Abstract
Dasatinib, a tyrosine kinase inhibitor, is widely used for patients with chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Although the drug has a potent immunosuppressive effect, infectious complications during dasatinib treatment have been reported rarely. We describe five patients who developed cytomegalovirus (CMV) colitis during dasatinib treatment, in whom the colitis was initially confused with other causes. The patients, three with chronic myeloid leukemia, and two with acute lymphoblastic leukemia, were diagnosed with CMV colitis based on endoscopic and histologic findings. The patients who examined blood CMV polymerase chain reaction were all positive. The patients received antiviral therapy in the form of either ganciclovir or valganciclovir, and the overall treatment outcome was fair. These cases suggest that physicians should consider the possibility of CMV reactivation when treating diarrhea and/or hematochezia in patients on dasatinib.
Keywords: Cytomegalovirus, Colitis, Dasatinib
Introduction
Dasatinib is an oral tyrosine BCR-ABL kinase inhibitor, and suppresses T-cell activation and proliferation [1]. It is used for the treatment of chronic myeloid leukemia (CML) [2], and relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), owing to its excellent efficacy [3,4]. Cytopenia and fluid retention are the major adverse reactions, although hemorrhagic colitis without thrombocytopenia and ulcers have also been reported during dasatinib treatment [5]. Despite the significant effects of dasatinib on the immune system, associated infectious complications were not observed in a large clinical study [6]. In the previous report, expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus (CMV) reactivation [7]. However, dasatinib associated CMV disease seems to be rare, and only few cases have been reported so far [8,9].
Recently, we encountered 5 cases of CMV colitis during dasatinib treatment for patients with CML or Ph+ ALL, mimicking dasatinib-induced hemorrhagic colitis. Distinguishing CMV colitis from dasatinib- related hemorrhagic colitis is not easy as the symptoms of diarrhea and hematochezia are present in both cases. Moreover, there was no guideline for treatment and preemptive monitoring of CMV disease such as CMV colitis during dasatinib treatment. Through these cases, we looked into the clinical characteristics, risk factors, treatments, and outcomes of CMV colitis during dasatinib. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital at the Catholic University of Korea, with a waiver of informed consent (Subject number: KC16ZISE0467).
Case Reports
Among the 231 CML or Ph+ ALL patients treated with dasatinib in 2014, we describe here five patients who suffered from diarrhea and/or hematochezia during the dasatinib treatment, and were diagnosed with CMV colitis. CMV colitis is defined by the presence of clinical symptoms, and demonstration of the virus in a biopsy tissue (through microscopic finding and immunohistochemical [IHC] staining).
1. Patient 1
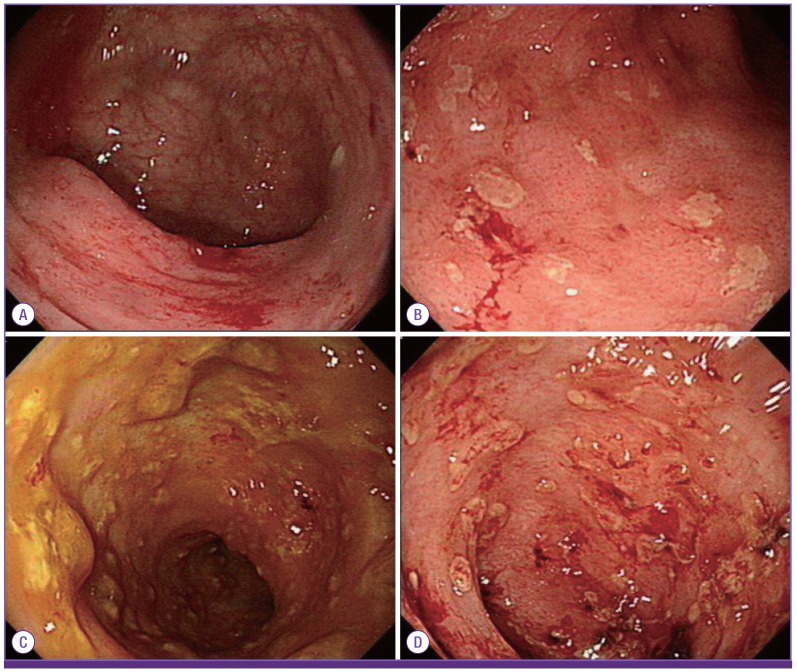
