Abstract
Individuals who have prior history of childhood traumatic experiences are at a high risk for a variety of psychological and behavioral problems throughout their lifetime. This study aimed to investigate whether such individuals exhibit altered cortical functional networks during a behavioral inhibition task. One hundred fifty-three non-clinical individuals were recruited and instructed to perform a Go/NoGo task during an electroencephalograph. Source-level weighted functional networks based on the graph theory were analyzed for NoGo-P3 processing. Based on their total scores on the childhood trauma questionnaire (CTQ) participants were divided into three groups: low CTQ, middle CTQ, and high CTQ. Results at the global level indicated decreased strength, clustering coefficient, and efficiency for the low and gamma bands in the high CTQ group. In addition, the path length of the low beta band was observed to be longer in the high CTQ group than the low CTQ group. At the nodal level, the nodal clustering coefficient of high CTQ group was decreased in left primary somatosensory cortex and middle occipital gyrus for the low beta band, and in left superior temporal gyrus for the gamma band. The nodal clustering coefficient of the left primary somatosensory cortex showed a significant negative correlation with the total CTQ score for the low beta band. In addition, the nodal clustering coefficient of the left middle occipital gyrus for the low beta band and superior temporal gyrus for the gamma band showed significant negative correlations with the emotional neglect score. Our results demonstrate an altered cortical functional network in individuals who experienced childhood trauma. In particular, the left primary somatosensory cortex, middle occipital gyrus, and superior temporal gyrus were found to be vulnerable in individuals who experienced childhood trauma, especially emotional neglect.
Introduction
Childhood trauma appears to be a crucial etiological factor in the development of various psychological and behavioral disorders across the lifespan1. In particular, childhood trauma has been associated with multiple adverse cognitive consequences, such as low IQ, decreased academic performance, and deficits in attention, emotion, and inhibitory functioning2.
Epidemiological studies reveal that children with early traumatic experiences are more susceptible to developing depression and/or anxiety disorders3. Childhood trauma may also play a role in the development of impulsivity that progress into substance abuse and suicidal behaviors4,5. Moreover, those with a history of childhood trauma demonstrate impaired behavioral inhibition6,7, which is an essential regulatory function that develops progressively from child- to adulthood8. Deficits of inhibitory control have been consistently observed in adults who experienced childhood sexual abuse9, adolescents with early life stress10, and maltreated and institutionalized children11–13. In addition, childhood adversity can alter reward processes central to the Behavioral Activation System. Studies have identified an insensitivity to rewards in maltreated children14, and less brain activation in regions associated with reward processes during the anticipation of reward in those with childhood trauma15,16.
Furthermore, childhood trauma has been associated with cognitive dysfunctions2,17, including attentional deficits in both children and adults7. Considering that inattention is one of the fundamental symptoms of attention deficit hyperactivity disorder (ADHD)18, it is arguable that the exposure to traumatic events during childhood is a risk factor for ADHD in childhood19 and later in adulthood20.
Meanwhile, the Go/NoGo task has been widely used to explore behavioral inhibition21,22. In the Go/Nogo task, individuals are instructed to respond to Go trials and to refrain responding (inhibit) to Nogo trials21. Go/NoGo event-related potentials (ERP) have been used as an informative tool to evaluate behavioral inhibitory capacities23. Typically, N2 and P3 components of ERPs are analyzed in the Go/NoGo task. These two components generally appear in sequence and are associated with the early and late phases of response inhibition, respectively24. NoGo-N2 has been suggested to reflect response inhibition25 or the process of conflict monitoring26, whereas NoGo-P3 has been proposed to primarily reflect the inhibitory process itself27. Recent studies have suggested that NoGo-P3 may play an important role in the post-response stage, reflecting processes of evaluation or monitoring of inhibition, error detection, and preparation for future trials28,29.
Childhood traumatic experiences may lead to long-term structural and functional changes in brain development30–33. An increasing number of researchers have started to focus on changes in the cortical functional network. Many of these studies have adopted the graph theory to quantify global and local changes in the cortical functional network using network indices such as the strength, clustering coefficient, path length, and efficiency34–37. In addition, different modalities, including the resting-state functional network using functional magnetic resonance imaging (fMRI)38, the structural cortical network based on cortical thickness39, and the structural connectivity network based on diffusion tensor imaging (DTI) tractography40 have reported altered brain networks in individuals with childhood trauma. However, no study has evaluated the cortical functional network using electroencephalography (EEG). EEG has significant advantages such as advanced time resolution for investigating the functional network of early neural processing (i.e., the N2 or P3 component) during the Go/NoGo task and cost-effectiveness. While EEG has some limitations such as low spatial resolution that originates from volume conduction41,42, and poor signal-to-noise ratios affected by various noises and artifacts43,44, they can be addressed through source-imaging methods. In particular, the spatial resolution of EEG can be substantially improved by mapping the scalp potential distribution onto the underlying cortical source space using source-imaging methods.
In the present study, brain cortical networks were evaluated using a source-level weighted functional network analysis during a Go/NoGo paradigm in individuals who had experienced childhood trauma. Source-level weighted network analysis allows observation of alternations in specific local cortical regions as well as in the patterns of the global brain network. We hypothesized that during a behavioral inhibition task, individuals with traumatic childhood experiences would exhibit an altered cortical functional network at both global and nodal levels, reflecting a loss of inhibitory control during a behavioral inhibition task. Furthermore, we hypothesized that the altered cortical network indices such as the strength, clustering coefficient, path length, and efficiency would be significantly correlated with the severity of childhood trauma.
