Abstract
Because primary antifungal prophylaxis is widely used for immunocompromised hosts, the incidences of unusual fungal infections have increased. Trichosporon asahii has emerged as an important life-threatening opportunistic systemic pathogen because of the increased use of cytotoxic or immunosuppressant agents, along with high mortality rates. Here, we describe a case of catheter-related T. asahii bloodstream infection with multiple septic skin nodules in both the arms and legs of the patient who was in the neutropenic period after allogeneic stem cell transplantation for myelodysplastic syndrome treated with prophylactic ciprofloxacin and itraconazole. We successfully treated her with intravenous voriconazole for more than a month without any complications. Clinicians should consider breakthrough Trichosporon infections when clinical progress in an immunocompromised patient with unexplained infection signs and symptoms does not improve despite proper treatment with antibiotics or various antifungal agents. In addition, voriconazole can be a good treatment choice for achieving better treatment results and prognosis.
Keywords: Trichosporon asahii, Catheter-related infection, Fungemia, Voriconazole, Stem cell transplantation
Introduction
Because high-intensity chemotherapy and immunosuppressive therapy have been commonly used in patients with hematologic malignancy, the incidence of invasive fungal infection has increased. Primary antifungal prophylaxis has reduced the incidence of invasive fungal infection and has improved the survival rate and prognosis in patients with prolonged neutropenia after chemotherapy and stem cell transplantation [1]. While primary antifungal prophylaxis has been generally used, the incidence of non-Aspergillus mold or non-Candida yeast infection has steadily increased that has not been observed before [2,3].
Trichosporon asahii is a urease-positive, non-encapsulated basidiomycetous yeast. In recent years, T. asahii has been reported in several cases as an important life-threatening opportunistic systemic pathogen with reduced susceptibility to antifungal therapy in immunocompromised and immunocompetent patients who were receiving broad-spectrum antibiotics for a long period, in diabetic patients, or in heavy alcoholics, and even in healthy individuals [4,5].
Here, we present a case of catheter-related T. asahii bloodstream infection with multiple septic skin nodules during the neutropenic period after allogeneic stem cell transplantation (SCT) that was successful treated with voriconazole. This study was approved by the institutional review board of Seoul St. Mary’s Hospital at the Catholic University of Korea, with a waiver of informed consent (subject no. KC13RISI0364).
Case report
