Introduction
Palbociclib, a selective cyclin-dependent kinase (CDK) 4/6 inhibitor, is currently under investigation for the treatment of multiple cancers. Here we describe a patient with metastatic breast cancer who had a Stevens-Johnson syndrome (SJS)-like reaction with a protracted course after 3 weeks of therapy with palbociclib. The patient's lesions improved with cessation of palbociclib and treatment with oral cyclosporine. This is the first report to our knowledge of a CDK 4/6 inhibitor associated with a severe cutaneous reaction.
Case presentation
A 63-year old woman with a history of metastatic breast cancer presented to the emergency room with a 3-week history of a worsening, painful rash that started on her face and spread to her trunk and extremities after 6 weeks of treatment with palbociclib and fulvestrant. She had last taken palbociclib 5 days and fulvestrant 1 week before her presentation. Associated symptoms included malaise, dysuria, and a gritty sensation in her eyes. She denied any other new medications.
This patient's medical history was notable for psoriatic arthritis. Her breast cancer was diagnosed 5 years ago and treated with lumpectomy, cyclophosphamide, and docetaxel followed by adjuvant aromatase inhibitor therapy. At the time of metastatic recurrence, the patient received fulvestrant for 2 years. Most recently, 1 year prior, she was placed on a clinical trial of entinostat or placebo plus an aromatase inhibitor. Because of progression, the patient was switched to palbociclib and fulvestrant therapy. She did not experience any dermatologic complications with prior therapies.
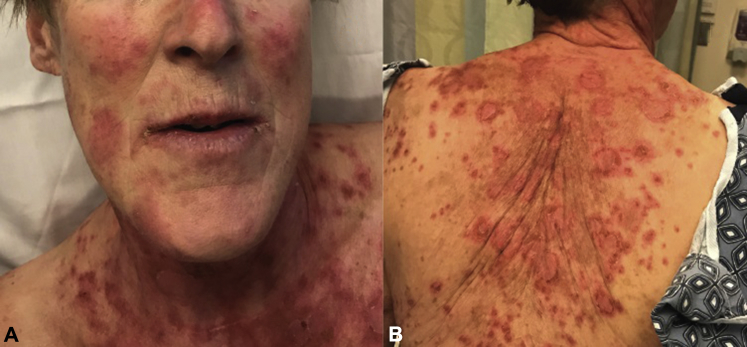
Physical examination found pink-to-violaceous, eroded, tender plaques with collarettes of scale on the face, trunk, and legs, some of which had yellow crust and dusky centers (Fig 1). Hemorrhagic crust was noted around the patient's lips (Fig 1, A). The oropharynx was spared. Nikolsky sign was initially negative but became positive the following day.
Fig 1.
Clinical images. A, Pink to violaceous, eroded plaques with collarettes of scale and dusky centers on the face. Hemorrhagic crust around the lips. B, Similar lesions on the back.
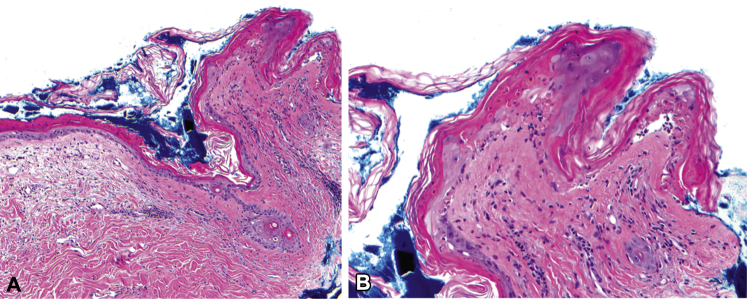
Skin biopsy found vacuolar interface dermatitis, focal full-thickness epidermal necrosis, and mild superficial perivascular lymphocytic infiltrate with pigment incontinence and dermal edema (Fig 2). Immunofluorescence studies did not find any immunoreactants.
Fig 2.
Histology. A, Biopsy of left upper back shows vacuolar interface dermatitis with focal full thickness epidermal necrosis and mild superficial perivascular lymphocytic infiltrate and dermal edema. B, Detail shows vacuolar interface and focal full thickness epidermal necrosis. A and B, Hematoxylin-eosin stain; original magnifications: A, ×40; B, ×200.)
The painful skin, ocular symptoms, mucocutaneous erosions, positive Nikolsky sign, and full-thickness epidermal necrosis seen on biopsy supported the diagnosis of SJS. Paraneoplastic pemphigus was considered but excluded given the lack of immunoreactants. The most likely cause of this patient's SJS was thought to be palbociclib. The patient had previously tolerated fulvestrant for 2 years without adverse effects. Herpes simplex virus IgM was negative, and there was no suggestion of Mycoplasma infection based on symptomatology. Oral cyclosporine at 5 mg/kg was started and slowed the progression of her lesions. The ophthalmology and gynecology departments were consulted because of concern for mucous membrane involvement; ultimately none was found.
