Introduction
Iododerma is a rare eruption caused by the inadvertent accumulation of iodine. Published sources of iodine excess producing iododerma include oral and intravenous iodinated contrast dyes, potassium iodide, amiodarone, and topical wound care products such as povidone iodine and iodoform gauze or in expectorants.1, 2, 3, 4, 5, 6, 7 Iododerma is more common in patients with renal dysfunction because of the impaired renal clearance and accumulation of iodine.8 One study of patients with end-stage renal disease who received iodinated contrast documented that the plasma half-life of the contrast was increased to 23 hours compared with 2 hours in those with normal kidney function.9 The pathophysiology of iododerma is currently unknown, but it is speculated to be the induction of a delayed hypersensitivity reaction via iodine acting as a hapten.1
Here we present a robust case of iododerma from exposure to iodinated contrast dye in the setting of renal insufficiency. No findings are pathognomonic for this condition; thus, meticulous clinical evaluation and investigation of exposure history are required to make the diagnosis.
Case report
A 47-year-old white woman with a history of transposition of the great arteries treated with Mustard procedure, multiple cerebrovascular accidents, diabetes mellitus, and chronic kidney disease had a new cutaneous eruption during a hospitalization for acute right leg weakness. Upon admission, she was started on heparin and underwent an abdominal aortogram in evaluation for a recurrent cerebrovascular or thrombolic event. Two days later, a hemorrhagic vesicopustular erupted on the face, circumferential neck, chest, and dorsal hands. Her oral and ocular mucosae were spared. Initial laboratory tests found a normal complete blood count with differential and liver function panel. Creatinine was elevated at 2.08 mg/dL (estimated glomerular filtration rate, 28 mL/min/1.73 m2). There were no associated fevers, chills, myalgias or arthralgias, and she had no history of this eruption previously (Figs 1 and 2).
Fig 1.
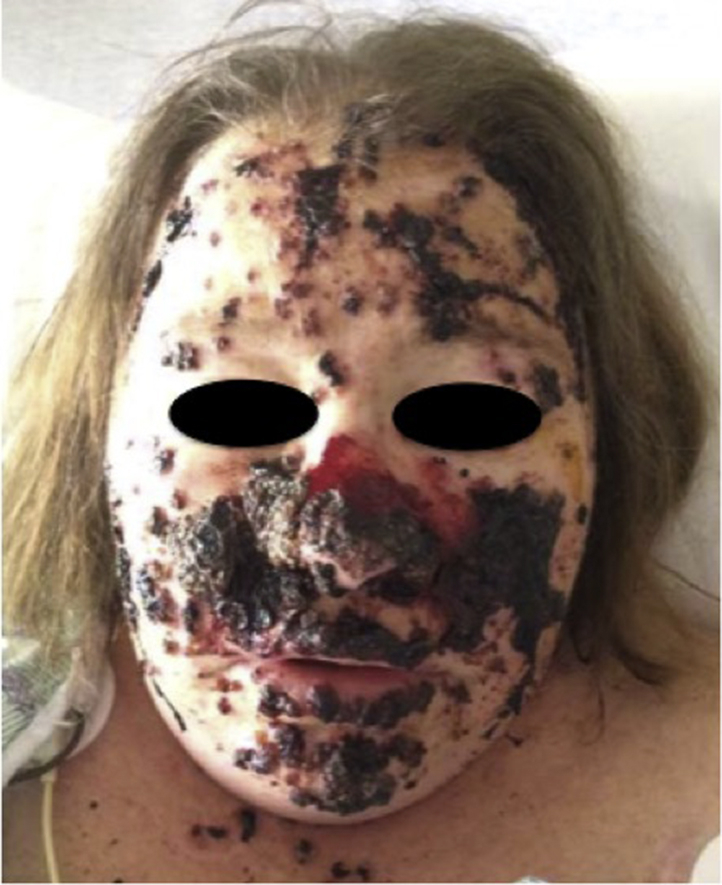
Vesicopustules with associated hemorrhage and crust on the face.
Fig 2.