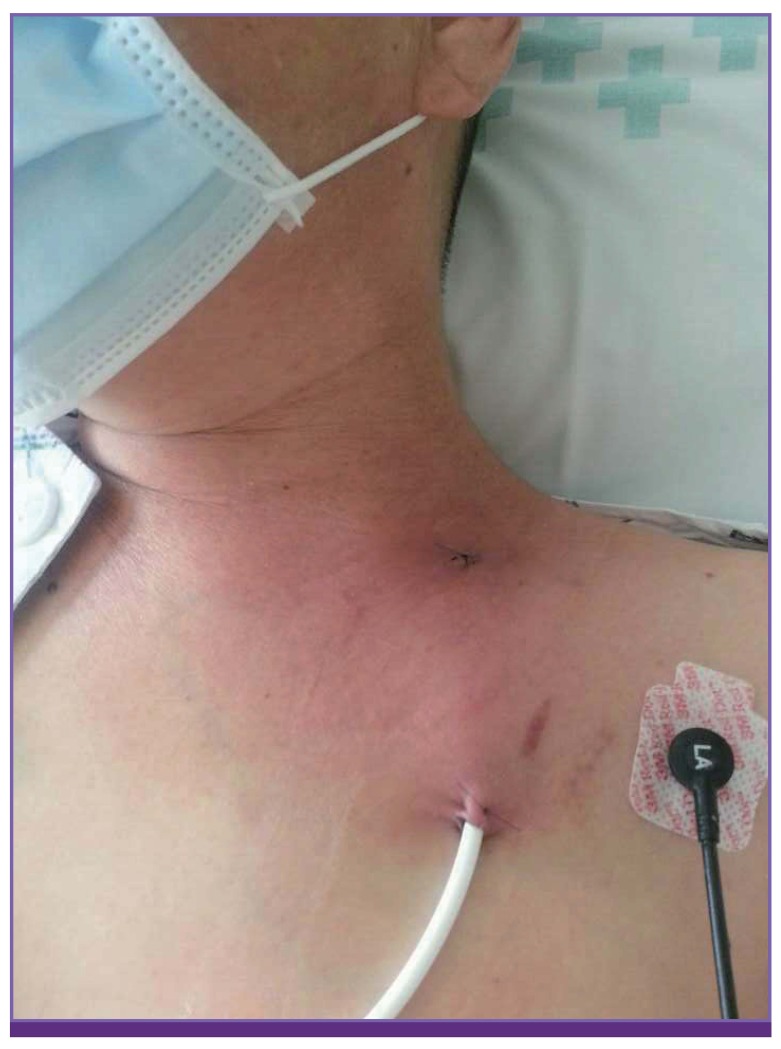
A 54-year-old woman was admitted for allogeneic SCT in March 2014. She was diagnosed as myelodysplastic syndrome 4 months before and was treated with steroid and methotrexate therapies for Crohn’s disease. On admission, her vital signs were stable, and her physical examination findings were unremarkable. The myeloablative conditioning regimen was initiated. Ciprofloxacin (1,000 mg/day) and itraconazole suspension (10 mg/kg/day) were administered as prophylaxis, and isoniazid (300 mg/day) was administered for latent tuberculosis. On day 5 of SCT, the patient complained of Hickmann catheter exit site pain and showed redness and swelling without fever (Fig. 1). Her blood cell count indicated neutropenia (absolute neutrophil count, 0.69 × 109/L), and her C-reactive protein (CRP) level was 4.13 mg/dL. Blood culture from the Hickmann catheter and peripheral line was performed, and teicoplanin (400 mg intravenous loading three times and then 400 mg once a day) was administered. The Hickmann catheter was removed, and a catheter tip culture was also performed. On day 6 of SCT, neutropenic fever developed, with increasing body temperature of up to 38.4°C. Her blood pressure and pulse rate were 110/70 mm Hg and 108 beats/min, respectively. No definite abnormal results were observe on her chest radiograph, blood chemistry tests, and urinalysis. Empirical antibiotics, 4 g/day cefepime and 400 mg/day isepamicin were administered, and itraconazole was substituted with 50 mg/day micafungin because of intolerance. On day 7 of SCT (33 hours after entering the automated blood culture system), blood culture from the Hickmann catheter was reported to be positive, and the Gram stain finding revealed a yeast-like organism. We decided to continue the micafungin therapy but increased the dose to 100 mg for suspicion of candidemia.
Figure 1. Catheter exit site.
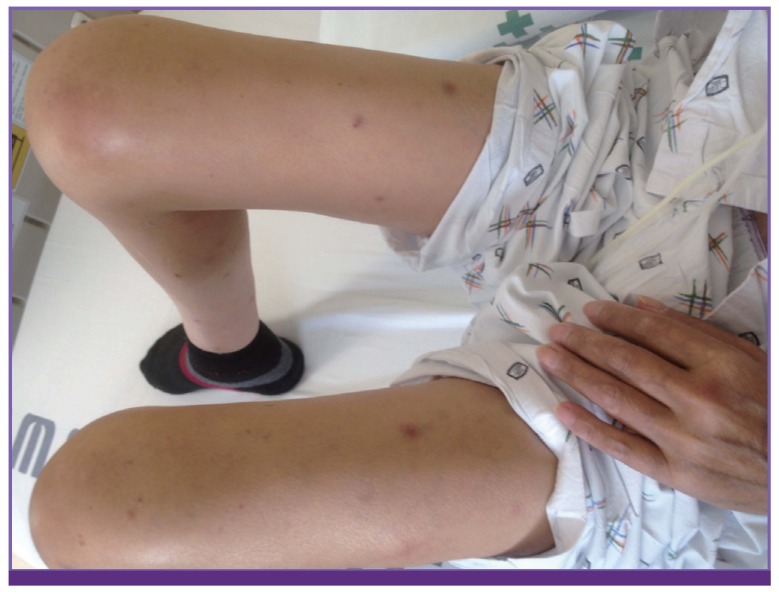
On day 8 of SCT, the fever persisted, and multiple skin nodules and erythema of various sizes on both legs and arms appeared (Fig. 2). The antibiotic agents were changed to meropenem (3 g/day). The results of the galactomannan and cryptococcal antigen tests were both repetitively negative. After 5 days of incubation (day 10 of SCT), white to cream-colored colonies (>15 colony-forming units) with raised surfaces on potato dextrose agar plates were observed (Fig. 3). On day 10 of SCT, blood culture from the Hickmann catheter and catheter tip culture finally yielded T. asahii, but nothing from the peripheral line. Intravenous voriconazole was administered as treatment for T. asahii fungemia (6 mg/kg iv twice daily for loading for 1 day and then 4 mg/kg iv twice a day for maintenance) instead of micafungin.