A 67-year-old male patient presented with a three-day history of diarrhea. He had received 14 months of dasatinib therapy (100 mg/day) for the treatment of CML, and He had chronic kidney disease. Physical examination revealed no fever and mild abdominal pain without any tenderness. At hospitalization, he had white blood cell (WBC) count of 19,430/mm3 (lymphocyte, 71%), hemoglobin (Hb) level of 8.0 g/dl, platelet count of 239,000/mm3, and a blood urea nitrogen/creatinine ratio of 85.2/3.08 mg/dl. On the 2nd day, his WBC count declined sharply to 8,720/mm3 (lymphocyte 20%), and he developed hematochezia without thrombocytopenia on 3rd day of admission. At this point, his blood and stool cultures tested negative. Dasatinib-induced hemorrhagic colitis was suspected, and a total colonoscopy revealed edematous and friable mucosa, along with a loss of vascularity from the cecum to rectum (Fig. 1A). His symptoms did not improve upon the discontinuation of dasatinib and with conservative care. After 4 days of admission, biopsy tissue showed positive CMV-specific IHC staining, and a blood CMV real time quantitative polymerase chain reaction (RQ-PCR) also tested positive (760 copies/ml). His symptoms subsided following 19 days of ganciclovir (5 mg/kg IV q12hr) administration. Dasatinib was re-started subsequently, and the CMV colitis did not relapse (15 months).
Figure 1. Colonoscopic finding of cytomegalovirus colitis (A) patient 1, edematous mucosa, friability, loss of vascularity. (B) patient 2, multiple erythema and shallow ulceration. (C) patient 4, edematous mucosa and shallow erosion. (D) patient 5, mild erythema, ulceration with blood clot.
2. Patient 2
A 43-year-old female patient complained of watery diarrhea and abdominal pain for two weeks. She had received two months of dasatinib (140 mg/day) for chronic phase CML and entecavir (0.5 mg/day) for chronic hepatitis B. Hematochezia and fever (38.1°C) developed on the day of admission. Her laboratory results were as follows: WBC count, 7,390/mm3 (lymphocyte, 34%); Hb, 8.4 g/dl; platelets, 141,000/mm3; and C-reactive protein, 2.7 mg/dl. Empirical antibiotic treatment (cefepime, metronidazole) was administered. However, the diarrhea and hematochezia persisted.
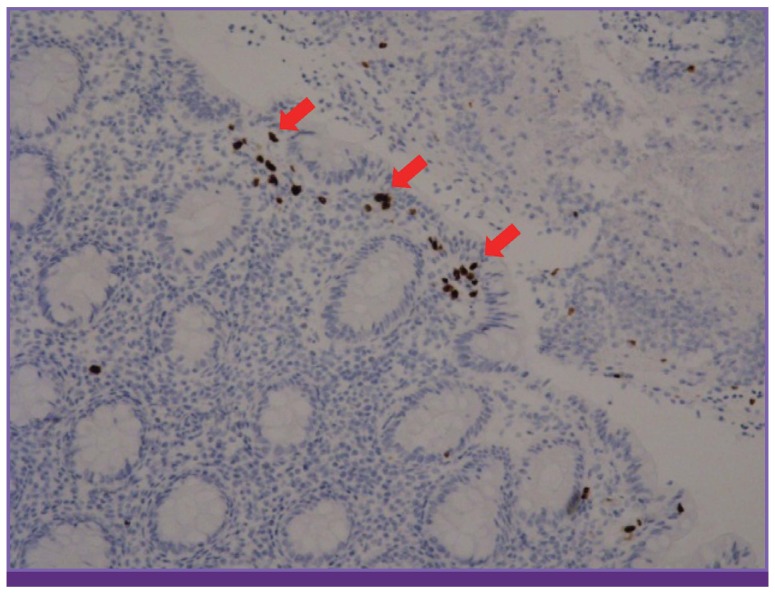
A sigmoidoscopy revealed multiple diffuse erythema, and shallow ulcerations with spontaneous bleeding involving the rectum and sigmoid colon (Fig. 1B). These findings indicated CMV colitis, dasatinib-induced hemorrhagic colitis, or both, and dasatinib was discontinued. On day 5, she showed decreased lymphocyte count (1,080/mm3), blood CMV load of 1,650 copies/ml, and positive CMV-specific IHC staining in the biopsied colon tissue (Fig. 2). CMV colitis was diagnosed and ganciclovir (5 mg/kg IV q12hr) was administered. Her dasatinib treatment was restarted during this time. After five days of ganciclovir treatment, she again developed hematochezia, and dasatinib was discontinued for a second time. A repeat sigmoidoscopy revealed improvements, and the CMV IHC staining and RQ-PCR both tested negative. However, a Clostridium difficile toxin assay tested positive. She was treated with metronidazole (1,500 mg/day PO) and vancomycin (500 mg/day PO). After 12 days of ganciclovir administration and antibiotic regimen for C. difficile-associated diarrhea (CDAD), she got better; dasatinib was restarted and she was discharged. The CMV colitis did not relapse (16 months).
