Abstract
Our study aimed to investigate the association between metabolic syndrome and postoperative survival in patients with esophageal squamous cell carcinoma, and evaluate whether metabolic syndrome can predict the prognosis in esophageal cancer patients. The retrospective study reviewed 519 patients with esophageal squamous cell carcinoma who had received esophagetomy and lymphnode dissections in the Department of Thoracic Surgery, Qilu Hospital of Shandong University between January 2007 and December 2011. All patients were followed up until December 2016. The median follow-up time was 39.59 months (range 0.25–72 months). The 3-year and 5-year survival rate was 51.4% and 37.0%, respectively. Kaplan–Meier survival analysis revealed a significant correlation between OS and obesity (P = 0.000), weight loss (P = 0.000), diabetes (P = 0.001) and dyslipidemia (P = 0.030). Multivariate analysis indicated that advanced TNM staging (P = 0.007, HR: 1.760, 95% CI: 1.167–2.654) and more weight loss (P = 0.000, HR: 1.961, 95% CI: 1.697–2.267) were independent factors for adverse prognosis of esophageal squamous carcinoma patients. In contrast, diabetes was a protective factor in the prognosis of patients with esophageal cancer (P = 0.018, HR: 0.668, 95% CI: 0.478–0.933). Our findings suggest that TNM staging, weight changes and diabetes were independent predictors for the prognosis of esophageal cancer patients.
Introduction
Esophageal cancer (EC) is one of the most common malignant gastrointestinal tumors in the world. With increasing incidence and mortality, esophageal cancer is the leading cause of death in China. The latest malignancy epidemiology data showed that the number of new cases was 477,900 people and the number of deaths was 375,000 people in China in 20151. Esophageal cancer has two main subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC)2. ESCC accounts for about 90% of cases of esophageal cancer worldwide3, which is predominant in Asia, Africa, and South America4. At present, the main treatment methods for esophageal squamous cell carcinoma include: surgical treatment, radiotherapy, chemotherapy, immunotherapy, comprehensive treatment and other treatment methods. In the early stage of esophageal cancer, there are no obvious clinical symptoms. Most of the patients are diagnosed in the middle or advanced stage. ESCC has high malignancy, rapid development process, poor therapeutic effect, and high rate of recurrence and metastasis. Although early detections and surgical techniques have improved, the five-year survival rate of esophageal cancer remains generally poor, according to the regional metastasis or distant metastasis5. Therefore, it is necessary to determine the factors that affect the postoperative prognosis and the recurrence or metastasis.
The metabolic syndrome (MS), including hyperglycaemia, dyslipidemia, obesityand hypertension, has become one of the major worldwide public-health challenges6. MS had been highlighted as a risk factor for ischemic heart disease and arteriosclerotic disease, it was also recently shown to be associated with several tumors7–9. Some studies have indicated that certain factors in MS were associated with risk of cancer, including liver, colorectal, bladder, pancreatic, breast and esophageal cancer10–12. Obesity was associated with an increased risk of EAC12 and a decreased risk of ESCC13. A prospective study included 1082 esophageal cancer showed that there was a strong inverse association between BMI and death from esophageal cancer13. Several studies have shown that preoperative weight loss in esophageal cancer resection might have a certain relationship with postoperative survival14,15.The evidence for the positive correlation between lipid metabolism and risk of esophageal cancer was presented16. There is now accumulating evidence that the MS may be not only risk factor for tumors but also cancer mortality marker10. However, the relationship between MS factor and prognosis of esophageal cancer is controversial. Some studies suggested that MS was associated with better prognosis of esophageal cancer17, while others failed to establish a connection18. Accordingly, the effects of MS and its related factors on overall survival (OS) and progression-free survival (PFS) of esophageal cancer need to be further discussed.
Therefore, we conducted this retrospective study to investigate the relationship between MS, weight loss and postoperative survival in patients with ESCC, and evaluate whether MS or weight loss can predict the prognosis in esophageal cancer patients.
Results
Patient characteristics
According to the classification of pathological types, there were 549 patients, including 519 patients with esophageal squamous cell carcinoma, 15 patients with esophageal adenocarcinoma, 10 patients with basal cell type squamous cell carcinoma and 5 patients with neuroendocrine carcinoma. Taking into account the number of samples, this experiment included only 519 patients with esophageal squamous cell carcinoma.