Figure 2. Skin lesions on both legs with multiple erythematous nodules.
Figure 3. (A) After 2 days of incubation. (B) After 5 days of incubation, white to cream-colored colonies with raised surfaces appeared on potato dextrose agar plates.
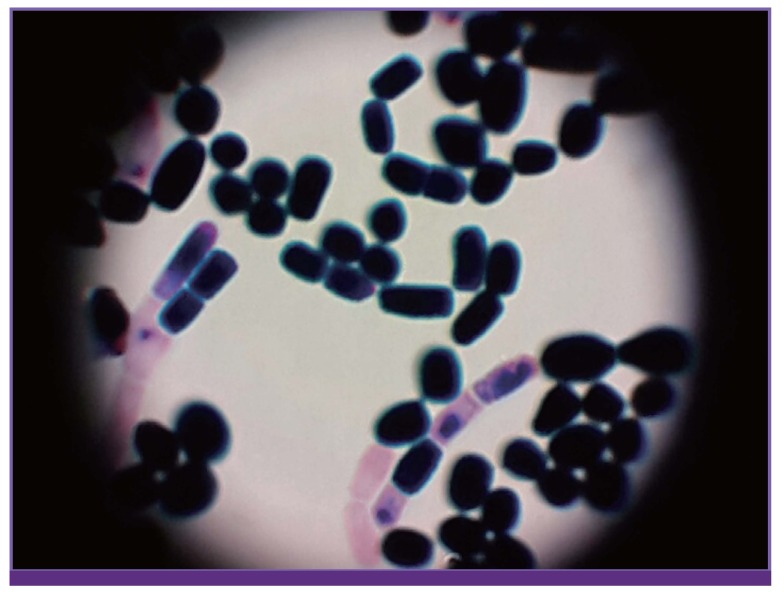
Figure 4. Microscopic morphology of T. asahii: septate hyphae and pseudohyphae with arthroconidia (Gram staining, ×1000).
In vitro studies of susceptibility to amphotericin B, fluconazole, itraconazole, caspofungin, and voriconazole were performed by using the microbroth dilution technique and the guidelines of the National Committee for Clinical Laboratory Standards [6]. The minimal inhibitory concentrations (MICs) were as follows: amphotericin B, 1 mg/mL; fluconazole, 8 mg/mL; itraconazole, 16 μg/mL; caspofungin, 16 μg/ml; and voriconazole, 0.5 μg/mL.
On day 14 of SCT (i.e., day 5 of voriconazole therapy), the neutrophil count recovered (absolute neutrophil count, 1.25 × 109/L), but follow-up blood culture persistently showed T. asahii fungemia, and the CRP level increased up to 33.1 mg/dL. On day 20 of SCT (day 12 of voriconazole therapy), fungemia disappeared with defervescence, and septic skin emboli were cleared. Intravenous voriconazole was changed to tablet form and continued for 30 days after negative conversion of fungemia. She is still alive without any complication 16 months after SCT.
Discussion
Trichosporon species are distributed in external environments, such as soil, wood, and air, and they are a part of the normal flora of the human skin (especially the peri-genital area), respiratory tract, and gastrointestinal tracts [7]. All pathogenic members of the genus Trichosporon were once regarded as a single species, T. beigelii [7]. However, the genus has undergone several reclassifications, and more recently, 50 species have been described, of which 16 species have been associated with human infections [7,8]. In most cases, T. asahii (74%) has emerged as an important life-threatening opportunistic systemic pathogen because of the increased use of cytotoxic or immunosuppressant agents. The clinical manifestations of Trichosporon infection represent various ranges from localized form or disseminated in multiple organs, particularly in immunocompromised patients [4,9,10]. The infection most commonly presents as fungemia (75%), and approximately 50% of cases are associated with metastatic skin lesions such as papular-purpuric, nodular, vesicular, or pustular lesions [10].