Figure 2. Pathologic finding of cytomegalovirus colitis: positive immunohistochemistry for cytomegalovirus (red arrow) (×100).
3. Patient 3
A 51-year-old male patient visited our outpatient clinic for consultation on CMV colitis. He had undergone a colonoscopy two weeks before at a local clinic for a small amount of hematochezia, and had received a CMV colitis diagnosis. He had started receiving dasatinib (140 mg/day) for chronic phase CML 14 months prior to the onset of hematochezia, and had also received 6 cycle of systemic chemotherapy for diffuse large B-cell lymphoma nine months before symptom onset. Dasatinib had been maintained subsequent to the end of chemotherapy. At the time of presenting as an outpatient, the hematochezia had subsided and he had no symptoms.
His laboratory results revealed WBC of 6,330/mm3 (lymphocyte, 54%) without lymphopenia, Hb of 11.4 g/dl, and platelets of 199,000/mm3. Because, he was in stable condition and his laboratory results were within normal limits, dasatinib was maintained and CMV colitis treatment was not initiated. Six weeks after presentation, he re-developed hematochezia. A colonoscopy was performed again and the results revealed erythema, friability, and easy touch bleeding from the ileocecal valve to the sigmoid colon. Further, tissue biopsy also showed positive CMV-specific IHC staining. Thereafter, he received three weeks of oral valganciclovir (900 mg BID) treatment, and his symptoms improved. Hematochezia developed again two weeks after the end of the valganciclovir treatment. A repeat colonoscopy showed improvements, and his symptoms of hematochezia became better than before. We therefore decided to observe him without retreatment for CMV. The dasatinib dose was maintained, and CMV colitis did not relapse (16 months).
4. Patient 4
A 54-year-old male patient presented with diarrhea and hematochezia. He was treated with dasatinib (100 mg/day) for three months for relapsed Ph+ ALL, and had also received salvage chemotherapy of high-dose cytarabine, mitoxantrone, and etoposide. The laboratory results revealed persistent lymphopenia and thrombocytopenia (lymphocyte count, 748/mm3; platelets, 18,000/mm3). A sigmoidoscopy showed edematous mucosa with erythema and shallow erosions. Although CMV colitis was diagnosed based on the presence of pathologic tissue that showed positive CMV-specific IHC staining, symptoms continued to improve with conservative care and empirical antibiotic treatment (piperacillin, tazobactam) in the absence of any antiviral agent.
Hematochezia developed again ten days later. CMV colitis was confirmed based on pathologic findings in a repeat sigmoidoscopy (Fig. 1C), and blood CMV load of 925 copies/ml. In addition, a diagnosis of CDAD was made following positive toxin assay result and negative results for diarrheagenic bacterial pathogens (in-house multiplex PCR). Metronidazole and ganciclovir (5 mg/kg IV q12hr) were initiated, and while the diarrhea improved. After eleven days to initiation of ganciclovir, He once again developed hematochezia. Then, the dasatinib was discontinued. Symptom subsided completely after 18 days of ganciclovir treatment, CMV blood viral load was not detectable, CMV-specific IHC staining was negative from the third sigmoidoscopy. CMV colitis did not relapse in 9 months, at which time the patient succumbed to refractory leukemia.
