencing.
Amplicon library construction
The library was constructed using the 16S Metagenomic Sequencing library preparation methodology (http://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html). Each sample with a gel-purified fragment (~ 4 ng) was used for amplifying V3-V4 region of 16S rRNA region with specific primers that has a tag sequence that are complementary to the Illumina sequence adapter and index primers from the Nextera XT Index kit V2 (Illumina, #FC-131-2002) which resulted ~ 430–480 bp amplicons. Amplification was verified on 1% agarose gel before proceeding for indexing PCR. In the next round of PCR (indexing PCR), Illumina sequencing adapters and dual-indexing barcodes were added using limited cycle PCR to generate a final product of ~ 570–620 bp. High Prep PCR post-PCR clean up system (MagBio Genomics, Inc., USA) was used to clean the libraries and quality control check of the library was performed using High Sensitivity Tape Station (Agilent Technologies, USA). PCR products with unique indices from each library were taken in equal (~ 4 ng) quantities and subjected to paired-end sequencing using Illumina sequencing platform.
Illumina adapter sequences:
5′AATGATACGGCGACCACCGAGATCTACAC [i5] TCGTCGGCAGCGTC 3′.
5′CAAGCAGAAGACGGCATACGAGAT [i7] GTCTCGTGGGCTCGG 3′.
[i5, i7]—unique dual index sequence to identify sample-specific sequencing data.
Sequencing and initial processing of sequence reads
The PCR products from each library having unique indices were taken in equal nanomolar quantities and were sequenced using Illumina MiSeq sequencer at Genotypic Technology Pvt. Ltd. (Bangalore, India). All the samples were pooled in a single lane followed by image analysis and base calling using Illumina Analysis pipeline V 2.2. The generated paired-end raw reads were demultiplexed using bcl2fastq v 2.17 tool by allowing and 1 bp mismatch in index1 and index2 barcodes, respectively. The reads from each sample were subjected to quality check using FastQC v 0.11.4. Reads having primer sequence and high-quality bases with phred score (> Q20) were processed further for analysis. Less than 2% of Ns were allowed in the sequence ends. Base quality < Q20 towards the end were removed from the sequence ends. Sequences were stitched by reverse complementing R2 reads and merging with R1 reads. These stitched reads were analyzed using QIIME-1.9.1 analysis workflow.
Data analysis
All sequences were processed using QIIME-1.9.1 software package. The reads were clustered at 97% identity against the chimera free Greengenes database (version 13.8) using UCLUST v1.2.22q. “Closed reference” approach was performed for this study where the reads that failed to match the reference sequences were discarded. Taxonomies were assigned from the reference database based upon the identity of the reference sequence, Operational taxonomic Unit (OTU) ids clustered against. The number of raw reads per sample were 165,936 (Sc.I), 208,264 (Sc.II), 178,895 (Sc.III), and 157,104 (Sc.IV) bps. The OTU table was rarefied to a sampling depth of 40,000 sequences per sample with step size of ten interactions. Alpha diversity indexes were determined based on the rarefied biome. The 97% OTUs phylogenetic tree supplied with Greengenes database was used for calculation of beta diversity using weighted and unweighted Unifrac distances. Supplementary S1 has software details on OTU picking analysis and graphical visualizations.
Graphical visualization and statistical analysis
Heatmap and stacked barcharts were generated at phylum and class level, respectively, with relative abundance values ≥ 1% for all the samples from various scenarios in R 3.3.0 environment. Heatmap was plotted using NMF package with a heatmap function applying pairwise distance correlation method and stacked barcharts was generated using ggplot2. STAMP (Statistical Analysis of Metagenomic Profiles) tool was used for significance testing between two samples at order level and the corresponding significant orders (P < 0.05) were plotted as extended error barcharts for a pair of scenarios using Fisher’s exact test with Storey-FDR correction and the difference between proportion was calculated using Newcombe–Wilson method (with confidence interval 95%) (Supplementary S2). Column barcharts generated using R ggplot2 were compared across four scenarios at order level for a few classes of Proteobacteria.
Soil chemical and physical data were subjected to analysis of variance (ANOVA) and using the general linear model (GLM) procedure of the SPSS window version 17.0 (SPSS Inc., Chicago, USA). Treatment means were separated by Duncan Multiple Range Test at 5% level of significance (P < 0.05).
Nucleotide sequence accession number
Paired-end Illumina sequence data were submitted to the NCBI sequence Read Archive (SRA) under Bioproject accession number PRJNA348170 which consists of four biosample accession numbers SAMN05905538 (Sc.I), SAMN05905539 (Sc.II), SAMN05905540 (Sc.III), and SAMN05905541 (Sc.IV).
Results
Soil chemical parameters in different scenarios
In this study, scenario 1 (Sc.I) was based on farmers practice and scenario 2 (Sc.II) was based on partial CA-based, whereas scenarios 3 and 4 (Sc.III and Sc.IV) were based on full CA-based practices (Table 1). Results indicated that, after 6 years of continuous cultivation, EC was not affected by management practices as it did not differ significantly among scenarios. Full CA-based system recorded the lower pH and higher organic carbon and nitrogen than the partial CA and the conventional based system. Soil pH was recorded lower in Sc.IV (7.61) compared to Sc.I (7.89) and Sc.II (7.83), and it was followed by Sc.III (7.70). The CA-based scenarios (Sc.II to IV) recorded higher organic carbon (OC) and nitrogen (N) content averaged 0.78% and 178.17 kg/ha, respectively, compared to Sc.I (0.47% and 130.9 kg/ha). OC content was increased by 45, 77, and 79% in Sc.II, Sc.III, and Sc.IV, respectively, when compared to Sc.I (0.47%). Similar trend was recorded with N content where it was increased by 21, 48, and 49% in Sc.II, Sc.III, and Sc.IV, respectively, compared to Sc.I (130.9 kg/ha). Similarly, lower bulk density was recorded with CA-based scenarios (Sc.II to IV) averaged 1.56 Mg/m3 compared to Sc.I (1.63 Mg/m3) (Table 2).