Results
Psychological and Behavioral measures
Table 1 shows the comparison of demographic and psychological characteristics between the low, middle, and high CTQ groups. The scores of the STAI, BDI, BIS (attentional impulsivity and motor impulsivity), CAARS (inattention/memory and hyperactivity/restlessness), and CTQ (physical, emotional, sexual abuse, physical, and emotional neglect) were significantly higher in the high CTQ group than in the low CTQ group (SAI: 32.45 ± 7.26 vs. 41.89 ± 7.93, p < 0.001; TAI: 34.27 ± 9.88 vs. 45.87 ± 9.11, p < 0.001; BDI: 5.39 ± 3.50 vs. 12.08 ± 7.68, p < 0.001; attentional impulsivity: 15.39 ± 3.21 vs. 17.66 ± 3.72, p = 0.009; motor impulsivity: 23.91 ± 4.98 vs. 26.42 ± 4.32, p = 0.044; inattention/memory: 22.20 ± 4.95 vs. 28.86 ± 6.30, p < 0.001; hyperactivity/restlessness: 16.84 ± 4.11 vs. 20.79 ± 4.62, p < 0.001). In contrast, the score of the Behavioral Activation System (drive) was significantly lower in the high CTQ group than in the low CTQ group (9.02 ± 1.91 vs. 7.79 ± 1.76, p = 0.003). However, there was no significant difference among the three groups in the NoGo false alarm rate (0.12 ± 0.11 vs. 0.12 ± 0.08 vs. 0.13 ± 0.10, p = 0.888). In addition, the three groups did not significantly differ in their reaction time and hit rate during the Go condition (Go reaction time: 373.78 ± 26.53 vs. 374.53 ± 25.00 vs. 381.25 ± 25.76, p = 0.343; Go hit rate: 0.95 ± 0.07 vs. 0.94 ± 0.08 vs. 0.92 ± 0.07, p = 0.116).
Table 1.
Comparison of baseline demographic, psychological, and behavioral characteristics in individuals with low, middle, and high childhood trauma questionnaire (CTQ) scores.
| Low CTQ (N = 44) |
Middle CTQ (N = 71) |
High CTQ (N = 38) |
p | Pairwise test, pa | |
|---|---|---|---|---|---|
| Mean ± SD or N (%) | Low vs. High | ||||
| Age (years) | 26.14 ± 5.92 | 28.48 ± 6.74 | 28.24 ± 6.21 | 0.142 | |
| Sex | |||||
| Male (%) | 20 (45.5) | 24 (33.8) | 12 (31.6) | 0.343 | |
| Female (%) | 24 (54.5) | 47 (66.2) | 26 (68.4) | ||
| Education (years) | 13.91 ± 1.88 | 14.56 ± 1.75 | 14.58 ± 1.54 | 0.109 | |
| Go reaction time (ms) | 373.78 ± 26.53 | 374.53 ± 25.00 | 381.25 ± 25.76 | 0.343 | |
| Go hit rate | 0.95 ± 0.07 | 0.94 ± 0.08 | 0.92 ± 0.07 | 0.116 | |
| Nogo false alarm rate | 0.12 ± 0.11 | 0.12 ± 0.08 | 0.13 ± 0.11 | 0.888 | |
| State Anxiety Inventory (SAI) | 32.45 ± 7.26 | 36.00 ± 6.97 | 41.89 ± 7.93 | <0.001 | <0.001 |
| Trait Anxiety Inventory (TAI) | 34.27 ± 9.88 | 39.17 ± 8.23 | 45.87 ± 9.11 | <0.001 | <0.001 |
| Beck Depression Inventory (BDI) | 5.39 ± 3.50 | 6.92 ± 4.37 | 12.08 ± 7.68 | <0.001 | <0.001 |
| Barratt Impulsivity Scale (BIS) | 58.07 ± 10.17 | 58.83 ± 8.67 | 62.95 ± 9.29 | 0.039 | 0.056 |
| Attentional impulsivity | 15.39 ± 3.21 | 15.77 ± 3.36 | 17.66 ± 3.72 | 0.006 | 0.009 |
| Motor impulsivity | 23.91 ± 4.98 | 25.28 ± 4.49 | 26.42 ± 4.32 | 0.049 | 0.044 |
| Non-planning impulsivity | 18.77 ± 4.19 | 17.77 ± 3.47 | 18.87 ± 3.83 | 0.236 | |
| Conners’ Adult ADHD rating scale (CAARS) | 70.68 ± 12.08 | 73.25 ± 15.38 | 82.87 ± 13.35 | <0.001 | <0.001 |
| Inattention/Memory | 22.20 ± 4.95 | 23.92 ± 6.59 | 28.86 ± 6.30 | <0.001 | <0.001 |
| Hyperactivity/Restlessness | 16.84 ± 4.11 | 17.86 ± 4.69 | 20.79 ± 4.62 | <0.001 | <0.001 |
| Impulsivity/Emotional lability | 17.27 ± 3.74 | 17.93 ± 4.91 | 19.47 ± 3.97 | 0.070 | |
| Problem with self-concept | 14.36 ± 3.14 | 13.55 ± 2.93 | 13.95 ± 3.00 | 0.368 | |
| Behavioral Inhibition System | 21.34 ± 2.80 | 21.08 ± 2.22 | 21.53 ± 1.69 | 0.612 | |
| Behavioral Activation System | 37.73 ± 7.81 | 34.85 ± 5.26 | 34.42 ± 3.92 | 0.016 | 0.035 |
| Drive | 9.02 ± 1.91 | 8.30 ± 1.36 | 7.79 ± 1.76 | 0.003 | 0.003 |
| Fun-Seeking | 10.57 ± 2.63 | 10.28 ± 1.92 | 10.63 ± 1.94 | 0.685 | |
| Reward Responsiveness | 18.14 ± 7.24 | 16.27 ± 4.81 | 16.00 ± 1.79 | 0.104 | |
| Childhood Trauma Questionnaire | 31.41 ± 2.06 | 40.44 ± 3.61 | 60.21 ± 10.00 | <0.001 | <0.001 |
| Physical abuse | 5.66 ± 1.10 | 6.54 ± 1.82 | 10.18 ± 3.91 | <0.001 | <0.001 |
| Emotional abuse | 5.07 ± 0.26 | 5.82 ± 1.36 | 9.18 ± 3.73 | <0.001 | <0.001 |
| Sexual abuse | 5.16 ± 0.65 | 5.41 ± 0.90 | 7.61 ± 3.40 | <0.001 | <0.001 |
| Physical neglect | 5.55 ± 1.25 | 6.31 ± 1.91 | 7.89 ± 2.99 | <0.001 | <0.001 |
| Emotional neglect | 9.98 ± 1.89 | 16.37 ± 3.87 | 25.34 ± 4.53 | <0.001 | <0.001 |
ap-values represent statistically significant differences between the low and high CTQ groups with post-hoc test using Bonferroni correction.