5. Patient 5
A 55-year-old female patient with Ph+ ALL was admitted for double cord blood transplantation. She had been treated with dasatinib (140 mg/day) 5 month between induction and consolidation chemotherapy. The patient showed hematochezia and fever (38.3°C) at admission. Laboratory results on admission day revealed WBC count of 10,470/mm3 (lymphocyte, 31.3%), Hb of 7.9 g/dl, and platelets of 57,000/mm3. On day 2, her lymphocyte count dropped to 251/mm3, and she had a blood CMV load of 2,500 copies/ml. Sigmoidoscopy revealed mild erythematous mucosa with blood clot (Fig. 1D), and CMV-specific IHC stain. Ganciclovir was started for CMV colitis; her transplantation was delayed, and the dasatinib was maintained during this time. After ten days of ganciclovir treatment, the patient experienced neutropenia, a ganciclovir adverse effect. As the patient did not have any other symptoms more, and a repeat sigmoidoscopy revealed significant improvements, ganciclovir was discontinued and she was discharged. One week later, dasatinib was also discontinued due to poor response. The patient received double cord blood transplantation three months after discharge, and the CMV colitis relapsed one month after transplantation. She received retreatment with ganciclovir, showed improvements, and has not experienced a CMV colitis relapse 11 months after therapy conclusion.
Discussion
Patients with suppressed cytotoxic T-cell mediated immunity have a higher risk of CMV reactivation and CMV diseases [6,10]. The CMV disease is common in immunocompromised patients with acquired immune deficiency syndrome, solid organ transplantation, hematopoietic stem cell transplantation (HSCT), etc. [11,12]. Immunosuppressive agents, including anti-cancer agents, have been reportedly related to CMV reactivation. In addition, antithymocyte globulin, alemtuzumab, and steroid use have been shown as independent risk factors for CMV reactivation [13]. Moreover, the advent of new immunosuppressive and chemotherapeutic agents may be associated with a change in the spectrum of opportunistic infections. It is important to continue to reevaluate the incidence of CMV disease in adult non-transplantation patients with cancer [10]. In the present study, five patients among the 231 dasatinib-receiving patients were diagnosed with CMV colitis.
In a previous study, proliferation of lymphocytes with large granular lymphocytes (LGLs) morphologic features has been reported in a subset of patients with CML or Ph+ ALL treated with dasatinib. These patients showed appearances of CMV-specific CD8+ T cells, and an approximately one-third of the patients with LGL lymphocytosis also exhibited symptomatic CMV reactivation [7]. This maybe immunologic mechanism through which dasatinib can cause CMV reactivation. However, another study reported a lack of relationship between LGLs and CMV reactivation in clinical setting [14]. Very few cases of CMV disease during dasatinib have been reported. Further evaluation of mechanism and risk factor of CMV reactivation during dasatinib treatment are needed.
CMV diseases including pneumonitis, encephalitis, and colitis, and can be fatal in immunocompromised patients [12,15,16]. Thus, physicians should endeavor to diagnose and treat CMV diseases as soon as possible. However, natural history of CMV disease in the immunocompetent host are generally self-limiting, there is no recommendation for treatment of CMV disease in the immunocompetent patient [17]. Also, the treatment of CMV disease is not defined clearly in patients with hematologic malignancies who have not undergone HSCT, since anti-CMV drug is highly toxic and insufficient to establish safety and efficacy [17]. However, historically, poor prognosis has been reported for severe CMV infection in immunocompetent patient in the absence of antiviral therapy, suggesting that an early initiation of specific treatment may be important in severe cases [17]. In addition, cases of CMV disease in patients with non-transplant hematologic malignancies has increased and report on association of CMV reactivation with mortality indicates that antiviral treatment is need [16].
We did a literature review to find case reports of CMV colitis during dasatinib treatment, and identified two such cases (Table 1). The first case was of a 26-year-old Japanese female suffering for CML chronic phase. She was hospitalized because of fever and hemorrhagic diarrhea during dasatinib maintenance therapy. She was diagnosed with CMV colitis by histologic exam, and received ganciclovir treatment. Although blood leukocyte CMV antigen and CMV staining in colonic mucosa were negative after ganciclovir treatment, hemorrhagic diarrhea did not improve. Hemorrhagic colitis, however, improved dramatically after discontinuation of dasatinib [9].