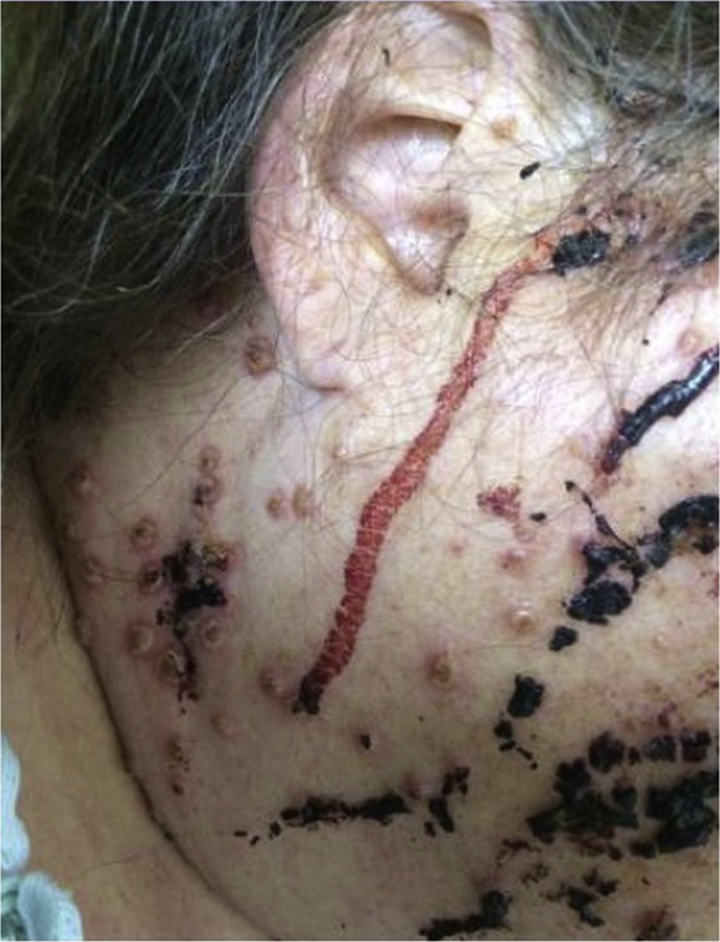
Hemorrhagic vesicopustules on the upper back and biopsy sites.
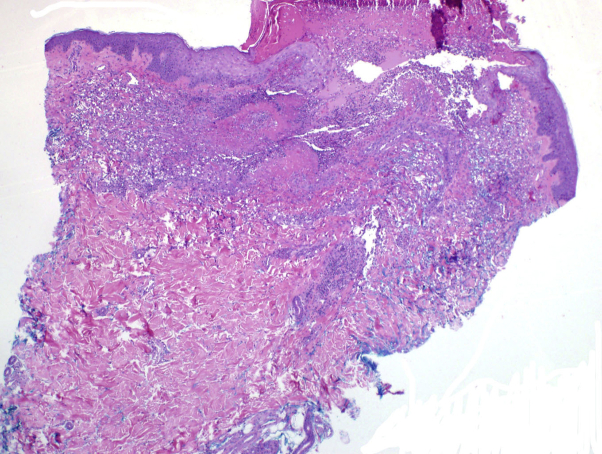
Biopsy of a neck lesion found a central ulcer with underlying neutrophils and histiocytes, and areas of dermal necrosis (Figs 3 and 4). The adjacent epidermis was acanthotic. Herpes simplex virus and varicella zoster virus immunohistochemical studies were negative. Gomori methenamine silver stain and periodic acid–Schiff stain for fungus showed no fungi. Fite and Ziehl Neelsen stains were negative for acid-fast bacilli, and no bacteria were identified on a Twort stain. Perilesional direct immunofluorescence showed no specific immune deposition pattern. Viral swab of the lesions sent for herpes simplex virus/varicella zoster virus polymerase chain reaction were negative as were blood and tissue cultures.
Fig 3.
A punch biopsy found an ulcer with a bed of neutrophils, histiocytes, and fibrinoid necrosis. (Original magnification: ×4.)
Fig 4.
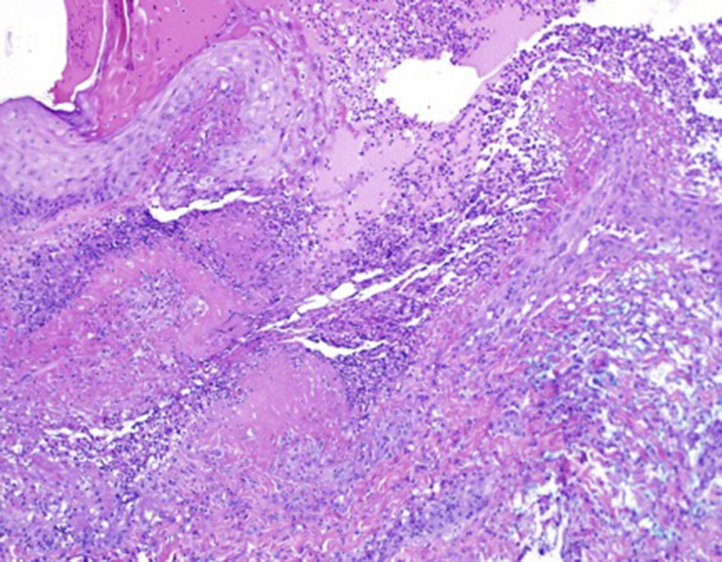
A punch biopsy found an ulcer with a bed of neutrophils, histiocytes, and fibrinoid necrosis. (Original magnification: ×100.)