Table 2.
Soil chemical and physical parameters
| Scenario | EC (dS/m) | pH | OC (%) | N (kg/ha) | BD (mg/m3) |
|---|---|---|---|---|---|
| Sc.I | 0.26 ± 0.03a | 7.89 ± 0.12a | 0.47 ± 0.04c | 130.9 ± 1.33c | 1.63 ± 0.01a |
| Sc.II | 0.23 ± 0.04a | 7.83 ± 0.13a | 0.68 ± 0.05b | 158.3 ± 2.06b | 1.57 ± 0.03b |
| Sc.III | 0.22 ± 0.05a | 7.70 ± 0.11ab | 0.83 ± 0.08a | 193.8 ± 1.09a | 1.56 ± 0.04b |
| Sc.IV | 0.22 ± 0.03a | 7.61 ± 0.18b | 0.84 ± 0.10a | 182.4 ± 2.01a | 1.55 ± 0.06b |
Different small letters within the same column show the significant difference at P = 0.05 according to Duncan Multiple Range Test for separation of mean
EC electric conductivity, OC organic carbon, BD bulk density
Bacterial abundance/ community composition in different scenarios
A total of 710,199 raw reads were obtained from all four scenarios and 478,913 stitched reads were considered for analysis after processing. A total of ~ 42% of the stitched reads from all four samples after processing were as assigned into different OTUs with reference to the Greengenes database. The average read length for all the samples were ~ 410 bp. A total of 10,570 unique OTUs were detected from all the samples at 97% sequence similarity including the singletons. Rarefaction curve generated at a read depth of 40,000 displayed an asymptotic curve indicating the saturation profiling of the bacterial communities (Supplementary S3).
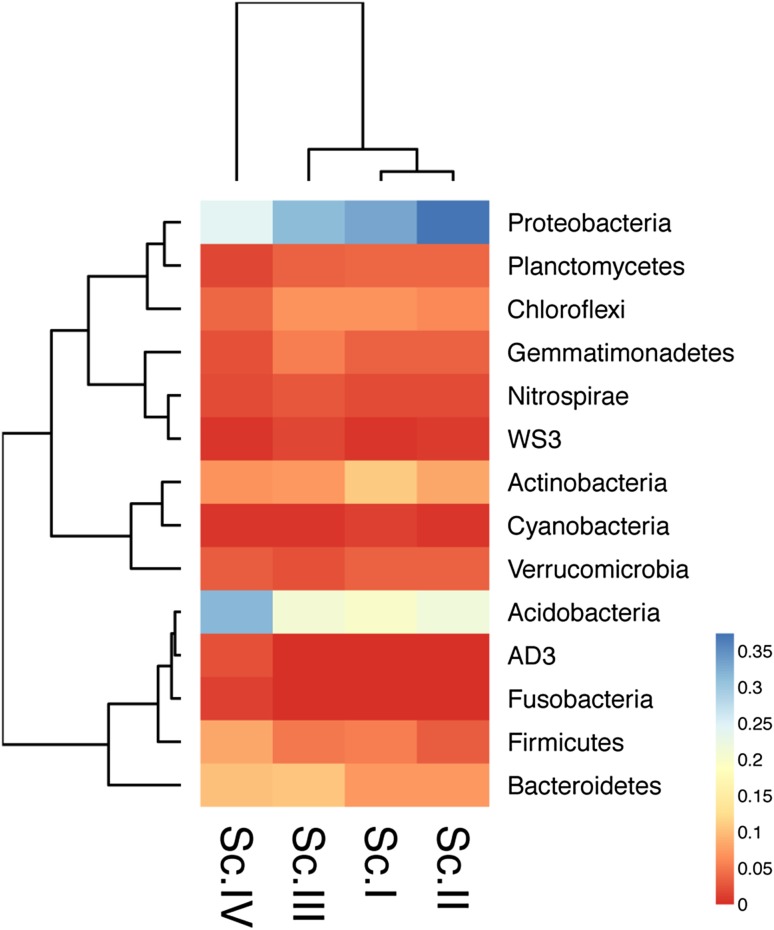
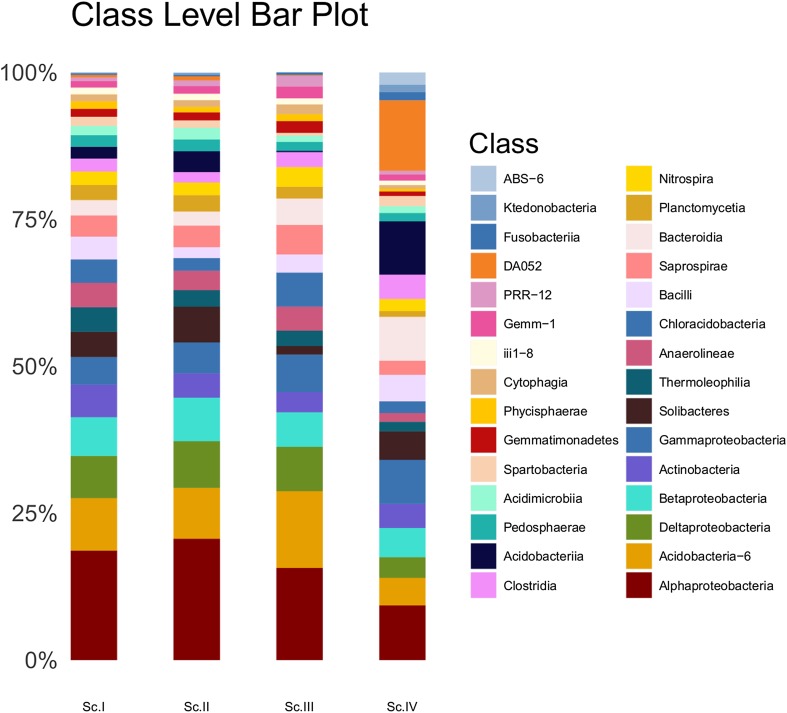
A total of 53 phyla were detected from all the samples; most of them were present in all scenarios. The predominant phyla in all scenarios were Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes which accounted for more than 70% of the identified phyla’s, in each scenario, present in soil (Table 3). First three scenarios (Sc.I–III) were dominated by phylum Proteobacteria and it was followed by Acidobacteria. Conversely, in Sc.IV, dominating phylum was Acidobacteria followed by Proteobacteria. Three phyla of archaea—Crenarchaeota, Euryarchaeota, and Parvarchaeota—were also reported but their abundance was very low (< 0.007%). To understand the clustering pattern for samples and their abundance profile across four scenarios, a biclustering heatmap (Fig. 1) was generated based on the relative abundance of the bacterial phyla contributing greater than 1%. This figure shows that bacterial community in Sc.I and Sc.II was closely clustered and distantly related to Sc.IV. Further stacked barchart was generated at class level (Fig. 2) to visualize the abundance percentage of the microbial communities residing across four scenarios. It was found that distribution of classes was similar in Sc.I, Sc.II, and Sc.III but differs in Sc.IV. Class Alphaproteobacteria of Proteobacteria was the most abundant class followed by Acidobacteria-6 of Acidobacteria in all scenarios except in Sc.IV. The most abundant class in Sc.IV is DA052 