Global level differences in cortical functional networks
Table 2 shows the comparison of global level indices, including the strength, clustering coefficient, path length, and efficiency of each frequency band among the low, middle, and high CTQ groups. The strength, clustering coefficient, and efficiency of the low beta and gamma bands were significantly decreased in the high CTQ group compared to the low CTQ group (strength: 179.14 ± 10.79 vs. 173.50 ± 7.60, p = 0.001; clustering coefficient: 0.57 ± 0.03 vs. 0.55 ± 0.02, p = 0.001; efficiency: 0.57 ± 0.03 vs. 0.55 ± 0.02, p = 0.001 for low beta band, strength: 134.70 ± 18.02 vs. 126.69 ± 12.83, p = 0.002; clustering coefficient: 0.42 ± 0.06 vs. 0.39 ± 0.04, p = 0.002; efficiency: 0.43 ± 0.06 vs. 0.40 ± 0.04, p = 0.002 for gamma band). On the other hand, the path length of the low beta band was significantly longer in the high CTQ group than in the low CTQ group (1.85 ± 0.09 vs. 1.90 ± 0.07, p = 0.002). However, there was no significant difference among the three groups in other frequency bands. The power was 0.95 to detect an effect size of 0.13 in comparison of the cortical network characteristics at the global level among the three groups.
Table 2.
Mean and standard deviation values of global network indices including strength, clustering coefficient, path length, and efficiency in each frequency band among low, middle, and high childhood trauma questionnaire (CTQ) groups.
| Low CTQ (N = 44) |
Middle CTQ (N = 71) |
High CTQ (N = 38) |
Effect size (η2) |
p | Pairwise test, pa | |
|---|---|---|---|---|---|---|
| Mean ± SD | Low vs. High | |||||
| Alpha band | ||||||
| Strength | 208.09 ± 9.51 | 205.74 ± 8.98 | 205.56 ± 8.74 | 0.027 | 0.139 | |
| Clustering coefficient | 0.66 ± 0.03 | 0.65 ± 0.03 | 0.65 ± 0.03 | 0.025 | 0.152 | |
| Path length | 1.56 ± 0.07 | 1.57 ± 0.06 | 1.57 ± 0.06 | 0.021 | 0.208 | |
| Efficiency | 0.66 ± 0.03 | 0.65 ± 0.03 | 0.65 ± 0.03 | 0.027 | 0.139 | |
| Low beta band | ||||||
| Strength | 179.14 ± 10.79 | 175.94 ± 8.94 | 173.50 ± 7.60 | 0.083 | 0.002 | 0.001 |
| Clustering coefficient | 0.57 ± 0.03 | 0.56 ± 0.03 | 0.55 ± 0.02 | 0.083 | 0.002 | 0.001 |
| Path length | 1.85 ± 0.09 | 1.87 ± 0.08 | 1.90 ± 0.07 | 0.079 | 0.002 | 0.002 |
| Efficiency | 0.57 ± 0.03 | 0.56 ± 0.03 | 0.55 ± 0.02 | 0.083 | 0.002 | 0.001 |
| High beta band | ||||||
| Strength | 151.16 ± 14.30 | 147.59 ± 10.95 | 145.42 ± 10.81 | 0.067 | 0.006 | |
| Clustering coefficient | 0.47 ± 0.04 | 0.46 ± 0.03 | 0.45 ± 0.03 | 0.066 | 0.007 | |
| Path length | 2.29 ± 0.17 | 2.33 ± 0.14 | 2.36 ± 0.15 | 0.062 | 0.009 | |
| Efficiency | 0.48 ± 0.05 | 0.47 ± 0.03 | 0.46 ± 0.03 | 0.067 | 0.006 | |
| Gamma band | ||||||
| Strength | 134.70 ± 18.02 | 130.38 ± 14.15 | 126.69 ± 12.83 | 0.079 | 0.002 | 0.002 |
| Clustering coefficient | 0.42 ± 0.06 | 0.40 ±± 0.04 | 0.39 ± 0.04 | 0.078 | 0.003 | 0.002 |
| Path length | 2.71 ± 0.29 | 2.78 ± 0.26 | 2.84 ± 0.24 | 0.072 | 0.004 | |
| Efficiency | 0.43 ± 0.06 | 0.42 ± 0.05 | 0.40 ± 0.04 | 0.080 | 0.002 | 0.002 |
ap-values represent statistically significant differences between the low and high CTQ groups with post-hoc test using Bonferroni correction.
Nodal level differences in cortical functional networks
Based on significant differences of the low beta and gamma band clustering coefficients among the three groups, we decided to examine possible differences at the local level in the low beta and gamma bands. The nodal clustering coefficient of the high CTQ group was significantly decreased in two nodes for the low beta band (primary somatosensory cortex (BA 1–3): 0.58 ± 0.04 vs. 0.55 ± 0.03, p < 0.001; middle occipital gyrus (BA 19): 0.57 ± 0.05 vs. 0.55 ± 0.04, p < 0.001) and one node for the gamma band (superior temporal gyrus (BA 41): 0.46 ± 0.07 vs. 0.42 ± 0.05, p < 0.001) (Table 3).
Table 3.
Mean and standard deviation values of clustering coefficients in nodal level for low beta and gamma bands among low, middle, and high childhood trauma questionnaire (CTQ) groups.
| Low CTQ (N = 44) |
Middle CTQ (N = 71) |
High CTQ (N = 38) |
Effect size (η2) |
p | Pairwise test, pa | |
|---|---|---|---|---|---|---|
| Mean ± SD | Low vs. High | |||||
| Low beta band | ||||||
| Primary somatosensory cortex (BA 1–3) |
0.58 ± 0.04 | 0.56 ± 0.03 | 0.55 ± 0.03 | 0.124 | <0.001 | <0.001 |
| Middle occipital gyrus (BA 19) |
0.57 ± 0.05 | 0.55 ± 0.04 | 0.55 ± 0.04 | 0.120 | <0.001 | <0.001 |
| Gamma band | ||||||
| Superior temporal gyrus (BA 41) |
0.46 ± 0.07 | 0.44 ± 0.06 | 0.42 ± 0.05 | 0.115 | <0.001 | <0.001 |
ap-values represent statistically significant differences between the low and high CTQ groups with post-hoc test using Bonferroni correction.