After 1 week of initial cyclosporine therapy, her dose was halved to 2.5 mg/kg for another 2 weeks given continued formation of new bullae. At a 1-month follow-up appointment, her initial SJS-like eruption had resolved but had been replaced with a psoriasiform dermatitis.
Discussion
SJS and toxic epidermal necrolysis (TEN) are life-threatening cutaneous reactions characterized by necrosis of the epidermis. In adults, most cases are caused by drug hypersensitivity.1 SJS/TEN is thought to be mediated by T cells activated by drug-related haptens or direct noncovalent interaction of the drug itself with major histocompatibility complex I or T-cell receptor.1
Onset of the patient's symptoms 3 weeks after palbociclib is consistent with the time course of SJS.2 The continued progression of her symptoms was likely caused by continued ingestion of the inciting medication. However, after discontinuation of the likely trigger, her eruption continued for more than 4 weeks, suggesting an SJS-like reaction with a more protracted course. The development of a psoriasiform dermatitis in areas of the resolving eruption could represent Koebner phenomenon given the patient's history of psoriatic arthritis.
We cannot completely exclude other potential causes of SJS. She had tolerated her other medications, including fulvestrant, for years without cutaneous adverse effects. Using the algorithm for drug causality for epidermal necrolysis developed by Sassolas et al,3 the score for palbociclib was 3, suggesting possible cause, and 1 for fulvestrant, suggesting unlikely cause.3
Palbociclib and other CDK 4/6 inhibitors, including ribociclib and abemaciclib, are being studied in several clinical trials for the treatment of a variety of malignancies, and all have approvals in breast cancer.4 In 2013, the US Food and Drug Administration granted palbociclib breakthrough therapy designation in combination with an aromatase inhibitor as initial therapy for hormone receptor (HR)+, human epidermal growth factor receptor 2 (HER2)− metastatic breast cancer for postmenopausal women. Palbociclib received accelerated approval in 2015 on the basis of a phase 3 confirmatory trial, PALOMA-2 (NCT01942135), that found improved progression-free survival (24.8 vs 14.5 months) in patients treated with palbociclib or placebo and letrozole.5 Two other CDK 4/6 inhibitors, ribociclib and abemaciclib, have also been approved for HR+/HER2- metastatic breast cancer.6, 7
CDK 4/6 inhibitors are well tolerated. In the phase 3 PALOMA-3 trial of palbociclib and fulvestrant for breast cancer, the most common severe adverse effects in the palbociclib-treated group were neutropenia and leukopenia, occurring in 27% and 55% of participants, respectively.8 Grade 3 rash (severe reaction, requiring hospitalization and limitation of activities) occurred in 1% of the participants receiving palbociclib plus fulvestrant, but grade 1 to 2 rash occurred in 14% of participants. No grade 4 or higher rashes (life-threatening reaction requiring urgent intervention) were reported.8 Thus far, ribociclib and abemaciclib have not been reported to be associated with SJS/TEN. Ribociclib is associated with transaminitis and QTc prolongation, and abemaciclib is associated with more fatigue and gastrointestinal-related toxicity compared with palbociclib.6, 7
Given the severity of the patient's presentation, palbociclib and fulvestrant were discontinued. Several alternative cancer therapy options are available including everolimus plus exemestane,9 tamoxifen, chemotherapy, and clinical trial therapies.
As palbociclib in combination with an aromatase inhibitor is approved for first-line endocrine-based therapy for postmenopausal women with HR+/HER2− advanced breast cancer, many patients are likely to come into contact with this drug.5, 10 Our observations suggest palbociclib may potentially elicit an SJS-like reaction. We report this case to raise awareness about the possible risk of palbociclib-induced SJS and the need for close monitoring for signs of a cutaneous drug reaction after initiation of this medication.
Footnotes
Drs Karagounis and Vallurupalli contributed equally to this article.
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Schwartz R.A., McDonough P.H., Lee B.W. Toxic epidermal necrolysis. J Am Acad Dermatol. 2013;69:173. doi: 10.1016/j.jaad.2013.05.003. e1-e173.e13. [DOI] [PubMed] [Google Scholar]
- 2.Revuz J., Penso D., Roujeau J.C. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol. 1987;123:1160–1165. doi: 10.1001/archderm.123.9.1160. [DOI] [PubMed] [Google Scholar]
- 3.Sassolas B., Haddad C., Mockenhaupt M. ALDEN, an algorithm for assessment of drug causality in Stevens–Johnson syndrome and toxic epidermal necrolysis: comparison with case–control analysis. Clin Pharmacol Ther. 2010;88:60–68. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 4.Sherr C.J., Beach D., Shapiro G.I. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6:353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn R.S., Martin M., Rugo H.S. PALOMA-2: Primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2– advanced breast cancer (ABC) J Clin Oncol. 2016;34:507. [Google Scholar]
- 6.Hortobagyi G.N., Stemmer S.M., Burris H.A. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 7.Sledge G.W., Toi M., Neven P. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 8.Cristofanilli M., Turner N.C., Bondarenko I. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 9.Piccart M., Hortobagyi G.N., Campone M. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn R.S., Crown J.P., Lang I. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2014;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]