of Acidobacteria followed by Alphaproteobacteria. Apart from higher abundance of DA052, abundance of Acidobacteriia was also exceptionally higher in Sc.IV. As mentioned above the most dominating phylum is Proteobacteria, which comprises four major classes—Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, and Deltaproteobacteria. Further comparative analysis was performed for these classes of Proteobacteria at order level (Fig. 3a–d). The class Alphaproteobacteria showed majority of reads of order Rhizobiales, Rhodospirillales, and Sphimgomonadales order. Deltaproteobateria was second most abundant class of Proteobacteria that was dominated by Myxococcales in all four scenarios and followed by Syntrophobacterales, Desulfuromonadales, and Entotheonellales. The order of Betaproteobacteria was dominated by Burkholderiales in all scenarios; MND1 was present in greater proportion in Sc.III, while Neisseriales showed more hits in Sc.IV compared to the others. Gammaproteobacteria had a higher proportion of Xanthomonadales followed by Thiotrichales and Pseudomonadales.
Table 3.
Distribution of dominant phyla in conservation agriculture scenarios
| Scenario | Dominant phyla (%) | |||
|---|---|---|---|---|
| Sc.I | Proteobacteria (33) | Acidobacteria (20) | Actinobacteria (11) | Bacteroidetes (7) |
| Sc.II | Proteobacteria (37) | Acidobacteria (22) | Actinobacteria (9) | Bacteroidetes (7) |
| Sc.III | Proteobacteria (31) | Acidobacteria (21) | Bacteroidetes (11) | Actinobacteria (7) |
| Sc.IV | Acidobacteria (32) | Proteobacteria (24) | Bacteroidetes (10) | Actinobacteria (7) |
Fig. 1.
Phylum level biclustering heatmap and this is performed on the top 1% of the Phyla based on the relative abundance values. The heatmap was generated using NMF package with a heatmap function with pairwise distance correlation method. The color code scale represents the relative abundance values (https://cran.r-project.org/web/packages/NMF/NMF.pdf)
Fig. 2.
Stacked barchart generated at class level for samples across four scenarios with relative values ≥ 1%
Fig. 3.
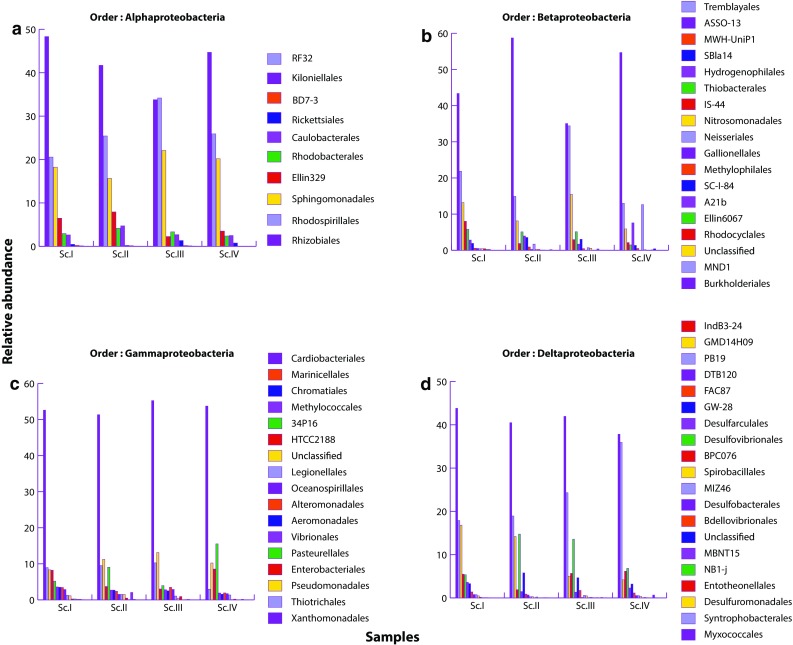
Column barcharts showing the relative abundance of top orders identified in a Alphaproteobacteria; b Betaproteobacteria; c Gammaproteobacteria; d Delta proteobacteria
Bacterial diversity in different scenarios
Different alpha diversity indices such as Shannon diversity index, chao1, PD_whole tree, and observed species were measured. Phylogenetic diversity of the individual samples was calculated for species richness (Chao1) and Faith’s diversity index (PD_whole tree). Converging trend in the alpha diversity values of chao1, PD_whole tree, and observed species were recorded. Shannon diversity index was 8.7% higher in Sc.I, 7.3% in Sc.II, and 2.6% in Sc.III compared to Sc.IV. The Chao1 diversity was also higher by 27.09% in Sc.I, 17.84% in Sc.II and almost similar in Sc.III as compared to Sc.IV (Table 4).
