Introduction
Psoriasis is largely a clinical diagnosis, and treatment can be initiated without histologic proof of diagnosis. Tumor necrosis factor (TNF)-α inhibitors revolutionized the treatment of recalcitrant and widespread psoriasis.1 TNF-α inhibitors are generally well tolerated but are not without potential treatment associated risks. Among these risks is an increased incidence of secondary malignancies during and after TNF-α inhibitor therapy, which is well documented in recent literature.2 Cutaneous T-cell lymphoma (CTCL) is the most common cutaneous lymphoma, but inflammatory skin diseases far exceed the prevalence of CTCL. The clinical features of mycosis fungoides (the most common variant of CTCL) can often resemble more common inflammatory dermatoses, and diagnosis can be delayed because of overlapping features.3, 4 Although the clinical course of early mycosis fungoides is generally indolent, some patients will experience systemic progression and life-threatening disease.5 Few cases of TNF-α inhibitor–induced, accentuated CTCL are known.6, 7, 8, 9 Here we present the unique case of adalimumab-induced rapid systemic progression of CTCL. This report highlights the importance of histologic confirmation in the diagnosis of atypical psoriasis before initiation of systemic immunosuppressive therapy.
Case report
The 52-year-old white man presented initially to an outside dermatologist 4 years prior with dermatitis involving the trunk and waistline and later the extremities. Per the clinical features, an eczematous process was suspected initially. Later, based on the clinical presentation, psoriasis was diagnosed at an outside facility. The localization and clinical characteristics of the disease never completely fit the classic, more frequent types of plaque, inverse, or guttate psoriasis. During the course of the disease, the patient tried clobetasol propionate 0.05% ointment to the body and mometasone furoate 0.1% cream twice a day to the face and intertriginous areas. Per the patient's report, there was only limited transient response. The morphology and distribution of the areas remained unchanged. Slowly, new plaques appeared, and no areas showed complete resolution. After 4 years of futile topical therapy, the patient was recommended TNF-α inhibitor treatment. The diagnosis was never confirmed by biopsy. Routine laboratory testing before initiation of TNF-α inhibitor treatment was within normal limits. During adalimumab treatment, the patient noted no improvement in any of the affected areas. Paradoxically, at approximately week 4 of treatment, thickening of the previously present plaques was noted. At this time the patient also noted a large mass on the left side of the scalp. Oral antibiotics were prescribed at the outside facility, and a skin biopsy of the left scalp mass was obtained. The biopsy found an atypical, epidermotropic T-cell infiltrate consistent with T-cell lymphoma. Adalimumab was discontinued 6 weeks after initiation of treatment, and the patient was referred to us for hematology/oncology and dermato-oncology evaluation and care.
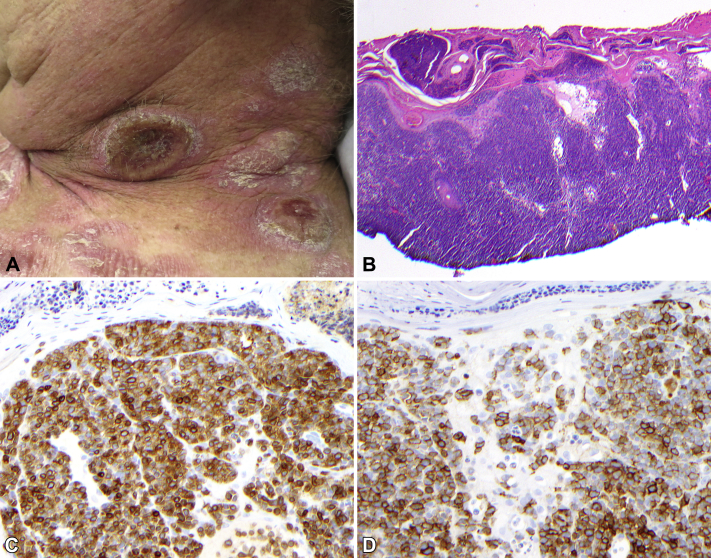
On physical examination, the patient presented with multiple salmon-colored, scaly, geographic plaques scattered on the trunk and extremities. There were multiple round, red nodules and tumors on the trunk, scalp, and neck (Fig 1, A). The left scalp tumor was ulcerated with cloudy white exudate. The total modified severity weighted assessment tool score (mSWAT) was 59, and 35% of the total body surface area was affected.
Fig 1.
A, Representative area on the left side of the neck with plaques and tumors at the time of biopsy. (B, Hematoxylin-eosin stain; C, CD3 immunohistochemistry [DAB]; D, CD30 immunohistochemistry [DAB]; original magnifications: B, ×20; C and D, ×200.)
Additional tissue sampling of both growing tumors and stable plaques showed cutaneous T-cell lymphoma, with large cell transformation of the left scalp tumor showing extensive CD30 expression (Fig 1, B). Lymphocytes were almost exclusively CD3+ and CD4+.