Correlation between network indices and psychological characteristics
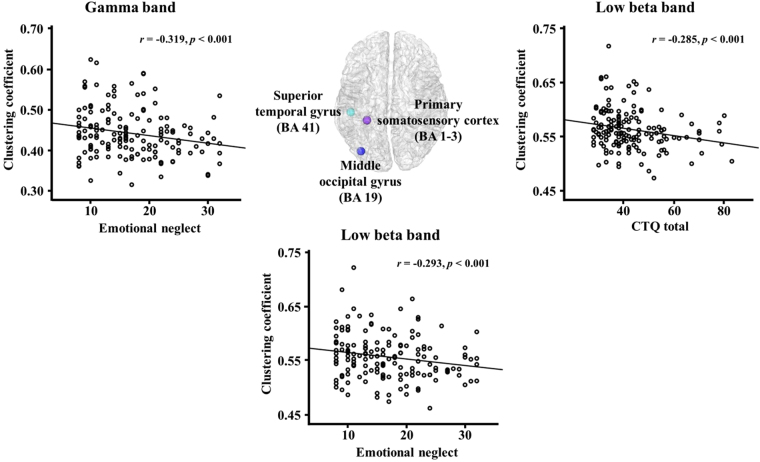
The relationships between the network indices at the global and nodal levels and childhood trauma-related measures were investigated in the low beta and gamma bands. The nodal clustering coefficient in the left primary somatosensory cortex (BA 1–3) significantly correlated with the total CTQ score for the low beta band (r = −0.285, p < 0.001). In addition, the nodal clustering coefficients in the left middle occipital gyrus (BA 19) for the low beta band and superior temporal gyrus (BA 41) for the gamma band were significantly correlated with emotional neglect (r = −0.293, p < 0.001; r = −0.319, p < 0.001, respectively) (Fig. 1).
Figure 1.
The correlations of the nodal clustering coefficients with childhood trauma-related measures in the low beta and gamma bands. The nodal clustering coefficient at the left primary somatosensory cortex (BA 1–3) showed significant correlations with the childhood trauma questionnaire (CTQ) total score for the low beta band. The nodal clustering coefficients at the left middle occipital gyrus (BA 19) for the low beta band and superior temporal gyrus (BA 41) for the gamma band were significantly correlated with the CTQ emotional neglect subscale score.
Discussion
This study evaluated whether individuals with higher CTQ scores show altered cortical functional networks during a behavioral inhibition task (i.e., NoGo-P3 processing of the Go/NoGo task). Our major findings, which pertained to the low beta and gamma bands, can be divided into the global and the nodal levels. First, at the global level, the strength, clustering coefficient, and efficiency of the low beta and gamma bands were significantly decreased in the high CTQ group compared to the low CTQ group. The path length of the low beta band was significantly longer in the high CTQ group than in the low CTQ group. Second, at the nodal level, the clustering coefficient of the low beta band was decreased in the left primary somatosensory cortex and middle occipital gyrus and that of the gamma band was decreased in the left superior temporal gyrus for the high CTQ group. Third, the nodal level low beta band clustering coefficient of the left primary somatosensory cortex was significantly negatively correlated with the total CTQ score. In addition, the nodal clustering coefficient in the left middle occipital gyrus for the low beta band and superior temporal gyrus for the gamma band was significantly negatively correlated with emotional neglect.
In this study, the scores of the STAI, BDI, BIS subscales (attentional impulsivity and motor impulsivity), and CAARS (inattention/memory and hyperactivity/restlessness) were significantly higher in the high CTQ group than in the low CTQ group. This is consistent with the previous literature that has identified significant associations between childhood trauma and the development of depressive and anxiety disorder in adulthood45,46, as well as behaviors related to impulsivity4,47. Specifically, Brodsky et al.4 noted that depressed patients with childhood trauma were not only more likely to have attempted suicide but also to have higher BIS scores. Narvaez et al.47 showed that childhood trauma was strongly associated with poor executive functioning and higher levels of impulsivity among crack cocaine users. Furthermore, Beers and De Bellis revealed that maltreated children with post-traumatic stress disorder showed decreased sustained attention and were more vulnerable to distractions48.
In contrast to the STAI, BDI, BIS subscales, and the CAARS, the scores of the Behavioral Activation System (drive) were found to be significantly lower in the high CTQ group. Previously, it has been suggested that childhood maltreatment could alter the sensitivity of the Behavioral Activation System49, and also lead to deficits in cognitive control and emotion regulation2,50. Our results support the previous findings that individuals with childhood trauma display various psychological and behavioral disruptions.
In this study, no significant differences were reported among the three groups in term of the NoGo false alarm rate. In addition, no significant relationships were found between the network indices and the NoGo false alarm rate. These results are similar to the previous study, which identified no significant correlation between the Go/NoGo task performance and the ratings on childhood trauma51. However, studies that have examined the influences of childhood trauma on the Go/NoGo task have not produced entirely consistent results and there have been studies that reported significant associations between childhood trauma and the performance in tasks using the Go/NoGo paradigm. For example, longer reaction times during inhibitory tasks have been reported in individuals with childhood maltreatment10. Tottenham et al.50 reported that institutionalized children, when compared to controls, were more likely to make false alarm errors to negatively valenced faces that appear in the NoGo condition. This suggests that these children may have difficulties regulating emotions.
Although our study did not find any significant effect of childhood trauma on the performance during the Go/NoGo task, we identified apparent differences between the network indices of the low and high CTQ groups. Moreover, the nodal level clustering coefficients showed significant correlations with childhood trauma-related measures during the Go/NoGo task.
The strength, clustering coefficient, and efficiency of the low beta and gamma frequency bands were significantly decreased in the high CTQ group compared to the low CTQ group. On the other hand, the path length of the low beta frequency band was significantly prolonged in the high CTQ group. Decreased clustering coefficient and increased path length could each imply decreased local connectedness and inefficiently increased connectedness of brain networks. Furthermore, decreased strength in the brain networks of the high CTQ group could reflect weaker connection strength (looser links) compared to that of the low CTQ group. Maltreatment during childhood has been associated with changes in the structure and functional connectivity at the network level. Teicher et al.39 reported that childhood maltreatment was associated with a decrease in the centrality of the left anterior cingulate region and an increase in the centrality of the right anterior insula and precuneus. An fMRI study assessing the inhibitory control network revealed that less inhibition of the right inferior frontal cortex, which was negatively modulated by the dorsal anterior cingulate cortex, improved inhibitory control ability in males with higher CTQ scores52. In addition, individuals with childhood trauma showed decreased network indices (degree, efficiency, and betweenness centrality) in the right ventrolateral prefrontal cortex and the dorsal anterior cingulate cortex compared to the control group38. The findings from these previous studies support our results that network characteristics are disrupted in individuals with childhood trauma.