Table 4.
Alpha diversity of bacteria in soils of conservation agriculture scenarios
| Scenario | Shannon diversity index | Chao1 | PD_whole_tree | Observed species |
|---|---|---|---|---|
| Sc.I | 11.133 | 8690.763 | 249.123 | 6000 |
| Sc.II | 10.990 | 8058.105 | 239.627 | 5675 |
| SC.III | 10.507 | 6898.574 | 217.194 | 4737 |
| Sc.IV | 10.240 | 6838.160 | 207.602 | 4650 |
Discussion
Soil chemical parameters in different scenarios
In full CA-based scenarios (Sc.III and Sc.IV), lower pH was observed which attributed to more accumulation of organic matter in the upper surface layer (0–15 cm) under CA-based management which causes increase in electrolytes concentration and reduction in pH. Similarly Rahman et al. (2008) also reported lower value of pH with no tillage compared to the conventional tillage. Higher content of OC and N was recorded with CA-based management scenarios due to zero-tillage coupled with huge residue retention of 64.3 t/ha in Sc.II, 64.8 t/ha in Sc.III and 80.4 t/ha in Sc.IV, respectively, when compared to Sc.I (no residue retention) in the last 5 years. Residue retention helped in increasing the soil physico-chemical and biological properties (Jat et al. 2015; Choudhary et al. 2018a, b) which results in improved OC and N content in soil. Similar findings of increased SOC and N were also reported by other researchers (Jat et al. 2017; Mu et al. 2016). The conventional tillage practices deteriorate soil structure resulted in lower SOC and total nitrogen due to higher mineralization and leaching rate (During et al. 2002). In CA-based practices, lower bulk density might be attributed to increase in soil organic matter, increase in activities of soil micro- and macroorganisms, and improved aggregation compared to the conventional tillage practices (Blanco-Canqui et al. 2005; Choudhary et al. 2018b; Gupta et al. 2000; Singh and Sidhu 2014).
Abundance pattern of bacterial phyla in scenarios
The cropping systems, management practices, and recycled residues affected the dominance of bacterial phyla. Proteobacteria, Acidobacteria, Actinobacteria, and Bacteroidetes were among the most abundant phyla in the all samples, this result is consistent with the previous studies (Navarro-Noya et al. 2013; Wang et al. 2016). Proteobacteria was the dominating phyla in rice-based cropping systems (Sc.I, Sc.II and Sc.III) where lower amount of residue recycled. However, Acidobacteria was found dominating in maize-based cropping system (Sc.IV) where higher amount of residue recycled. Proteobacteria is the largest and phenotypically most diverse division amongst prokaryotes (Gupta 2000), and found in many different shapes which reflect enormous morphological and physiological diversity within this phylum (Stackebrandt et al. 1988). Acidobacteria is one of the most distributed bacterial groups which is abundantly present in the environment (Hugenholtz et al. 1998), but its abundance is usually reported lower than Proteobacteria (Zhao et al. 2014). In the study of Meisinger et al. (2007), it was found that some members of Acidobacteria were always associated with Gammaproteobacteria. This association is the result of ecological relationship between Proteobacteria and Acidobacteria which may influence each other’s position in the community (Kielak et al. 2016) as, in our study, both were found in dominance. These bacteria may be an important contributor to agriculture ecosystems, as they are particularly abundant within soils. Although high abundance and diversity of Acidobacteria were reported, but relatively less information about the activities and ecology of the members of this phylum are available, because majority of these are difficult to cultivate and lack in bacterial culture collections (Eichorst et al. 2011; Navarrete et al. 2013). Acidobacteria and Bacteroidetes tend to inhabit eco-niches supplied with plant-derived organic matter, where these are specializing in their degradation (Naumoff and Dedysh 2012) as it was evident from the presence of exceptionally higher abundance of members of Acidobacteria (DA052 and Acidobacteriia) and Bacteroidetes (Bacteroidia) in Sc.IV (Fig. 2). In the Sc.IV (maize-based systems), large quantities of residue were recycled which might be responsible for making soil environment more acidic than other scenarios (Jat et al. 2017). The lower pH may favor the occurrence of some groups of Acidobacteria (DA052 and Acidobacteriia) (Shen et al. 2013; Fierer et al. 2007; Navarrete et al. 2013). Apart from amount of residues, SOC was also higher in Sc.IV that might be resulted in higher abundance of Acidobacteria (Table 3), and theses are in line of work done by Navarrete et al. (2013) and Liu et al. (2016). A decline in proteobacterial population was observed in organically managed soils compared to the conventional managed soils (Aparna et al. 2014; Chinnadurai et al. 2014). Smit et al. (2001) hypothesized that Proteobacteria and Acidobacteria (P/A) ratio may provide insight into the general soils nutrient status; high and low P/A ratio would be indicative of copiotrophic and oligotrophic soils, respectively. In our study, low P/A ratio was observed in maize-based system (Sc.IV). In Sc.IV, higher amount of maize residue were present than any other crop residue. Maize residues (maize stalks) are not easily decomposes because of wide C/N ratio and less contact with surface compared to rice residue, and this may create oligotrophic conditions as it was evident by the presence of exceptionally higher abundance of Ellin6513 and Acidobacteriales which are orders under Acidobacteria in Sc.IV (Supplementary S2f). Acidobacteriales was dominated by Koribacteraceae. Ellin6513 and Koribacteraceae are considered as oligotrophs by some researchers (Fierer et al. 2007). Negative correlation between abundance of Acidobacteria and carbon (C) mineralization rates, and positive correlation of β-Proteobacteria and Bacteroidetes abundances with C mineralization were reported by Fierer et al. (2007). Similarly, in our study, positive correlation of C mineralization and β-Proteobacteria was found in Sc.II where the highest C mineralization rate and highest abundance of β-Proteobacteria (Fig. 3b) were recorded. However, no correlation was found between abundance of Acidobacteria and C mineralization rates in other scenarios. Highest abundance of Acidobacteria (Table 3) was recorded with Sc.IV, while the lowest C mineralization was recorded with Sc.I (61.4 µg C g−1) compared to other scenarios (Sc.II-91.5 µg C g−1, Sc.III-70.74 µg C g−1 and Sc.IV-83.64 µg C g−1), while the highest C mineralization was recorded in Sc.II which has the highest abundance of β-Proteobacteria (Fig. 3b).