The diagnosis of invasive Trichosporon infection is based on microscopy in wet mount or tissue biopsy specimen and culture [7]. The microscopic morphology of T. asahii shows septated hyphae, pseudohyphae, and blastoconidia, with cylinder-shaped arthroconidia [7]. Colonies on solid media are white with raised farinose surfaces, but T. asahii on CHROM agar forms characteristic dirty-green colonies. However, tissue biopsy may be difficult for patients with hematologic malignancy because of thrombocytopenia and culture-based phenotypic methods are insensitive, with low positivity rates. Non-culture-based methods for diagnosis of invasive fungal infection are gradually needed to overcome the limitations of test insensitivity and delayed results [7]. A recent report of 33 cases of Trichosporon fungemia in Japan indicated that (1–3)-β-D-glucan levels may have serious limitations for the early detection of invasive trichosporonosis unlike candidiasis and aspergillosis [11,12]. Trichosporon species share antigens with Cryptococcus and Aspergillus. Many previous reports have demonstrated a cross reaction for the cryptococcal and galactomannan antigens [8,11]. Therefore, dual positivity in these tests may be interpreted as an indicator of invasive trichosporonosis, but the sensitivity and specificity of these tests have not been defined [8]. More recently, molecular tests, including polymerase chain reaction-based methods, flow cytometry assays, and proteomics, are being developed [7].
Definite treatment guidelines for invasive T. asahii infections are yet to be established. In previous cases, Trichosporon species are resistant to flucytosine or echinocandins and show various susceptibilities in vitro and limited activity to amphotericin B in vivo [4,13]. However, recent study results suggest that azoles are superior to other antifungal drug classes in prophylaxis and treatment for Trichosporon infections with low MICs [10,12]. On the basis of a head-to-head comparison of five triazoles, voriconazole showed the best therapeutic effect in terms of in vitro and in vivo activities and good results for treating disseminated T. asahii infection [14]. The combination of voriconazole with amphotericin B or echinocandin appears to have some synergistic antifungal effects in some reports [15].
We describe the case of a breakthrough catheter-related T. asahii bloodstream infection during itraconazole prophylaxis. This is the second reported case of T. asahii bloodstream infection case in Korea, after subsequent reclassification [16]. Although the patient received triazole as prophylaxis, we treated her with voriconazole single as primary therapy, which showed successful treatment results without any complications. As primary antifungal prophylaxis is widely used, the invasive fungal infection epidemiology has changed steadily [2]. Moreover, in the past, many antifungal agents used for prophylactic purposes were prescribed with a treatment aim; thus, physicians should consider antifungal resistance and treatment failure due to prior exposure. Therefore, physicians should be concerned about voriconazole treatment failure in breakthrough T. asahii fungemia during triazole prophylaxis even if this is known to be the best treatment agent for T. asahii infection.
To identify other cases of breakthrough T. asahii fungemia in patients with hematologic malignancy with antifungal use, we searched the Medline database (National Library of Medicine, Bethesda, MD, USA) and found reports of 13 cases [4,5,9,13,14,15,17,18,19,20] (Table 1). Breakthrough trichosporonosis has been reported during the administration of various antifungal agents except voriconazole. Treatment outcomes varied. However, voriconazole-containing regimens seemed to have better treatment results, even if the patients received prophylaxis with triazole [5,9]. MICs of antifungal agents were observed in 8 patients. All the strains isolated from the 8 patients examined were highly resistant to echinocandin. The MICs for amphotericin B deoxycholate and fluconazole varied, whereas the MICs for itraconazole and voriconazole were relatively low. Resistance to one triazole does not seem to imply resistance against the whole class of drugs. However, only few data demonstrate the relationship between prophylactic antifungal use and drug resistance; thus, future investigations in larger patient cohorts are needed.