The left scalp tumor continued to rapidly progress, as did multiple plaques and new tumors on the trunk and neck. Computed tomography (CT) scan found bilateral axillary, subpectoral, pelvic and inguinal adenopathy consistent with systemic involvement. Positron emission tomography (PET)-CT identified extensive metabolic lesions predominantly involving superficial soft tissues and lung, consistent with advanced, aggressive systemic T-cell lymphoma. Surprisingly, peripheral blood flow cytometry showed 1:2 CD4/CD8 ratio and lacked other evidence of T-cell lymphoma in the peripheral blood. The bone marrow showed polyclonal T-cell populations. Based on the clinical presentation of rapidly growing, subcutaneous, ulcerated tumors with histologic findings of transformed CTCL and the PET showing extensive systemic involvement, transformed mycosis fungoides, clinical stage IV was diagnosed. The patient was initiated on cyclophosphamide, liposomal doxorubicin, vincristine, etoposide, and prednisone (CHOEP). Skin lesions showed marked improvement by cycle 2 of chemotherapy. He completed 4 cycles of CHOEP, achieving initial partial response but later was found to have progressive systemic disease, with overall increase in volume and intensity of soft tissue and lung lesions on PET-CT. The patient was then switched to brentuximab vedotin and concurrent total skin electron beam therapy (TSEBT), total dose of 18 Gy. After radiation therapy he completed 5 cycles of brentuximab vedotin, achieving a partial response with disappearance of the visceral disease (mSWAT, 2.5). Allogeneic bone marrow transplantation is planned to consolidate his response.
Discussion
CTCL is the most common lymphoma affecting the skin.4 Most cases of CTCL are quite indolent with protracted clinical courses.4 Affected skin areas often resemble eczematous or psoriasiform dermatitis clinically. The diagnosis of CTCL is frequently delayed by years given its resemblance to other dermatoses, often relatively banal clinical appearance and indolent clinical course. CTCL is often initially misdiagnosed as an eczematous or psoriasiform process, and repeated biopsies are frequently needed to confirm the diagnosis even in cases of clinically suspected CTCL.3, 4 Further diagnostic hardship arises from the response of CTCL to topical medications used for the treatment of common inflammatory dermatoses. In most cases, the diagnostic difficulty is not thought to affect long-term patient outcomes. Unfortunately, in rare cases, CTCL heralds a potentially life-threatening systemic T-cell lymphoma, and immunosuppression may increase the risk of lymphoproliferative diseases.4
TNF-α inhibitors have revolutionized the treatment of psoriasis.1 However, TNF-α inhibitors have also been found to increase the risk of lymphoma.2 Previously, etanercept, infliximab, and adalimumab treatment were found to be associated with the emergence of CTCL,6, 7, 8, 9 although others also reported the safety of etanercept in CTCL.10
The case we presented here describes the rapid progression of a misdiagnosed CTCL to symptomatic, systemic transformed T-cell lymphoma within weeks of adalimumab initiation. This case highlights the importance of considering clinical mimickers of common inflammatory dermatoses. It provides evidence for the importance of histologic verification of the diagnosis of psoriasis before initiation of systemic treatment that may detrimentally affect the course of CTCL.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Hernandez-Ilizaliturri receives honorarium for speaking engagements and consulting fees from Seattle Genetics but no conflicting or competing interests are present for material published in this report. The rest of the authors have no conflicts to disclose.
References
- 1.Langley R.G., Strober B.E., Gu Y., Rozzo S.J., Okun M.M. Benefit-risk assessment of tumour necrosis factor antagonists in the treatment of psoriasis. Br J Dermatol. 2010;162:1349–1358. doi: 10.1111/j.1365-2133.2010.09707.x. [DOI] [PubMed] [Google Scholar]
- 2.Deepak P., Sifuentes H., Sherid M., Stobaugh D., Sadozai Y., Ehrenpreis E.D. T-cell non-Hodgkin's lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-alpha) inhibitors: results of the REFURBISH study. Am J Gastroenterol. 2013;108:99–105. doi: 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 3.Nashan D., Faulhaber D., Stander S., Luger T.A., Stadler R. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;156:1–10. doi: 10.1111/j.1365-2133.2006.07526.x. [DOI] [PubMed] [Google Scholar]
- 4.Jawed S.I., Myskowski P.L., Horwitz S., Moskowitz A., Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e201–205.e216. doi: 10.1016/j.jaad.2013.07.049. quiz 221-202. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.H., Jensen R.A., Watanabe G.L., Varghese A., Hoppe R.T. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Arch Dermatol. 1996;132:1309–1313. [PubMed] [Google Scholar]
- 6.Dalle S., Balme B., Berger F., Hayette S., Thomas L. Mycosis fungoides-associated follicular mucinosis under adalimumab. Br J Dermatol. 2005;153:207–208. doi: 10.1111/j.1365-2133.2005.06686.x. [DOI] [PubMed] [Google Scholar]
- 7.Lafaille P., Bouffard D., Provost N. Exacerbation of undiagnosed mycosis fungoides during treatment with etanercept. Arch Dermatol. 2009;145:94–95. doi: 10.1001/archdermatol.2008.526. [DOI] [PubMed] [Google Scholar]
- 8.Mahe E., Descamps V., Grossin M., Fraitag S., Crickx B. CD30+ T-cell lymphoma in a patient with psoriasis treated with ciclosporin and infliximab. Br J Dermatol. 2003;149:170–173. doi: 10.1046/j.1365-2133.2003.05384.x. [DOI] [PubMed] [Google Scholar]
- 9.Suga H., Sugaya M., Toyama T. A case of mycosis fungoides with large cell transformation associated with infliximab treatment. Acta Derm Venereol. 2014;94:233–234. doi: 10.2340/00015555-1675. [DOI] [PubMed] [Google Scholar]
- 10.Tsimberidou A.M., Giles F.J., Duvic M., Kurzrock R. Pilot study of etanercept in patients with relapsed cutaneous T-cell lymphomas. J Am Acad Dermatol. 2004;51:200–204. doi: 10.1016/j.jaad.2003.05.009. [DOI] [PubMed] [Google Scholar]