Table 1. Clinical characteristics, laboratory findings, treatment, and outcome of patients with CMV colitis during dasatinib treatment.
| [Ref]/ Patient | Age/Sex | Hematologic disease | Dasatinib duration | Lymphocyte | Coinfection pathogen | Serum CMV DNA titer (copies/ml) | Dasatinib during treatment | Treatment | Dasatinib after antiviral treatment | Relapse | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [8] | F/39 | CML, CP | 30 month | Not mentioned | None | Not mentioned | Stop | Ganciclovir for 6 weeks | Restart | None | |
| 1 | M/67 | CML, CP | 14 month | 1,744/mm3 | None | 760 | Stop | Ganciclovir for 19 days | Restart | None | |
| 2 | F/43 | CML, CP | 2 month | 1,115/mm3 | Clostridium difficile | 1,650 | Stop | Ganciclovir for 12 days | Restart | None | |
| 3 | M/51 | CML, CP | 14 month | 1st episode | 4,928/mm3 | None | unchecked | Continued | None | Continued | Relapsed after 6 weeks |
| 2nd episode | 4,190/mm3 | None | unchecked | Continued | Valganciclovir for 21 day | Continued | None | ||||
| 4 | M/54 | Ph (+) ALL | 3 month | 1st episode | 300/mm3 | None | unchecked | Continued | None | Continued | relapsed after 2 weeks |
| 2nd episode | 1,398/mm3 | Clostridium difficile | 925 | Stop | Ganciclovir for 19 days | Restart | None | ||||
| 5 | F/55 | Ph (+) ALL | 5 month | 251/mm3 | None | 2500 | Continued | Ganciclovir for 10 days | Stop | None | |
CMV, cytomegalovirus; F, female; CML, chronic myeloid leukemia; CP, chronic phase; M, male; Ph (+) ALL, Philadelphia chromosome-positive acute lymphoblastic leukemia.
Case of reference 9 was not included in the table because only abstract written in English was available.
The second case was a 39-year-old Ethiopian female patient who received dasatinib as treatment for chronic phase CML. She experienced chronic diarrhea for two months, which progressed into hemorrhagic colitis due to CMV. Dasatinib was stopped immediately and diarrhea improved spontaneously after 5 days. The patient also received ganciclovir for a total of 6 weeks [8].
In this case report series, two patients were discontinued dasatinib, consider dasatinib induced hemorrhagic colitis. The remaining three patients continued using dasatinib, and two of them did not receive any antiviral treatment, due to a self-limited disease course. However, both these patients exhibited recurrence of CMV colitis. Although dasatinib was restarted after antiviral treatment completion, recurrence of colitis was not observed. Our observations suggest that antiviral therapy is necessary for treatment of CMV colitis during dasatinib treatment, even as the disease seems to have a self-limiting course. A discontinuation of dasatinib may aid in CMV colitis treatment, and dasatinib can be restarted after the CMV colitis has resolved.
In conclusion, dasatinib treatment is a potential risk factor for CMV reactivation and development of CMV disease. The possibility of CMV reactivation should be considered in unexplained inflammatory responses during dasatinib treatment.
Footnotes
Conflicts of interest: No conflicts of interest.