Table 1. Summary of cases of breakthrough Trichosporon asahii fungemia in patients with hematologic disease who were receiving antifungal agents (including the present case).
| Underlying disease | Predisposing condition | Site of infection | Previous antifungal Tx. | Definite Tx. of trichosporonosis | Outcome | AMB | FLC | ITC | VRC | CAF | MIF | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myelodysplastic syndrome | SCT | Catheter | ITC | VRC | Survived | 1 | 8 | 16 | 0.5 | 16 | In this case | |
| AML | CTx | Primary fungemia | CAF | L-AMB + VRC | Expired | >32 | 4 | 0.5 | >0.015 | 16 | [4] | |
| AML | CTx | Primary fungemia | ITC | VRC | Survived | 1 | 32 | 1 | 0.5 | >16 | [5] | |
| Thalassemia | SCT | Catheter | FLC | VRC | Survived | 0.25 | 3 | 0.04 | >32 | [9] | ||
| AML | CTx | Catheter | FLC | AMB + CAF | Survived | 0.06 | >64 | [13] | ||||
| Hematologic malignancy | SCT | Primary fungemia | MIF | L-AMB | Survived | S | S | S | S | R | R | [14] |
| AML | CTx | Catheter | FLC | L-AMB + CAF | Survived | [15] | ||||||
| AML | CTx | GI tract | PSC | VRC + AMB | [17] | |||||||
| AML | CTx | Catheter | ITC | AMB + FLC | Survived | [18] | ||||||
| Myelofibrosis | CTx | Primary fungemia | MIF | L-AMB | Expired | [19] | ||||||
| Myelodysplastic syndrome | CTx | Catheter | MIF | FLC | Expired | [20] | ||||||
| AML | SCT | Primary fungemia | MIF | FLC | Expired | [20] | ||||||
| AML | CTx | Pneumonia | AMB + CAF | VRC | Expired | 0.5 | 8 | 1 | 16 | [20] | ||
| AML | CTx | Primary fungemia | MIF | VRC | Survived | 1 | 32 | 1 | 16 | [20] |
AMB, amphotericin B deoxycholate; FLC, fluconazole; ITC, itraconazole; VRC, voriconazole; AML, acute myeloid leukemia; CAF, caspofungin; MIF, micafungin; SCT, stem cell transplantation; CTx, chemotherapy; L-AMB, liposomal amphotericin B; PSC, posaconazole.
Blank, not commented in the reference article.
Clinicians should be aware that cases of disseminated Trichosporon infections have been increasingly reported worldwide, and these infections may develop in immunocompromised patients, particularly those who have intravascular devices and develop symptoms and signs of a breakthrough infection while receiving primary antifungal prophylaxis. Further research should be performed to investigate in vitro and in vivo activities of antifungal drugs against T. asahii, resistance mechanisms, non-culture-based diagnostic methods, and more detailed treatment strategies, including combination therapy and treatment duration. Based on this case, we emphasize that early suspicion and diagnosis of breakthrough T. asahii infection are important in high-risk patients, even in those receiving antifungal agents, and can lead to proper therapeutic choice and improved prognosis.
Footnotes
Conflicts of interest: No conflicts of interest.
References
- 1.Malani AN, Kauffman CA. Changing epidemiology of rare mould infections: implications for therapy. Drugs. 2007;67:1803–1812. doi: 10.2165/00003495-200767130-00001. [DOI] [PubMed] [Google Scholar]
- 2.Auberger J, Lass-Florl C, Aigner M, Clausen J, Gastl G, Nachbaur D. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: real-life data from a single-centre institutional retrospective observational study. J Antimicrob Chemother. 2012;67:2268–2273. doi: 10.1093/jac/dks189. [DOI] [PubMed] [Google Scholar]
- 3.Kang SH, Kim HS, Bae MN, Kim J, Yoo JY, Lee KY, Lee DG. Fatal breakthrough mucormycosis in an acute myelogenous leukemia patient while on posaconazole prophylaxis. Infect Chemother. 2015;47:49–54. doi: 10.3947/ic.2015.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayramoglu G, Sonmez M, Tosun I, Aydin K, Aydin F. Breakthrough Trichosporon asahii fungemia in neutropenic patient with acute leukemia while receiving caspofungin. Infection. 2008;36:68–70. doi: 10.1007/s15010-007-6278-6. [DOI] [PubMed] [Google Scholar]