Previous studies have reported significant association between altered beta and gamma band activities and childhood trauma. In addition, the presence of beta and gamma bands have been related to symptoms of anxiety and anxiety related disorders53–55. Increased beta power is known to occur during sleep in individuals who have experienced childhood maltreatment56. Individuals with childhood trauma have demonstrated significantly higher beta coherence over the right central and temporal regions than those with no trauma history57. A recent study by our research group showed increased beta and gamma power in individuals with childhood trauma, which suggests that the increased power of individuals with childhood trauma may reflect cognitive deficits of the brain. Furthermore, it revealed that childhood trauma might be associated with high anxiety states that cause the increase of gamma band power in the brain58. In addition, increased resting state EEG beta powers in bereaved family members of accident victims have been observed; this increased beta power was interpreted to reflect a coping strategy for extremely complex and stressful situations. The same study also reported that gamma activity was significantly higher in bereaved family members with high anxiety scores59. In sum, these findings support the hypothesis that the beta and gamma frequency bands reflect vulnerability to anxiety, stress, and childhood trauma.
In the high CTQ group, the nodal clustering coefficients of the low beta band were decreased in the left primary somatosensory cortex and middle occipital gyrus; in the left superior temporal gyrus, that of the gamma frequency band was decreased. The nodal clustering coefficient of the left primary somatosensory cortex for the low beta band was negatively correlated with the CTQ total score. In addition, the nodal clustering coefficients of the left middle occipital gyrus for the low beta band and the superior temporal gyrus for the gamma band were negatively correlated with the emotional neglect subscale score. Childhood maltreatment has been associated with abnormal development of the sensory systems that relay adverse sensory experiences. For instance, women with childhood maltreatment display thinner left primary somatosensory cortex60. In addition, a recent meta-analysis demonstrated that childhood maltreatment was associated decreased primary somatosensory volume61. In terms of the left middle occipital gyrus, a recent meta-analysis revealed that individuals with childhood maltreatment had larger gray matter volumes in the region61.
In addition, the superior temporal gyrus has been closely associated with childhood trauma. Individuals with childhood maltreatment revealed reduced gray matter volume in the superior temporal gyrus61. Another study suggested that childhood maltreatment was associated with altered symmetry in the superior temporal gyrus62. Fisher et al.63 reported that when incorrect NoGo trials were compared to the resting baseline period, foster children who had been maltreated showed significantly stronger activation than non-maltreated children in a number of brain regions, including the left superior temporal gyrus. Furthermore, given that the superior temporal gyri develops relatively late64,65, the region may be more susceptible to impairment in individuals with childhood trauma. Taken together, these regions are highly related to childhood trauma and our results support these previous findings.
The clustering coefficient represents how strongly each node is connected with its neighbors, and provides information about the level of local connectedness within a network. A higher clustering coefficient represents higher local efficiency of information transfer66,67, while a lower clustering coefficient represents loose coupling and a rapid shift towards network randomness68,69. Therefore, reduced clustering coefficient in the left primary somatosensory cortex, the middle occipital gyrus, and the superior temporal gyrus indicates that the regions became less connected with its neighbors in accordance with the severity of childhood trauma.
The limitations of this study were as follows. First, the present study did not use a structured clinical interview to screen possible psychiatric illnesses. Second, although the CTQ has been widely used in research for clinical and non-clinical individuals70, the CTQ may not precisely reflect the individual’s traumatic childhood experiences because of its retrospective nature. Third, our results may not extrapolate to clinical individuals.
Despite these limitations, our results are meaningful considering that our study was the first to analyze the source-level small-world network in individuals with childhood trauma using an inhibitory task. Our results demonstrate that the functional clustering of the left primary somatosensory cortex, the middle occipital gyrus, and the superior temporal gyrus was vulnerable and dysfunctional in individuals with childhood trauma, especially in those who had experienced emotional neglect. In addition, our results suggest that source-level network analysis of EEG signals might be used as correlates in individuals with childhood trauma.
Methods
Participants
The study was performed on 153 non-smoking non-clinical volunteers (56 males and 97 females) with a mean age of 27.75 ± 6.43 years. Participants were recruited from the local community through newspapers and posters. Participants, who revealed any history of neurological or psychological diseases during the initial screening interview, were excluded from the study. All participants had normal or corrected-to-normal vision, as determined by a check of visual acuity with the Snellen chart71. The participants were divided into 3 subgroups based on the 25% and 75% quartiles of the Childhood Trauma Questionnaire (CTQ)72 total score (34.0 and 47.5, respectively): low CTQ group (lower 25%, n = 44, 31.41 ± 2.06), middle CTQ group (25–75%, n = 71, 40.44 ± 3.61), and high CTQ group (upper 25%, n = 38, 60.21 ± 10.00). The Institutional Review Board at Inje University Ilsan Paik Hospital approved the study and all of its experimental protocols (2015-07-026-001). The study was performed in accordance with approved guidelines and an informed consent was obtained from all individuals included in the study.
Psychological measures
Anxiety and depression was measured through the State-Trait Anxiety Inventory (STAI)73,74 and the Beck Depression Inventory (BDI)75. The STAI is a self-rating scale of state and trait anxiety74. It consists of a state anxiety inventory (SAI) and a trait anxiety inventory (TAI), which are comprised of 20 items73. The BDI is a self-rating scale composed of 21 items that measures the severity of symptoms related to depression75.
The Barratt Impulsiveness Scale (BIS)76,77 and Conners’ Adult ADHD rating scale (CAARS)78 were used to assess impulsivity-related traits. The BIS consists of 30 items, and is designed to assess the personality/behavioral construct of impulsiveness. It is consisted of three sub-factors: attentional, motor, and non-planning impulsivity76. The CAARS is designed to assess manifestations of ADHD in adults, and is composed of 42 items that are divided into four subscales: inattention/memory, hyperactivity/restlessness, impulsivity/emotional lability, and problems with self-concept78.
Behavioral Inhibition System and Behavioral Activation System scales were used to measure the self-reported dysregulations of behavioral inhibition and activation. The Behavioral Inhibition System and Behavioral Activation System scales are 20-item self-rating scales with good psychometric properties79,80. The scales assess the Behavioral Inhibition System (7 items) and three subdomains of the Behavioral Activation System: Drive (4 items), Fun-Seeking (4 items), and Reward Responsiveness (5 items).
The CTQ72 was used to assess traumatic childhood experiences. The CTQ consists of 28 items (25 clinical and 3 validity items) that measure 5 categories of childhood maltreatment, including physical, emotional, and sexual abuse, as well as physical and emotional neglect. Each subscale has 5 items with a 5-point frequency of occurrence ranging from 5 to 25.
