Introduction
Hidradenitis suppurativa (HS) is a chronic disease marked by suppuration and scarring in intertriginous regions of the groin, axillae, and breasts. Chronic disease may progress to ulceration, sinus tract formation, and progressive scarring. Furthermore, chronic wounds in HS can rarely predispose to the development of squamous cell carcinomas (SCCs) within these lesions.
Humoral hypercalcemia of malignancy (HHM) is hypercalcemia related to factors produced by malignant cells. Although SCCs of the lung, breast, oropharynx, and esophagus are commonly associated with HHM, SCCs of cutaneous origin are rarely implicated in HHM.1
Here, we present a case of paraneoplastic hypercalcemia from parathyroid hormone–related peptide (PTHrP) production by an SCC arising in the context of severe, long-standing HS in a white man.
Case
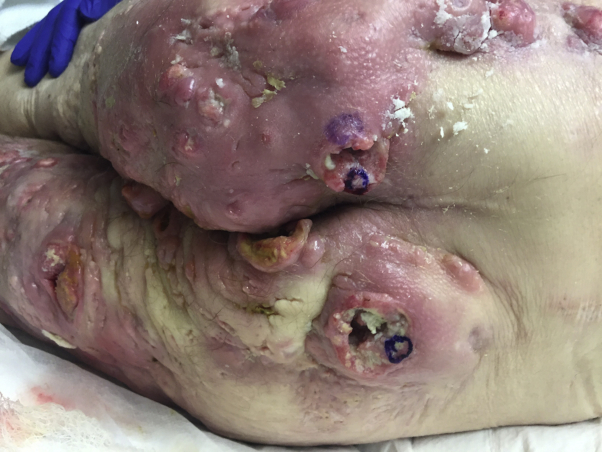
A 63-year-old man with an approximately 40-year history of severe HS recalcitrant to oral antibiotics and systemic retinoids presented to our clinic with severe disease of the groin and buttocks. We prescribed adalimumab, but his insurance denied him despite multiple appeals. He began doxycycline, 100 mg twice daily. Four months later, he presented to the emergency department with a flare of his HS. He also complained of constipation, weakness, and anorexia with resultant weight loss. On examination he had ulcerated tumors on the buttocks and bilateral groin (Fig 1), which had not been present at his previous outpatient appointment. Biopsies performed of his right thigh, right buttock, and left buttock were consistent with moderately to poorly differentiated SCC with evidence of angiolymphatic invasion. Radiographic imaging showed likely metastases to lymph nodes.
Fig 1.
Multiple SCCs developing within long-standing hidradenitis.
On admission, the patient's serum calcium level was 11.1 mg/dL (normal, 8.6-10.2 mg/dL), with a value of 12.4 mg/dL when corrected for hypoalbuminemia. Despite aggressive hydration, his calcium level continued to increase during his hospital stay, peaking at 13.6 mg/dL. He had confusion, hyperreflexia, and muscle cramping, all hallmarks of hypercalcemia. His intact parathyroid hormone (PTH) level was undetectable at less than 6.0 pg/mL (normal, 15-65 pg/mL), raising suspicion for HHM. Other causes of hypercalcemia were ruled out (Table I). Several days later, his PTHrP returned markedly elevated at 69.6 pmol/L (normal: 0.0 – 2.3 pmol/L), supporting the diagnosis of HHM.
Table I.
Notable serum values
| Patient's value (normal range) | |
|---|---|
| PTHrP | 69.6 (0-2.3 pmol/L) |
| Phosphatase | 3.6 (2.7-4.5 mg/dL) |
| TSH | 1.32 (0.27-4.20 μIU/mL) |
| iPTH | <6 (15-65 pg/mL) |
| 25-OH vitamin D2 | 31 (20-60 ng/mL) |
| 1,25-dihydroxy vitamin D | 42 (19.9-79.3) |
| Alkaline phosphatase bone fraction | 20.7 (6.5-20.1 μg/L) |
| Alkaline phosphatase | 168 (40-130 μ/L) |
Surgical resection was deemed inappropriate for this patient's treatment given the extensive involvement of the tumor. There were no viable treatment options that could be recommended by medical oncology given the extent of disease and his poor performance status. Radiation oncology offered palliative radiation. Given the lack of curative options, altered mental status, difficult-to-manage pain from his HS, and symptomatic electrolyte abnormalities, the family opted to pursue comfort measures only. He died in inpatient hospice 3 weeks after his hospital presentation.