- 5.Asada N, Uryu H, Koseki M, Takeuchi M, Komatsu M, Matsue K. Successful treatment of breakthrough Trichosporon asahii fungemia with voriconazole in a patient with acute myeloid leukemia. Clin Infect Dis. 2006;43:e39–41. doi: 10.1086/505970. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute (CLSI) Reference method for broth dilution antifungal susceptibility testing of yeast; 4th informational supplement, M27-S4. Wayne, PA: CLSI; 2012. [Google Scholar]
- 7.Colombo AL, Padovan AC, Chaves GM. Current knowledge of Trichosporon spp. and Trichosporonosis. Clin Microbiol Rev. 2011;24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group. European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 9.Dua V, Yadav SP, Oberoi J, Sachdeva A. Successful treatment of Trichosporon asahii infection with voriconazole after bone marrow transplant. J Pediatr Hematol Oncol. 2013;35:237–238. doi: 10.1097/MPH.0b013e318279b21b. [DOI] [PubMed] [Google Scholar]
- 10.Ruan SY, Chien JY, Hsueh PR. Invasive trichosporonosis caused by Trichosporon asahii and other unusual Trichosporon species at a medical center in Taiwan. Clin Infect Dis. 2009;49:e11–e17. doi: 10.1086/599614. [DOI] [PubMed] [Google Scholar]
- 11.Liao Y, Hartmann T, Ao JH, Yang RY. Serum glucuronoxylomannan may be more appropriate for the diagnosis and therapeutic monitoring of Trichosporon fungemia than serum β -D-glucan. Int J Infect Dis. 2012;16:e638. doi: 10.1016/j.ijid.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Nakase K, Kyo T, Kohara T, Sugawara Y, Shibazaki T, Oka K, Tsukada T, Katayama N. Fatal Trichosporon fungemia in patients with hematologic malignancies. Eur J Haematol. 2010;84:441–447. doi: 10.1111/j.1600-0609.2010.01410.x. [DOI] [PubMed] [Google Scholar]
- 13.Menezes EA, Marinho JA, Angelo MR, Cunha Mda C, Cunha FA, Vasconcelos Júnior AA. Isolation and antifungal susceptibility testing of Trichosporon asahii in Ceará, Brazil. Rev Inst Med Trop São Paulo. 2012;54:1–3. doi: 10.1590/s0036-46652012000100001. [DOI] [PubMed] [Google Scholar]
- 14.Nachbaur D, Angelova O, Orth-Höller D, Ditlbacher A, Lackner M, Auberger J, Lass-Flörl C. Primary antifungal prophylaxis with micafungin in patients with haematological malignancies: real-life data from a retrospective single-centre observational study. Eur J Haematol. 2015;94:258–264. doi: 10.1111/ejh.12426. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Chen F, Wang Y, Yang LY, Miao M, Han Y, Wu DP. Use of combination therapy to successfully treat breakthrough Trichosporon asahii infection in an acute leukemia patient receiving voriconazole. Med Mycol Case Rep. 2014;6:55–57. doi: 10.1016/j.mmcr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YJ, Kim SI, Kim YR, Park YM, Park YJ, Kang MW. Successful treatment of septic shock with purpura fulminans caused by Trichosporon asahii in an immunocompetent patient. Ann Clin Lab Sci. 2007;37:366–369. [PubMed] [Google Scholar]
- 17.Rieger C, Geiger S, Herold T, Nickenig C, Ostermann H. Breakthrough infection of Trichosporon asahii during posaconazole treatment in a patient with acute myeloid leukaemia. Eur J Clin Microbiol Infect Dis. 2007;26:843–845. doi: 10.1007/s10096-007-0366-5. [DOI] [PubMed] [Google Scholar]
- 18.Jang MJ, Lee YK, Han KC, Hong SG, Kang MS, Oh D, Chong SY. A case of Trichosporon beigelii fungemia treated with mphotericin B and fluconazole in a patient with acute myelogenous leukemia. Korean J Hematol. 2004;39:109–112. [Google Scholar]
- 19.Shimono J, Tsutsumi Y, Ohigashi H. Acute renal tubular damage caused by disseminated Trichosporon infection in primary myelofibrosis. Rinsho Ketsueki. 2015;56:21–24. doi: 10.11406/rinketsu.56.21. [DOI] [PubMed] [Google Scholar]
- 20.Matsue K, Uryu H, Koseki M, Asada N, Takeuchi M. Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin Infect Dis. 2006;42:753–757. doi: 10.1086/500323. [DOI] [PubMed] [Google Scholar]