Recording and Preprocessing of electroencephalography (EEG)
EEG was recorded using a NeuroScan SynAmps amplifier (Compumedics USA, Charlotte, NC) with 64 Ag-AgCl electrodes mounted on a Quik-Cap using an extended 10–20 placement scheme. The ground electrode was placed on the forehead and the physically linked reference electrode was attached to both mastoids. The vertical electrooculogram (EOG) channels were positioned above and below the left eye, and the horizontal EOG channels were recorded at the outer canthus of each eye. The impedance was maintained below 5 kΩ. All data were processed with a 0.1–100 Hz band pass filter and sampled at 1000 Hz.
The recorded EEG data were preprocessed using CURRY 7 (Compumedics USA, Charlotte, NC). Gross artifacts such as movement artifacts were rejected by visual inspection by a trained individual with no prior information regarding the origin of the data. Artifacts related to eye movement or eye blinks were removed using the mathematical procedure81 implemented in the preprocessing software of CURRY 7. The data were filtered using a 0.1–55 Hz bandpass filter and epoched from 100 ms pre-stimulus to 900 ms post-stimulus. The epochs were rejected from further analysis if they contained significant physiological artifacts (amplitude exceeding ± 75 μV) at any site over the 62 electrodes. For the analysis of the Go/NoGo task, only epochs corresponding to correct responses were used. The number of Go/NoGo epochs used for the analysis did not significantly differ among the low, middle, and high CTQ groups (Go condition: 207.16 ± 22.61 vs. 202.15 ± 26.36 vs. 201.76 ± 24.77, p = 0.514, NoGo condition: 48.18 ± 7.43 vs. 47.27 ± 6.77 vs. 47.29 ± 7.98, p = 0.784).
Go/NoGo experiment
Participants were seated approximately 60 cm away from a computer screen (Mitsubishi, 22-inch CRT monitor). Stimuli for the Go/NoGo task, which consisted of the numbers 1–8, were presented randomly on the screen. The participants were instructed to press a space bar as accurately and quickly as possible when the Go stimuli (even numbers: 2, 4, 6, and 8) appeared at the center of the screen and to not respond when the NoGo stimuli (odd numbers: 1, 3, 5, and 7) were displayed. There were 300 trials comprising the Go (80% probability) condition and the NoGo (20% probability) condition. For each trial, a fixation cross was presented for 100 ms. Following intervals of 700–1000 ms, the Go or NoGo targets appeared for 500 ms. In between trials, there was a 500 ms interval. These stimuli were generated by E-Prime software (Psychology Software Tools, Pittsburgh, PA, USA).
Source localization
The minimum-norm estimation, which was implemented in eConnectome toolbox (Biomedical Functional Imaging and Neuroengineering Laboratory, University of Minnesota, Minneapolis, MN), was used to estimate the time series of source activities82. A three-layer boundary element method (BEM) model, constructed from the MNI 152 standard template, was used to compute the lead field matrix. Cortical current density values at 7,850 cortical vertices were evaluated for every time point of each epoch. After estimating the cortical current density at every time point, 314 nodes were extracted as evenly as possible from the original cortical surface model69. The time series of the cortical sources at each of the 314 nodes were bandpass filtered and divided into four frequency bands: alpha (8–12 Hz), low beta (12–18 Hz), high beta (18–30 Hz), and gamma (30–55 Hz). Lower frequency bands such as delta (1–4 Hz) and theta (4–8 Hz) were not considered in the analysis because only one or two full cycles of these frequency components were included in the analysis time window (500 ms), and inclusion may have caused significant bias in the phase-locking analysis results. The time interval, which was based on previous studies and included the P300 component, was set from 200 to 700 ms after the NoGo target stimulus onset21,83. Visual inspection of the grand-averaged waveforms at the 4 electrodes of interest (Fz, FCz, Cz, and Pz), all of which were located on the midline84, was performed.
Connectivity and network analysis
The functional connectivity between each pair of nodes was evaluated using phase-locking values (PLVs)85. PLVs were used as the measure of synchronization because they range from 0 to 1, and can be directly used to represent the connection strength in the weighted network analysis without further modifications.
In this study, we performed weighted network analysis based on the graph theory35,36. The use of weighted networks is not only free from ambiguity in determining the threshold values, but can also preserve the unique traits of the original network without distortion. A network is composed of several nodes that are connected to each other at their edges. Four different global level weighted network indices were evaluated. First, ‘strength’ refers to the degree of connection strength in the network. It is estimated by summing up the weight of links connected to the brain regions. Second, ‘clustering coefficient’ indicates the degree of which a node is clustered with its neighboring nodes. Clustering coefficient was calculated for the whole network. Third, ‘path length’ indicates overall connectedness of the whole network and is calculated as the sum of lengths between two nodes in the entire network. Fourth, ‘efficiency’ refers to the efficiency of information processing in the brain. Additionally, the weighted nodal clustering coefficient was evaluated for each node (supplementary).
Statistical analysis
A one-way analysis of variance (ANOVA) was conducted to compare the scores of psychological and behavioral data among the three groups. In addition, a multivariate ANOVA (MANOVA) was conducted to compare the cortical network characteristics at the global level for each frequency band among the three groups with STAI, BDI, and CAARS as covariates. The multiple comparison problems were solved through the Bonferroni correction with an adjusted p-value of 0.05/16 = 0.003125. The identical analysis was conducted at the nodal level, followed by the Bonferroni correction with an adjusted p-value of 0.05/314 = 0.000159. The variables showing significant differences among three groups were further analyzed using post hoc pairwise comparisons, namely the Bonferroni correction. Effect sizes were expressed as partial eta squared (η2). In addition, the relationships between network indices and childhood trauma-related measures were analyzed by a partial Pearson’s correlation in which STAI, BDI, and CAARS were controlled for. The correlation analysis was followed by the Bonferroni correction with an adjusted p-value of 0.05/60 = 0.000833. Statistical analyses were performed using SPSS 21 (SPSS, Inc., Chicago, IL). The power of cortical network characteristic differences at the global level among the three groups was calculated using the G*Power 3.1.9 software86.
Electronic supplementary material
Acknowledgements
We wish to acknowledge Yeonsoo Park for kind proofreading and language editing of the manuscript. This research was supported by the Brain Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2015M3C7A1028252), and the Korea Science and Engineering Foundation (KOSEF), funded by the Korean government (NRF-2018R1A2A2A05018505).