Discussion
Our case presents humoral hypercalcemia of malignancy related to the production of PTHrP from SCC arising within long-standing, severe HS. HS is an uncommon disease, with a prevalence of 0.1% in a large, heterogeneous US population sample.2 Chronic inflammation from lesions in apocrine gland–bearing areas predispose to the development of SCCs, with mortality rates greater than 40% consistently shown in reviews of the literature.3, 4 Malignant transformation of HS to SCC, however, is rare, with approximately 80 published cases to date. Interestingly, involvement of the buttocks, especially in men, confers the greatest malignant potential.5 Furthermore, impedance of immune cells by disruption of lymphatic microcirculation and altered immune function in scarred areas have been proposed as additional mechanisms for production of squamous cell carcinomas in HS.6
The incidence of hypercalcemia at some point during disease course is common in cancer patients, often estimated around 20% to 30%, especially at advanced stages.7 There are many mechanisms for hypercalcemia of malignancy: osteolytic lesions, increased vitamin D via production of 1-α hydroxylase by tumor, PTH production, and PTHrP production. PTHrP production accounts for approximately 80% of hypercalcemia of malignancy.7 PTHrP is present in many normal tissues and has important paracrine activity, including effects on mammary and cartilage development, keratinocyte differentiation, placental calcium transport, and lactation.8 However, circulating levels in adults seem to be found only in malignancy and lactation and a handful of cases from benign tumors of the uterus and ovaries.8, 9 A portion of the PTHrP molecule has significant homology to PTH, and therefore can act at the PTH receptors in bone and kidney to increase serum calcium levels.
Despite the high rate of hypercalcemia in malignancy in general, it is rare in cutaneous SCCs. There are reports of HHM arising from SCC transformation of chronic wounds such as decubitus ulcers10 and burns; however, the association of HHM with HS-related SCC is even rarer.
This case highlights the mortality risk of uncontrolled HS and the necessity for appropriate treatment and follow-up. The dramatic malignant transformation and subsequent hypercalcemia in this patient were abrupt, clinically manifesting within a 4-month interval from his last dermatology visit to his fatal hospital course. Given the severe and often debilitating scarring and ulceration that can occur during the natural history of long-standing HS, our case brings to light the clinical challenge in assessing malignancy within long-standing HS. No guidelines exist to date regarding increased surveillance for malignancy in HS. Because no data exist to support immunomodulation preventing this complication of chronic HS, further investigation is needed. We emphasize heightened awareness of the malignant potential of HS along with special consideration of systemic symptoms that can be associated with HHM.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Kaur M.R., Marsden J.R., Nelson H.M. Humoral hypercalcaemia of malignancy associated with primary cutaneous squamous cell carcinoma. Clin Exp Dermatol. 2007;32(2):237–238. doi: 10.1111/j.1365-2230.2006.02316.x. [DOI] [PubMed] [Google Scholar]
- 2.Garg A., Kirby J.S., Lavian J. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pena Z.G., Sivamani R.K., Konia T.H., Eisen D.B. Squamous cell carcinoma in the setting of chronic hidradenitis suppurativa; report of a patient and update of the literature. Dermatol Online J. 2015;21(4) [PubMed] [Google Scholar]
- 4.Constantinou C., Widom K., Desantis J. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74(12):1177–1181. [PubMed] [Google Scholar]
- 5.Herschel S., Laske J., Stein A. Squamous cell carcinoma arising in hidradenitis suppurativa. J Dtsch Dermatol Ges. 2014;12(5):417–419. doi: 10.1111/ddg.12279. [DOI] [PubMed] [Google Scholar]
- 6.Caccavale S., Caccavale T., La Montagna M. Hidradenitis suppurativa complicated by squamous cell carcinoma: isoscartopic response. Int Wound J. 2017;14:1397–1398. doi: 10.1111/iwj.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart A.F. Hypercalcemia associated with cancer. N Engl J Med. 2005;352(4):373. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 8.McCauley L.K., Martin T.J. Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. J Bone Miner Res. 2012;27(6):1231–1239. doi: 10.1002/jbmr.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcha A.S., Gumaste P., Cherian S., Khanna A. Hypercalcemia: an unusual manifestation of uterine leiomyoma. Case Rep Med. 2013;2013:815252. doi: 10.1155/2013/815252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed M.A., George G.A., Lajara S., Perkins B., Knight C.M. A rare case of hypercalcemic crisis secondary to a squamous cell carcinoma arising from a non-healing ulcer. AACE Clin Case Rep. 2016 In-Press. [Google Scholar]