Bacterial community composition at class and lower level
Soil bacterial community composition was influenced by CA-based management practices. In this study, it was observed that, within phylum Proteobacteria, the most abundant class was Alphaproteobacteria which was followed by Deltaproteobacteria, Betaproteobacteria, and Gammaproteobacteria in all scenarios. Similar trend of abundance of classes was also reported by Spain et al. (2009) in their crop fields. The Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, and Deltaproteobacteria were dominated by the members of Rhizobiales, Burkholderiales, Xanthomonadales, and Myxococcales, respectively. Castro et al. (2010) also reported the same trend of dominance except for Gammaproteobacteria, which was dominated by Chromatiales and Enterobacteriales in their study. Alphaproteobacteria are one of the most studied bacterial classes (Ettema and Andersson 2009) which have different roles in ecosystem like- plant-growth promoter (Azospirillum), plant symbiont (Rhizobium, Azorhizobium, Mesorhizobium, and Sinorhizobium), phototrophs (Rhodobacter), and pathogens (Agrobacterium, Brucella, and Rickettsia). The Betaproteobacteria dominating by Burkholderia plays different roles in soils like biological control of pathogens (Parke and Gurian-Sherman 2001), plant-growth promotion (Nehra and Choudhary 2015), and nitrogen fixation (Wong-Villarreal and Caballero-Mellado 2010). Chemolithotrophic genera related to nitrification, including Nitrosomonas and Nitrosospira, are also important members of the Betaproteobacteria (Schmidt and Bock 1997; Shaw et al. 2006). The member of Deltaproteobacteria may also plays important role in the availability of micronutrients for plants and soil microbiota, as they reported to be involved in the reduction of iron and sulphate (Foti et al. 2007; Hori et al. 2010). The Gammaproteobacteria class comprises several important bacteria like Xanthomonas and Pseudomonas spp. Pseudomonas is one of the most commonly described genera possessing plant-growth promoting activities (Nehra and Choudhary 2015), whereas Xanthomonas campestris is widely known as pathogenic bacterium of soil (Boch and Bonas 2010). Although, by the high-throughput sequencing methods, we are able to study more bacterial groups than the traditional cultural methods but very limited information is available on these bacterial groups, because it is very often that many of these sequences do not match with known sequences of bacterial species. Only fractions of the soil bacterial species are known, and hence, it is a major challenge for researchers to identify and culture them to study their role in their eco-niches.
Diversity of bacteria in scenarios
Soil microbial diversity is considered to be critical to the integrity, function, and long-term sustainability of soil ecosystems (Kennedy and Smith 1995). Bacterial diversity was more in the conventional practices (Sc.I) compared to CA-based scenarios (Sc.II to IV). Degrune et al. (2016) and Lienhard et al. (2014) also reported similar trend as in the conventional practices bacterial richness and diversity increases, whereas some reported inverse of this trend that they the found highest levels of bacterial diversity and richness in soils under CA (zero-tillage with crop residue retention)-based system (Constancias et al. 2014; Ceja-Navarro et al. 2010). In full CA-based scenarios (Sc.III and IV), high stability of aggregates was observed after 5 years of continuous cultivation (Choudhury et al. 2014) which may provide the stable environment to the microbes. Once the soil stabilizes a particular type of bacterial communities, this, in turn, may dominate any other variety of communities which may narrow down the bacterial diversity. Higher diversity in the conventional till soil may be due to the disturbance and exposure of soil to the different climatic conditions which causes release of organic matter, making it available for bacterial activities (Degrune et al. 2015).
Conclusion
Conservation agriculture (CA)-based management practices in cereal-based cropping system influenced the bacterial diversity and abundance. Higher bacterial diversity was recorded with farmers’ practice compared to CA-based practices. Most abundant phylum in rice and maize-based systems were Proteobacteria and Acidobacteria, respectively. The high abundance of Acidobacteria in Sc.IV was governed by tillage, crop rotation, and amount of crop residues recycled. At class level, Proteobacteria was dominated by Alphaproteobacteria, and it was closely followed by Deltaproteobacteria, Betaproteobacteria, and Gammaproteobacteria. In this study, a number of reads belongs to many bacterial classes were recorded, but very little is known about their individual functions and role in the soil, and hence, more research is required to understand the influence of layering of crop management practices on bacterial species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge that this research was undertaken in collaboration with International Maize and Wheat Improvement Centre (CIMMYT) under Cereal Systems Initiative for South Asia (CSISA) project supported by Bill and Melinda Gates Foundation (BMGF), USAID and CGIAR Research Programs on Climate Change, Agriculture and Food Security (CCAFS), and Wheat Agri-food Systems (WHEAT). We also acknowledge the support received from the Director, ICAR-CSSRI, Karnal.
Author contributions
MC, PS, and HS designed the study; AM, ML, and HS gave the idea of study and supported the design; MC conducted the research; AD and BR analyzed the data; MC wrote the manuscript and HS assisted with revising the draft manuscript. All authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1317-9) contains supplementary material, which is available to authorized users.