Despite a 9-day interval from an abdominal aortogram at the outside institution, urine studies documented an elevated iodine level at 91,975 μg/L (normal range, 26-705 μg/L). At the time of the aortogram, she had a creatinine level of 2.08 mg/mL (estimated glomerular filtration rate, 28 mL/min/1.73 m2) and had received 97 mL of iopamidol contrast dye. She was not exposed to any additional topical or systemic iodinated agents after angiography. The robust hemorrhage within the lesions was likely exacerbated by the heparin that the patient took during the eruption. The patient was treated with diuresis and intravenous methylprednisolone at 1 mg/kg/d. The urine iodine level was monitored, and 2 days later it had trended down to 56,118 μg/L. Clinically, she had progressive improvement of the skin lesions and was transitioned to a prolonged prednisone taper over 6 weeks at hospital discharge. Follow-up of the patient at 6 months found complete resolution of the skin lesions without postinflammatory hyperpigmentation or scarring other than at the biopsy sites.
Discussion
The most common presentation of iododerma is an acneiform eruption; however, edematous, bullous, or hemorrhagic lesions can develop and evolve to vegetative nodules in advanced disease.1, 2, 9 Skin lesions in iododerma localize to the regions of the skin with high sebaceous gland density, and satellite lesions are seen on the extremities, especially the hands.3, 4, 9 A similar presentation can be seen with the intake of other halogenated compounds containing bromine and fluorine. The pathophysiology of this condition remains elusive. The consensus in the literature to date is that iodide functions as a hapten that, when bound to serum proteins, produces a delayed-type hypersensitivity reaction.1 Likely requirements for producing this reaction are the accumulation of the offending agent to a critical level and doing so in a susceptible immune milieu. This finding is supported by the reported risk factors, which are exposures to iodine-containing products and impaired clearance in the form of acute or chronic renal failure. Further study is needed to confirm or negate this notion.
The diagnosis is dependent on clinical evaluation and exposure history, as no laboratory or histopathologic finding is pathognomonic.6 Elevated blood or urine iodine levels and histologic findings are supportive of the diagnosis. In the setting of acute iododerma, histopathologic findings are nonspecific but can include a polymorphonuclear cell infiltrate with a few eosinophils, mast cells, and plasma cells.5 The presence of follicular involvement of the inflammatory infiltrate can also be supportive. In chronic lesions, pseudoepitheliomatous hyperplasia and a mixed inflammatory infiltrate are seen.10
Treatment entails excretion of the iodine and avoidance of additional exposure. Some patients require only supportive treatment, and the lesions will resolve over 4 to 6 weeks with postinflammatory hyperpigmentation or dermal atrophy.4, 6 Others have responded to systemic glucocorticoids, cyclosporine, and hemodialysis.8, 10
Footnotes
Funding sources: This case report is funded by the Department of Dermatology at the University of Michigan.
Conflicts of interest: None disclosed.
References
- 1.Rosenberg F.R., Einbinder J., Walzer R.A. Vegetating iododerm: an immunologic mechanism. Arch Dermatol. 1972;105:900–905. [PubMed] [Google Scholar]
- 2.Frankel A.J., Ahmad M., Perl M., Yao J., Pereira F. Acute onset of a vesiculopustular rash in an ICU patient. Dermatol Online J. 2014;20(1):21249. [PubMed] [Google Scholar]
- 3.Vaillant L., Pengloan J., Blanchier D., Demuret A., Lorette G. Iododerma and acute respiratory distress with leukocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232–233. doi: 10.1111/j.1365-2230.1990.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 4.Stavert R., Bunick C.G., Modi B. Vegetative plaques and hemorrhagic pustules. Iododerma. JAMA Dermatol. 2013;149(10):1231–1232. doi: 10.1001/jamadermatol.2013.4156. [DOI] [PubMed] [Google Scholar]
- 5.Soria C., Allegue F., España A. Vegetating iododerma with underlying systemic diseases: report of three cases. J Am Acad Dermatol. 1990;22:418–422. doi: 10.1016/0190-9622(90)70057-o. [DOI] [PubMed] [Google Scholar]
- 6.Chang M.W., Miner J.E., Moiin A., Hashimoto K. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014–1016. doi: 10.1016/s0190-9622(97)80291-5. [DOI] [PubMed] [Google Scholar]
- 7.Masse M., Falanga V., Zhou L.H. Use of topical povidone-iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744–747. doi: 10.1111/j.1346-8138.2008.00554.x. [DOI] [PubMed] [Google Scholar]
- 8.Lauret P., Godin M., Bravard P. Vegetating iodides after an intravenous pyelogram. Dermatologica. 1985;171:463–468. doi: 10.1159/000249474. [DOI] [PubMed] [Google Scholar]
- 9.Young A.L., Grossman M.E. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170(6):1377–1379. doi: 10.1111/bjd.12852. [DOI] [PubMed] [Google Scholar]
- 10.Miranda-Romero A., Sánchez-Sambucety P., Esquivias Gómez J.I. Vegetating iododerma with fatal outcome. Dermatology. 1999;198(3):295–297. doi: 10.1159/000018134. [DOI] [PubMed] [Google Scholar]