Author Contributions
S.K. analyzed the data and wrote the paper. S.H.L. designed the study and wrote the paper. J.S.K. and M.S. collected the data. S.H.L. and C.H.I. reviewed and revised the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28329-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Terr LC. Childhood traumas: An outline and overview. Focus. 2003;1:322–334. doi: 10.1176/foc.1.3.322. [DOI] [PubMed] [Google Scholar]
- 2.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological psychiatry. 2001;49:1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky BS, et al. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158:1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- 5.Tucci AM, Kerr-Corrêa F, Souza-Formigoni MLO. Childhood trauma in substance use disorder and depression: an analysis by gender among a Brazilian clinical sample. Child Abuse & Neglect. 2010;34:95–104. doi: 10.1016/j.chiabu.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Anda RF, et al. The enduring effects of abuse and related adverse experiences in childhood. European archives of psychiatry and clinical neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart, H. & Rubia, K. Neuroimaging of child abuse: a critical review. Frontiers in human neuroscience6 (2012). [DOI] [PMC free article] [PubMed]
- 8.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental psychology. 1999;35:205. doi: 10.1037/0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Navalta CP, Polcari A, Webster DM, Boghossian A, Teicher MH. Effects of childhood sexual abuse on neuropsychological and cognitive function in college women. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:45–53. doi: 10.1176/jnp.18.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Mueller SC, et al. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mezzacappa E, Kindlon D, Earls F. Child abuse and performance task assessments of executive functions in boys. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:1041–1048. doi: 10.1111/1469-7610.00803. [DOI] [PubMed] [Google Scholar]
- 12.DePrince AP, Weinzierl KM, Combs MD. Executive function performance and trauma exposure in a community sample of children. Child abuse & neglect. 2009;33:353–361. doi: 10.1016/j.chiabu.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Pollak SD, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child development. 2010;81:224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyer AE, et al. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- 15.Dillon DG, et al. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta MA, et al. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of cognitive neuroscience. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 17.Perez CM, Widom CS. Childhood victimization and long-term intellectual and academic outcomes. Child abuse & neglect. 1994;18:617–633. doi: 10.1016/0145-2134(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 18.Ford JD, et al. Child maltreatment, other trauma exposure, and posttraumatic symptomatology among children with oppositional defiant and attention deficit hyperactivity disorders. Child maltreatment. 2000;5:205–217. doi: 10.1177/1077559500005003001. [DOI] [PubMed] [Google Scholar]
- 19.Stevens SE, et al. Inattention/overactivity following early severe institutional deprivation: presentation and associations in early adolescence. Journal of abnormal child psychology. 2008;36:385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- 20.Evren C, Umut G, Bozkurt M, Evren B, Agachanli R. Mediating role of childhood emotional abuse on the relationship between severity of ADHD and PTSD symptoms in a sample of male inpatients with alcohol use disorder. Psychiatry research. 2016;239:320–324. doi: 10.1016/j.psychres.2016.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology. 2001;112:2224–2232. doi: 10.1016/S1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- 22.Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug & Alcohol Dependence. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Benvenuti SM, Sarlo M, Buodo G, Mento G, Palomba D. Influence of impulsiveness on emotional modulation of response inhibition: An ERP study. Clinical Neurophysiology. 2015;126:1915–1925. doi: 10.1016/j.clinph.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Ramautar J, Kok A, Ridderinkhof K. Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain and cognition. 2004;56:234–252. doi: 10.1016/j.bandc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clinical neurophysiology. 2008;119:704–714. doi: 10.1016/j.clinph.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Bruin K, Wijers A, Van Staveren A. Response priming in a go/nogo task: do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clinical Neurophysiology. 2001;112:1660–1671. doi: 10.1016/S1388-2457(01)00601-0. [DOI] [PubMed] [Google Scholar]
- 28.Beste C, Willemssen R, Saft C, Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48:366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Schmiedt-Fehr C, Basar-Eroglu C. Event-related delta and theta brain oscillations reflect age-related changes in both a general and a specific neuronal inhibitory mechanism. Clinical Neurophysiology. 2011;122:1156–1167. doi: 10.1016/j.clinph.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biological psychiatry. 2000;48:778–790. doi: 10.1016/S0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 31.Bremner JD. Traumatic stress: effects on the brain. Dialogues in clinical neuroscience. 2006;8:445. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco L, et al. Neurological changes in brain structure and functions among individuals with a history of childhood sexual abuse: A review. Neuroscience & Biobehavioral Reviews. 2015;57:63–69. doi: 10.1016/j.neubiorev.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 34.Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear biomedical physics. 2007;1:3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature reviews. Neuroscience. 2009;10:186. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 36.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, et al. Decreased P300 current source density in drug-naive first episode schizophrenics revealed by high density recording. International Journal of Psychophysiology. 2010;75:249–257. doi: 10.1016/j.ijpsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Cisler J, et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological medicine. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- 39.Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biological psychiatry. 2014;76:297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puetz V, et al. Altered brain network integrity after childhood maltreatment: A structural connectomic DTI‐study. Human brain mapping. 2017;38:855–868. doi: 10.1002/hbm.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Broek SP, Reinders F, Donderwinkel M, Peters M. Volume conduction effects in EEG and MEG. Electroencephalography and clinical neurophysiology. 1998;106:522–534. doi: 10.1016/S0013-4694(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 42.Nolte G, et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clinical neurophysiology. 2004;115:2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Lange DH, Inbar GF. A robust parametric estimator for single-trial movement related brain potentials. IEEE transactions on biomedical engineering. 1996;43:341–347. doi: 10.1109/10.486254. [DOI] [PubMed] [Google Scholar]
- 44.Lemm S, Curio G, Hlushchuk Y, Muller K-R. Enhancing the signal-to-noise ratio of ICA-based extracted ERPs. IEEE Transactions on Biomedical Engineering. 2006;53:601–607. doi: 10.1109/TBME.2006.870258. [DOI] [PubMed] [Google Scholar]