References
- Aparna K, Pasha MA, Rao DLN, Krishnaraj PU. Organic amendments as ecosystem engineers: microbial, biochemical and genomic evidence of soil health improvement in a tropical arid zone field site. Ecol Eng. 2014;71:268–277. doi: 10.1016/j.ecoleng.2014.07.016. [DOI] [Google Scholar]
- Ashworth AJ, DeBruyn JM, Allen FL, Radosevich M, Owens PR. Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Biol Biochem. 2017;114:210–219. doi: 10.1016/j.soilbio.2017.07.019. [DOI] [Google Scholar]
- Blake GR, Hartge KH (1986) Bulk density. In: Methods of soil analysis. Klute A (ed). ASA and SSSA, Madison, Wisconsin, USA, 363–375
- Blanco-Canqui H, Lal R, Owens LB, Post WM, Izaurralde RC. Strength properties and organic carbon of soil in the North Appalachian region. Soil Sci Soc Am J. 2005;69:663–673. doi: 10.2136/sssaj2004.0254. [DOI] [Google Scholar]
- Boch J, Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phyto pathol. 2010;48:419–436. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW. Soil microbial community responses to multiple experimental climate change drivers. Appl Environ Microbiol. 2010;76:999–1007. doi: 10.1128/AEM.02874-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceja-Navarro JA, Rivera-Orduna FN, Patino-Zúniga L, Vila-Sanjurjo A, Crossa J, Govaerts B, Dendooven L. Phylogenetic and multivariate analyses to determine the effects of different tillage and residue management practices on soil bacterial communities. Appl Environ Microbiol. 2010;76:3685–3691. doi: 10.1128/AEM.02726-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai C, Gopalaswamy G, Balachandar D. Impact of long-term organic and inorganic nutrient managements on the biological properties and eubacterial community diversity of the Indian semi-arid Alfisol. Arch Agron Soil Sci. 2014;60(4):531–548. doi: 10.1080/03650340.2013.803072. [DOI] [Google Scholar]
- Choudhary M, Jat HS, Datta A, Yadav AK, Sapkota TB, Mondal S, Meena RP, Sharma PC, Jat ML. Sustainable intensification influences soil quality, biota, and productivity in cereal-based agroecosystems. Appl Soil Ecol. 2018;126:189–198. doi: 10.1016/j.apsoil.2018.02.027. [DOI] [Google Scholar]
- Choudhary M, Datta A, Jat HS, Yadav AK, Gathala MK, Sapkota TB, Das AK, Sharma PC, Jat ML, Singh R, Ladha JK. Changes in soil biology under conservation agriculture based sustainable intensification of cereal systems in Indo-Gangetic Plains. Geoderma. 2018;313:193–204. doi: 10.1016/j.geoderma.2017.10.041. [DOI] [Google Scholar]
- Choudhury SG, Srivastava S, Singh R, Chaudhari SK, Sharma DK, Singh SK, Sarkar D. Tillage and residue management effects on soil aggregation, organic carbon dynamics and yield attribute in rice–wheat cropping system under reclaimed sodic soil. Soil Tillage Res. 2014;136:76–83. doi: 10.1016/j.still.2013.10.001. [DOI] [Google Scholar]
- Constancias F, Prévost-Bouré NC, Terrat S, Aussems S, Nowak V, Guillemin JP, Bonnotte A, Biju-Duval L, Navel A, Martins JM, Maron PA. Microscale evidence for a high decrease of soil bacterial density and diversity by cropping. Agron Sustain Dev. 2014;34:831–840. doi: 10.1007/s13593-013-0204-3. [DOI] [Google Scholar]
- Degrune F, Dufrêne M, Colinet G, Massart S, Taminiau B, Bodson B, Hiel MP, Daube G, Nezer C, Vandenbol M. A novel sub-phylum method discriminates better the impact of crop management on soil microbial community. Agron Sustain Dev. 2015;35(3):1157–1166. doi: 10.1007/s13593-015-0291-4. [DOI] [Google Scholar]
- Degrune F, Theodorakopoulos N, Dufrêne M, Colinet G, Bodson B, Hiel MP, Taminiau B, Nezer C, Daube G, Vandenbol M. No favorable effect of reduced tillage on microbial community diversity in a silty loam soil (Belgium) Agric Ecosyst Environ. 2016;224:12–21. doi: 10.1016/j.agee.2016.03.017. [DOI] [Google Scholar]
- Dong W, Liu E, Yan C, Tian J, Zhang H, Zhang Y. Impact of no tillage vs. conventional tillage on the soil bacterial community structure in a winter wheat cropping succession in northern China. Eur J Soil Biol. 2017;80:35–42. doi: 10.1016/j.ejsobi.2017.03.001. [DOI] [Google Scholar]
- During RA, Thorsten H, Stefan G. Depth distribution and bioavailability of pollutants in long-term differently tilled soils. Soil Till Res. 2002;66:183–195. doi: 10.1016/S0167-1987(02)00026-0. [DOI] [Google Scholar]
- Eichorst SA, Kuske CR, Schmidt TM. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria. Appl Environ Microbiol. 2011;77:586–596. doi: 10.1128/AEM.01080-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema TJ, Andersson SG. The α-proteobacteria: the darwin finches of the bacterial world. Biol Let. 2009;5:429–432. doi: 10.1098/rsbl.2008.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO Conservation Agriculture Program (2014) CA adoption worldwide. http://www.fao.org/ag/ca/6c.html. Downloaded 26
- Fierer N, Bradford MA, Jackson RB. Toward an ecological classification of soil bacteria. Ecology. 2007;88:1354–1364. doi: 10.1890/05-1839. [DOI] [PubMed] [Google Scholar]
- Foti M, Sorokin DY, Lomans B, Mussmann M, Zacharova EE, Pimenov NV, Kuenen JK, Muyzer G. Diversity, activity and abundance of sulfate-reducing bacteria in saline and hypersaline soda lakes. Appl Environ Microbio. 2007;73:2093–2100. doi: 10.1128/AEM.02622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathala MK, Ladha JK, Saharawat YS, Kumar V, Kumar V, Sharma PK. Effect of tillage and crop establishment methods on physical properties of a medium-textured soil under a seven-year rice–wheat rotation. Soil Sci Soc Am J. 2011;75:1851–1862. doi: 10.2136/sssaj2010.0362. [DOI] [Google Scholar]