References
- 1.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–1377. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, Cervantes F, Deininger M, Gratwohl A, Guilhot F, Hochhaus A, Horowitz M, Hughes T, Kantarjian H, Larson R, Radich J, Simonsson B, Silver RT, Goldman J, Hehlmann R. European LeukemiaNet. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA, Rege-Cambrin G, Radich J, Hochhaus A, Apanovitch AM, Gollerkeri A, Coutre S. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 4.de la Hoz RE, Stephens G, Sherlock C. Diagnosis and treatment approaches of CMV infections in adult patients. J Clin Virol. 2002;25(Suppl 2):S1–12. doi: 10.1016/s1386-6532(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 5.Kmira Z, Nesrine BS, Houneida Z, Wafa BF, Aida S, Yosra BY, Monia Z, Sriha B, Abderrahim K. Severe hemorrhagic colitis in a patient with chronic myeloid leukemia in the blastic phase after dasatinib use. World J Gastrointest Pathophysiol. 2013;4:59–62. doi: 10.4291/wjgp.v4.i3.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres HA, Chemaly RF. Viral infection or reactivation in patients during treatment with dasatinib: a call for screening? Leuk Lymphoma. 2007;48:2308–2309. doi: 10.1080/10428190701760086. [DOI] [PubMed] [Google Scholar]
- 7.Kreutzman A, Ladell K, Koechel C, Gostick E, Ekblom M, Stenke L, Melo T, Einsele H, Porkka K, Price DA, Mustjoki S, Seggewiss R. Expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus reactivation. Leukemia. 2011;25:1587–1597. doi: 10.1038/leu.2011.135. [DOI] [PubMed] [Google Scholar]
- 8.Yassin MA, Nashwan AJ, Soliman AT, Yousif A, Moustafa A, AlBattah A, Mohamed SF, Mudawi DS, Elkourashy S, Asaari DR, Gutierrez HL, Almusharaf M, Hussein RM, Moustafa AH, Derhoubi HE, Boukhris S, Kohla S, AlDewik N. Cytomegalovirus-induced Hemorrhagic colitis in a patient with chronic myeloid leukemia (chronic phase) on dasatinib as an upfront therapy. Clin Med Insights Case Rep. 2015;8:77–81. doi: 10.4137/CCRep.S25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunami Y, Sato E, Ichikawa K, Yasuda H, Komatsu N. Hemorrhagic colitis caused by dasatinib following cytomegalovirus enterocolitis in a patient with chronic myelogenous leukemia in the second chronic phase. Rinsho Ketsueki. 2011;52:282–286. [PubMed] [Google Scholar]
- 10.Mera JR, Whimbey E, Elting L, Preti A, Luna MA, Bruner JM, Williams T, Jr, Bodey GP, Goodrich JM. Cytomegalovirus pneumonia in adult nontransplantation patients with cancer: review of 20 cases occurring from 1964 through 1990. Clin Infect Dis. 1996;22:1046–1050. doi: 10.1093/clinids/22.6.1046. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Lee HJ, Kim SM, Jung JH, Shin S, Kim YH, Sung H, Lee SO, Choi SH, Kim YS, Woo JH, Han DJ. Diagnostic usefulness of cytomegalovirus (CMV)-specific T cell immunity in predicting CMV infection after kidney transplantation: a pilot proof-of-concept study. Infect Chemother. 2015;47:105–110. doi: 10.3947/ic.2015.47.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemonovich TL, Watkins RR. Update on cytomegalovirus infections of the gastrointestinal system in solid organ transplant recipients. Curr Infect Dis Rep. 2012;14:33–40. doi: 10.1007/s11908-011-0224-6. [DOI] [PubMed] [Google Scholar]
- 13.Ko JH, Peck KR, Lee WJ, Lee JY, Cho SY, Ha YE, Kang CI, Chung DR, Kim YH, Lee NY, Kim KM, Song JH. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60:e20–e26. doi: 10.1093/cid/ciu969. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka H, Nakashima S, Usuda M. Rapid and sustained increase of large granular lymphocytes and rare cytomegalovirus reactivation during dasatinib treatment in chronic myelogenous leukemia patients. Int J Hematol. 2012;96:308–319. doi: 10.1007/s12185-012-1132-8. [DOI] [PubMed] [Google Scholar]
- 15.Chang H, Tang TC, Hung YS, Lin TL, Kuo MC, Wang PN. Cytomegalovirus infection in non-transplant patients with hematologic neoplasms: a case series. Chang Gung Med J. 2011;34:65–74. [PubMed] [Google Scholar]
- 16.Torres HA, Kontoyiannis DP, Aguilera EA, Younes A, Luna MA, Tarrand JJ, Nogueras GM, Raad II, Chemaly RF. Cytomegalovirus infection in patients with lymphoma: an important cause of morbidity and mortality. Clin Lymphoma Myeloma. 2006;6:393–398. doi: 10.3816/CLM.2006.n.016. [DOI] [PubMed] [Google Scholar]
- 17.Eddleston M, Peacock S, Juniper M, Warrell DA. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 1997;24:52–56. doi: 10.1093/clinids/24.1.52. [DOI] [PubMed] [Google Scholar]