- 45.Kendler KS, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Archives of general psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 46.Hovens JG, et al. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatrica Scandinavica. 2010;122:66–74. doi: 10.1111/j.1600-0447.2009.01491.x. [DOI] [PubMed] [Google Scholar]
- 47.Narvaez JC, et al. Childhood trauma, impulsivity, and executive functioning in crack cocaine users. Comprehensive Psychiatry. 2012;53:238–244. doi: 10.1016/j.comppsych.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 48.Beers SR, De Bellis MD. Outcomes of child abuse. Neurosurgery Clinics of North America. 2002;13:235–241. doi: 10.1016/S1042-3680(01)00003-1. [DOI] [PubMed] [Google Scholar]
- 49.Pearson R, McGeary JE, Beevers CG. Association between serotonin cumulative genetic score and the behavioral approach system (BAS): moderation by early life environment. Personality and individual differences. 2014;70:140–144. doi: 10.1016/j.paid.2014.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howells FM, Stein DJ, Russell VA. Childhood trauma is associated with altered cortical arousal: insights from an EEG study. Frontiers in integrative neuroscience. 2012;6:120. doi: 10.3389/fnint.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elton A, et al. Childhood maltreatment is associated with a sex‐dependent functional reorganization of a brain inhibitory control network. Human brain mapping. 2014;35:1654–1667. doi: 10.1002/hbm.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knott VJ, Bakish D, Lusk S, Barkely J, Perugini M. Quantitative EEG correlates of panic disorder. Psychiatry Research: Neuroimaging. 1996;68:31–39. doi: 10.1016/S0925-4927(96)02962-9. [DOI] [PubMed] [Google Scholar]
- 54.Sachs G, Anderer P, Dantendorfer K, Saletu B. EEG mapping in patients with social phobia. Psychiatry Research: Neuroimaging. 2004;131:237–247. doi: 10.1016/j.pscychresns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Oathes DJ, et al. Worry, generalized anxiety disorder, and emotion: Evidence from the EEG gamma band. Biological psychology. 2008;79:165–170. doi: 10.1016/j.biopsycho.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bader K, Schäfer V, Nissen L, Schenkel M. Heightened beta EEG activity during nonrapid eye movement sleep in primary insomnia patients with reports of childhood maltreatment. Journal of Clinical Neurophysiology. 2013;30:188–198. doi: 10.1097/WNP.0b013e3182767c4a. [DOI] [PubMed] [Google Scholar]
- 57.Cook F, Ciorciari J, Varker T, Devilly GJ. Changes in long term neural connectivity following psychological trauma. Clinical Neurophysiology. 2009;120:309–314. doi: 10.1016/j.clinph.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Lee S-H, Park Y, Jin MJ, Lee YJ, Hahn SW. Childhood trauma associated with enhanced high frequency band powers and induced subjective inattention of adults. Frontiers in behavioral neuroscience. 2017;11:148. doi: 10.3389/fnbeh.2017.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang, K. I. et al. Increased beta power in the bereaved families of the Sewol ferry disaster, a paradoxical compensatory phenomenon?: A two‐channel electroencephalography study. Psychiatry and Clinical Neurosciences (2017). [DOI] [PubMed]
- 60.Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. American Journal of Psychiatry. 2013;170:616–623. doi: 10.1176/appi.ajp.2013.12070950. [DOI] [PubMed] [Google Scholar]
- 61.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. American Journal of Psychiatry. 2014;171:854–863. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 62.De Bellis MD, et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biological psychiatry. 2002;51:544–552. doi: 10.1016/S0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- 63.Fisher, P. A., Bruce, J., Abdullaev, Y., Mannering, A. M. & Pears, K. C. In Inhibitory Control and Drug Abuse Prevention 229–247 (Springer, 2011).
- 64.Østby Y, et al. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’networks. nature. 1998;393:440. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 67.Dai Z, He Y. Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer’s disease. Neurosci Bull. 2014;30:217–232. doi: 10.1007/s12264-013-1421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Haan W, et al. Functional neural network analysis in frontotemporal dementia and Alzheimer’s disease using EEG and graph theory. BMC neuroscience. 2009;10:101. doi: 10.1186/1471-2202-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shim M, Kim D-W, Lee S-H, Im C-H. Disruptions in small-world cortical functional connectivity network during an auditory oddball paradigm task in patients with schizophrenia. Schizophrenia research. 2014;156:197–203. doi: 10.1016/j.schres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Grassi-Oliveira R, et al. Childhood Trauma Questionnaire (CTQ) in Brazilian samples of different age groups: findings from confirmatory factor analysis. Plos One. 2014;9:e87118. doi: 10.1371/journal.pone.0087118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lovie‐Kitchin JE. Validity and reliability of viscual acuity measurements. Ophthalmic and physiological optics. 1988;8:363–370. doi: 10.1111/j.1475-1313.1988.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 72.Bernstein DP, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child abuse & neglect. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 73.Kim J, Shin D. A study based on the standardization of the STAI for Korea. New Med J. 1978;21:69–75. [Google Scholar]
- 74.Spielberger, C. D. Manual for the State-Trait Anxiety Inventory STAI (form Y) (“self-evaluation questionnaire”) (1983).
- 75.Lee M, et al. A standardization study of beck depression inventory (I): Korean version (K-BDI): reliability land factor analysis. Korean J Psychopathol. 1995;4:77–95. [Google Scholar]
- 76.Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 77.Lee S-R, et al. The study on Reliability and Validity of Korean Version of the Barratt Impulsiveness Scale-11-Revised in nonclinical adult subjects. Journal of Korean Neuropsychiatric Association. 2012;51:378–386. doi: 10.4306/jknpa.2012.51.6.378. [DOI] [Google Scholar]
- 78.Park J-S, Lee WH, Lee S-R, Kim S-M, Bahn GH. Reliability and validity of Korean version of the conners adult attention deficit hyperactivity disorder scale in general population. Journal of Korean Neuropsychiatric Association. 2013;52:342–352. doi: 10.4306/jknpa.2013.52.5.342. [DOI] [Google Scholar]
- 79.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of personality and social psychology. 1994;67:319. doi: 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- 80.Kim K, Kim WS. Korean-BAS/bis scale. Korean J Health Psychol. 2001;6:19–37. [Google Scholar]
- 81.Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 82.He B, et al. eConnectome: A MATLAB toolbox for mapping and imaging of brain functional connectivity. Journal of neuroscience methods. 2011;195:261–269. doi: 10.1016/j.jneumeth.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sehlmeyer C, et al. ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia. 2010;48:2488–2495. doi: 10.1016/j.neuropsychologia.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Omura K, Kusumoto K. Sex differences in neurophysiological responses are modulated by attentional aspects of impulse control. Brain and cognition. 2015;100:49–59. doi: 10.1016/j.bandc.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Lachaux J-P, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human brain mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior research methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.