The clinicopathological characteristics of the patients are presented in Table 1. The median age at diagnosis was 62.08 years (ranged from 32 to 86 years), including 425 males (81.9%) and 94 females (18.1%). The median follow-up time was 39.59 months (range 0.25–72 months). The 3-year and 5-year survival rate was 51.4% and 37.0%, respectively. According to TNM stage, the number of patients in phase I, II and III was 73 (14.1%), 256 (49.3%), 190 (36.6%), separately. All patients underwent surgical treatment without receiving neoadjuvant chemotherapy or radiotherapy. About 59.3% of the patients received surgery alone, while the others received adjuvant postoperative therapy including radiotherapy (12.9%), chemotherapy (12.5%) and chemoradiotherapy (15.2%).
Table 1.
Clinical and pathological characteristics of patients.
| Characteristics | Classification | Patients (n) | Patients (percent) |
|---|---|---|---|
| Age | <60 years | 201 | 38.7% |
| ≥60 years | 318 | 61.3% | |
| Gender | Male | 425 | 81.9% |
| Female | 94 | 18.1% | |
| Smoking | Ever | 253 | 48.7% |
| Never | 266 | 51.3% | |
| Drinking | Ever | 218 | 42.0% |
| Never | 301 | 58.0% | |
| Family history | Yes | 33 | 6.4% |
| No | 486 | 93.6% | |
| Location | Cervical | 39 | 7.5% |
| Upper | 34 | 6.6% | |
| Middle | 269 | 51.8% | |
| Lower | 177 | 34.1% | |
| Length | <4 cm | 270 | 52.0% |
| ≥4 cm | 249 | 48.0% | |
| Differentiation | GX | 16 | 3.1% |
| G1 | 100 | 19.3% | |
| G2 | 204 | 39.3% | |
| G3 | 199 | 38.3% | |
| T stage | Tis | 16 | 3.1% |
| T1 | 57 | 11.0% | |
| T2 | 152 | 29.3% | |
| T3 | 285 | 54.9% | |
| T4 | 9 | 1.7% | |
| LNM | Yes | 225 | 43.4% |
| No | 294 | 56.6% | |
| N stage | N0 | 294 | 56.6% |
| N1 | 130 | 25.0% | |
| N2 | 67 | 12.9% | |
| N3 | 28 | 5.4% | |
| TNM | I | 73 | 14.1% |
| II | 256 | 49.3% | |
| III | 190 | 36.6% | |
| Treatment | S | 308 | 59.3% |
| S + RT | 67 | 12.9% | |
| S + CT | 65 | 12.5% | |
| S + RCT | 79 | 15.2% | |
| BMI | <18.5 | 86 | 16.6% |
| 18.5~25 | 347 | 66.9% | |
| >25 | 86 | 16.6% | |
| Weight loss | No | 96 | 18.5% |
| A little | 89 | 17.1% | |
| Middle | 239 | 46.1% | |
| Much | 95 | 18.3% | |
| Hypertension | No | 410 | 79.0% |
| Yes | 109 | 21.0% | |
| Diabetes | No | 430 | 82.9% |
| Yes | 89 | 17.1% | |
| Dyslipidemia | No | 434 | 83.6% |
| Yes | 85 | 16.4% | |
| HDL | <0.9 | 49 | 9.4% |
| ≥0.9 | 470 | 90.6% | |
| TG | <1.7 | 475 | 91.5% |
| ≥1.7 | 44 | 8.5% | |
| MS | No | 466 | 89.8% |
| Yes | 53 | 10.2% |
Abbreviations: LNM, lymph node metastasis; S, surgery; RT, radiotherapy; CT, chemotherapy; CRT, chemoradiotherapy; BMI, Body Mass Index; HDL, high density lipoprotein; TG, triglyceride; MS, metabolic syndrome.
There were 53 patients with metabolic syndrome and 466 patients with normal metabolic levels. The distribution of BMI was as follows: lower in 86 (16.6%) patients, normal in 347 (66.9%) patients, higher in 86 (16.6%) patients. 35.6% of patients had no significant changes in body weight during treatment, 46.1% of patients had middle weight loss, while 18.3% of patients lost weight significantly. In addition, the proportion of patients with hypertension, diabetes and dyslipidemia was 21.0%, 17.1% and 16.4%.
Associations of BMI, weight loss, hypertension, diabetes, dyslipidemia and metabolic syndrome with clinicopathological characteristics
The relationship between clinicopathological features and metabolic syndrome was statistically analyzed and the results were shown in Table 2. The decrease of BMI level had a certain correlation with the progress of tumor differentiation grade (P = 0.010), N stage (P = 0.039), lymph node metastasis (P = 0.010) and TNM stage (P = 0.010). In addition, weight loss was positive associated with increased T stage (P = 0.047), N stage (P = 0.000), lymph node metastasis (P = 0.000) and TNM stage (P = 0.000). In addition, hypertension, diabetes, dyslipidemia and MS had no significant relationship with clinicopathological characteristics. A correlation analysis between patients with esophageal cancer who are accompanied with obesity, diabetes, dyslipidemia, and hypertension and weight loss can be found as Supplementary Table S1.