- Gathala M, Kumar V, Sharma PC, Saharawat Y, Jat HS, Singh M, Kumar A, Jat ML, Humphreys E, Sharma DK, Sharma S, Ladha JK. Optimizing intensive cereal-based cropping systems addressing current and future drivers of agricultural change in the northwestern Indo-Gangetic Plains of India. Agric Ecosyst Environ. 2013;177:85–97. doi: 10.1016/j.agee.2013.06.002. [DOI] [Google Scholar]
- Ghestem M, Sidle RC, Stokes A. The influence of plant root systems on subsurface flow: Implications for slope stability. Bioscience. 2011;61:869–879. doi: 10.1525/bio.2011.61.11.6. [DOI] [Google Scholar]
- Gupta RS. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol Rev. 2000;24:367–402. doi: 10.1111/j.1574-6976.2000.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Halvorson AD, Peterson GA, Reule CA. Tillage system and crop rotation effects on dryland crop yields and soil carbon in the central Great Plains. Agron J. 2002;94:1429–1436. doi: 10.2134/agronj2002.1429. [DOI] [Google Scholar]
- Hobbs PR, Sayre K, Gupta R. The role of conservation agriculture in sustainable agriculture. Philos Trans R Soc B: Biol Sci. 2008;363:543–555. doi: 10.1098/rstb.2007.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Muller A, Igarashi Y, Conrad R, Friedrich MW. Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J. 2010;4:267–278. doi: 10.1038/ismej.2009.100. [DOI] [PubMed] [Google Scholar]
- Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indoria AK, Rao CS, Sharma KL, Reddy KS (2017) Conservation agriculture—a panacea to improve soil physical health. Curr Sci 112(1):52
- Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall of India Pvt. Ltd.; 1973. [Google Scholar]
- Jat RK, Sapkota TB, Singh RG, Jat ML, Kumar M, Gupta RK. Seven years of conservation agriculture in a rice–wheat rotation of eastern gangetic plains of South Asia: yield trends and economic profitability. Field Crops Res. 2014;164:199–210. doi: 10.1016/j.fcr.2014.04.015. [DOI] [Google Scholar]
- Jat HS, Singh G, Singh R, Choudhary M, Gathala MK, Jat ML, Sharma DK. Management influence on maize–wheat system performance, water productivity and soil biology. Soil Use Manage. 2015;31:534–543. doi: 10.1111/sum.12208. [DOI] [Google Scholar]
- Jat HS, Datta A, Sharma PC, Kumar V, Yadav AK, Choudhary M, Choudhary V, Gathala MK, Sharma DK, Jat ML, Yaduvanshi NPS (2017) Assessing soil properties and nutrient availability under conservation agriculture practices in a reclaimed sodic soil in cereal-based systems of North-West India. Arch Agron Soil Sci 1–15 [DOI] [PMC free article] [PubMed]
- Kennedy AC, Smith KL (1995) Soil microbial diversity and the sustainability of agricultural soils. In: The significance and regulation of soil biodiversity. Springer Netherlands, 75–86
- Kielak AM, Barreto CC, Kowalchuk GA, van Veen JA, Kuramae EE. The ecology of acidobacteria: moving beyond genes and genomes. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kladivko EJ. Tillage systems and soil ecology. Soil Till Res. 2001;61:61–76. doi: 10.1016/S0167-1987(01)00179-9. [DOI] [Google Scholar]
- Lienhard P, Terrat S, Prévost-Bouré NC, Nowak V, Régnier T, Sayphoummie S, Panyasiri K, Tivet F, Mathieu O, Levêque J, Maron PA. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron Sustain Dev. 2014;34:525–525 33. doi: 10.1007/s13593-013-0162-9. [DOI] [Google Scholar]
- Liu J, Sui Y, Yu Z, Yao Q, Shi Y, Chu H, Jin J, Liu X, Wang G. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol Biochem. 2016;95:212–222. doi: 10.1016/j.soilbio.2015.12.021. [DOI] [Google Scholar]
- Lohan SK, Jat HS, Yadav AK, Sidhu HS, Jat ML, Choudhary M, Peter JK, Sharma PC. Burning issues of paddy residue management in north-west states of India. Renew Sust Energ Rev. 2018;81:693–706. doi: 10.1016/j.rser.2017.08.057. [DOI] [Google Scholar]
- Meisinger DB, Zimmermann J, Ludwig W, Schleifer KH, Wanner G, Schmid M, Bennett PC, Engel AS, Lee NM. In situ detection of novel Acidobacteria in microbial mats from a chemolithoautotrophically based cave ecosystem (Lower Kane Cave, WY, USA) Environ Microbiol. 2007;9(6):1523–1534. doi: 10.1111/j.1462-2920.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- Mu X, Zhao Y, Liu K, Ji B, Guo H, Xue Z, Li C. Responses of soil properties, root growth and crop yield to tillage and crop residue management in a wheat–maize cropping system on the North China Plain. Eur J Agron. 2016;78:32–43. doi: 10.1016/j.eja.2016.04.010. [DOI] [Google Scholar]
- Naumoff DG, Dedysh SN. Lateral gene transfer between the Bacteroidetes and Acidobacteria: the case of α-l-Rhamnosidases. Fed Eur Biochem Soc Lett. 2012;586:3843–3851. doi: 10.1016/j.febslet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Navarrete AA, Kuramae EE, de Hollander M, Pijl AS, van Veen JA, Tsai SM. Acidobacterial community responses to agricultural management of soybean in Amazon forest soils. FEMS Microbiol Ecol. 2013;83:607–621. doi: 10.1111/1574-6941.12018. [DOI] [PubMed] [Google Scholar]
- Navarro-Noya YE, Gómez-Acata S, Montoya-Ciriaco N, Rojas-Valdez A, Suárez-Arriaga MC, Valenzuela-Encinas C, Jiménez-Bueno N, Verhulst N, Govaerts B, Dendooven L. Relative impacts of tillage, residue management and crop-rotation on soil bacterial communities in a semi-arid agroecosystem. Soil Biol Biochem. 2013;65:86–95. doi: 10.1016/j.soilbio.2013.05.009. [DOI] [Google Scholar]
- Nehra V, Choudhary M. A review on plant growth promoting rhizobacteria acting as bioinoculants and their biological approach towards the production of sustainable agriculture. J Appl Natl Sci. 2015;7:540–556. doi: 10.31018/jans.v7i1.642. [DOI] [Google Scholar]
- Parke JL, Gurian-Sherman D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopatho. 2001;39:225–258. doi: 10.1146/annurev.phyto.39.1.225. [DOI] [PubMed] [Google Scholar]
- Pascault N, Nicolardot B, Bastian F, Thiebeau P, Ranjard L, Maron PA. In situ dynamics and spatial heterogeneity of soil bacterial communities under different crop residue management. Microbial Ecol. 2010;60:291–303. doi: 10.1007/s00248-010-9648-z. [DOI] [PubMed] [Google Scholar]
- Rahman MH, Okubo A, Sugiyama S, Mayland HF. Physical, chemical and microbiological properties of an Andisol as related to land use and tillage practice. Soil Till Res. 2008;101:10–19. doi: 10.1016/j.still.2008.05.006. [DOI] [Google Scholar]
- Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, Daroub SH, Camargo FAO, Farmerie WG, Triplett EW. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007;1:283–290. doi: 10.1038/ismej.2007.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt I, Bock E. Anaerobic ammonia oxidation with nitrogen dioxide by Nitrosomonas eutropha. Arch Microbiol. 1997;167:106–111. doi: 10.1007/s002030050422. [DOI] [PubMed] [Google Scholar]
- Sharma N, Dadhwal VK, Kant Y, Mahesh P, Mallikarjun K, Gadavi H, Sharma A, Ali MM. Atmospheric CO2 variations in two contrasting environmental sites over India. Air Soil Water Res. 2014;7:61–68. doi: 10.4137/ASWR.S13987. [DOI] [Google Scholar]
- Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbio. 2006;8:214–222. doi: 10.1111/j.1462-2920.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Liang W, Chu H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem. 2013;57:204–211. doi: 10.1016/j.soilbio.2012.07.013. [DOI] [Google Scholar]
- Silva AP, Babujia LC, Matsumoto LS, Guimarães MF, Hungria M. Microbial diversity under different soil tillage and crop rotation systems in an oxisol of southern Brazil. Open Agric J. 2013;7(Suppl 1-M6):40–47. doi: 10.2174/1874331501307010040. [DOI] [Google Scholar]
- Singh Y, Sidhu HS. Management of cereal crop residues for sustainable rice-wheat production system in the Indo-Gangetic Plains of India. Proc Indian Natl Sci Acad. 2014;80:95114. [Google Scholar]
- Six J, Frey SD, Thiet RK, Batten KM. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J. 2006;70:555–569. doi: 10.2136/sssaj2004.0347. [DOI] [Google Scholar]
- Smit E, Leeflang P, Gommans S, Broek J, van Mil S, Wernars K. Diversity and seasonal fluctuations of the dominant members of the bacterial soil community in a wheat field as determined by cultivation and molecular methods. Appl Environ Microbiol. 2001;67:2284–2291. doi: 10.1128/AEM.67.5.2284-2291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR, Blair PL, Boyd C, Cody B, Hazel A, Hedrick A, Kathuria H, Khurana P, Kramer B, Muterspaw K, Peck C. Microbial community responses to soil tillage and crop rotation in a corn/soybean agroecosystem. Ecolo Evol. 2016;6(22):8075–8084. doi: 10.1002/ece3.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RC, Mendes IC, Reis-Junior FB, Carvalho FM, Nogueira MA, Vasconcelos ATR, Vicente VA, Hungria M. Shifts in taxonomic and functional microbial diversity with agriculture: how fragile is the Brazilian Cerrado? BMC Microbiol. 2016;16(1):42. doi: 10.1186/s12866-016-0657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain AM, Krumholz LR, Elshahed MS. Abundance, composition, diversity and novelty of soil Proteobacteria. The ISME J. 2009;3:992–1000. doi: 10.1038/ismej.2009.43. [DOI] [PubMed] [Google Scholar]
- Stackebrandt ER, Murray GE, Trüper HG. Proteobacteria classis nov a name for the phylogenetic taxon that includes the “purple bacteria and their relatives. Int J Syst Bacteriol. 1988;38:321–325. doi: 10.1099/00207713-38-3-321. [DOI] [Google Scholar]
- Subbiah BV, Asija GL. A rapid procedure 744 for the determination of available nitrogen in soils. Curr Sci. 1956;25:259–260. [Google Scholar]
- Walkely A, Black IA. An experiment of the Degtareff method for determination of soil organic matter and a proposed modification of the chronic acid titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- Wang Z, Liu L, Chen Q, Wen X, Liao Y. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron Sustain Dev. 2016;36(2):28. doi: 10.1007/s13593-016-0366-x. [DOI] [Google Scholar]
- Wong-Villarreal A, Caballero-Mellado J. Rapid identification of nitrogen-fixing and legume-nodulating Burkholderia species based on PCR 16S rRNA species-specific oligonucleotides. Syst Appl Microbiol. 2010;33:35–43. doi: 10.1016/j.syapm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang R, Xue C, Xun W, Sun L, Xu Y, Shen Q. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb Ecol. 2014;67:443–453. doi: 10.1007/s00248-013-0322-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.