Table 2.
Clinicopathological characteristics of 519 esophageal squamous cell carcinoma patients grouped by BMI, weight loss, hypertension, diabetes, dyslipidemia and metabolic syndrome.
| Characteristics | BMI | Weight loss | Hypertension | Diabetes | Dyslipidemia | MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5~25 | >25 | No | A little | Middle | Much | No | Yes | No | Yes | No | Yes | No | Yes | |
| 86 | 347 | 86 | 96 | 89 | 239 | 95 | 410 | 109 | 430 | 89 | 434 | 85 | 466 | 53 | |
| Differentiation | |||||||||||||||
| GX | 4 | 6 | 6 | 5 | 2 | 8 | 1 | 13 | 3 | 13 | 3 | 12 | 4 | 14 | 2 |
| G1 | 12 | 63 | 25 | 19 | 25 | 43 | 13 | 75 | 25 | 81 | 19 | 85 | 15 | 86 | 14 |
| G2 | 40 | 136 | 28 | 39 | 35 | 90 | 40 | 157 | 47 | 162 | 42 | 168 | 36 | 182 | 22 |
| G3 | 30 | 142 | 27 | 33 | 27 | 98 | 41 | 165 | 34 | 174 | 25 | 169 | 30 | 184 | 15 |
| P value | 0.010 | 0.238 | 0.339 | 0.178 | 0.684 | 0.350 | |||||||||
| T stage | |||||||||||||||
| Tis | 1 | 8 | 7 | 6 | 4 | 5 | 1 | 13 | 3 | 12 | 4 | 12 | 4 | 12 | 4 |
| T1 | 5 | 40 | 12 | 16 | 15 | 19 | 7 | 45 | 12 | 50 | 7 | 45 | 12 | 51 | 6 |
| T2 | 22 | 106 | 24 | 30 | 27 | 67 | 28 | 122 | 30 | 119 | 33 | 131 | 21 | 135 | 17 |
| T3 | 56 | 187 | 42 | 44 | 42 | 142 | 57 | 223 | 62 | 243 | 42 | 238 | 47 | 260 | 25 |
| T4 | 2 | 6 | 1 | 0 | 1 | 6 | 2 | 7 | 2 | 6 | 3 | 8 | 1 | 8 | 1 |
| P value | 0.060 | 0.047 | 0.990 | 0.153 | 0.611 | 0.327 | |||||||||
| LNM | |||||||||||||||
| Yes | 49 | 146 | 30 | 25 | 29 | 122 | 49 | 178 | 47 | 192 | 33 | 190 | 35 | 204 | 21 |
| No | 37 | 201 | 56 | 71 | 60 | 117 | 46 | 232 | 62 | 238 | 56 | 244 | 50 | 262 | 32 |
| P value | 0.010 | 0.000 | 0.956 | 0.189 | 0.658 | 0.563 | |||||||||
| N stage | |||||||||||||||
| N0 | 37 | 201 | 56 | 71 | 60 | 117 | 46 | 232 | 62 | 238 | 56 | 244 | 50 | 262 | 32 |
| N1 | 24 | 87 | 19 | 15 | 19 | 74 | 22 | 98 | 32 | 108 | 22 | 106 | 24 | 115 | 15 |
| N2 | 17 | 44 | 6 | 8 | 5 | 38 | 16 | 58 | 9 | 60 | 7 | 58 | 9 | 64 | 3 |
| N3 | 8 | 15 | 5 | 2 | 5 | 10 | 11 | 22 | 6 | 24 | 4 | 26 | 2 | 25 | 3 |
| P value | 0.039 | 0.000 | 0.342 | 0.387 | 0.446 | 0.423 | |||||||||
| TNM | |||||||||||||||
| I | 6 | 48 | 19 | 21 | 20 | 24 | 8 | 55 | 18 | 65 | 8 | 58 | 15 | 64 | 9 |
| II | 39 | 172 | 45 | 57 | 47 | 108 | 44 | 201 | 55 | 202 | 54 | 215 | 41 | 227 | 29 |
| III | 41 | 127 | 22 | 18 | 22 | 107 | 43 | 154 | 36 | 163 | 27 | 161 | 29 | 175 | 15 |
| P value | 0.010 | 0.000 | 0.575 | 0.052 | 0.570 | 0.403 | |||||||||
The relationship between BMI, weight loss, diabetes, dyslipidemia and OS, PFS of ESCC
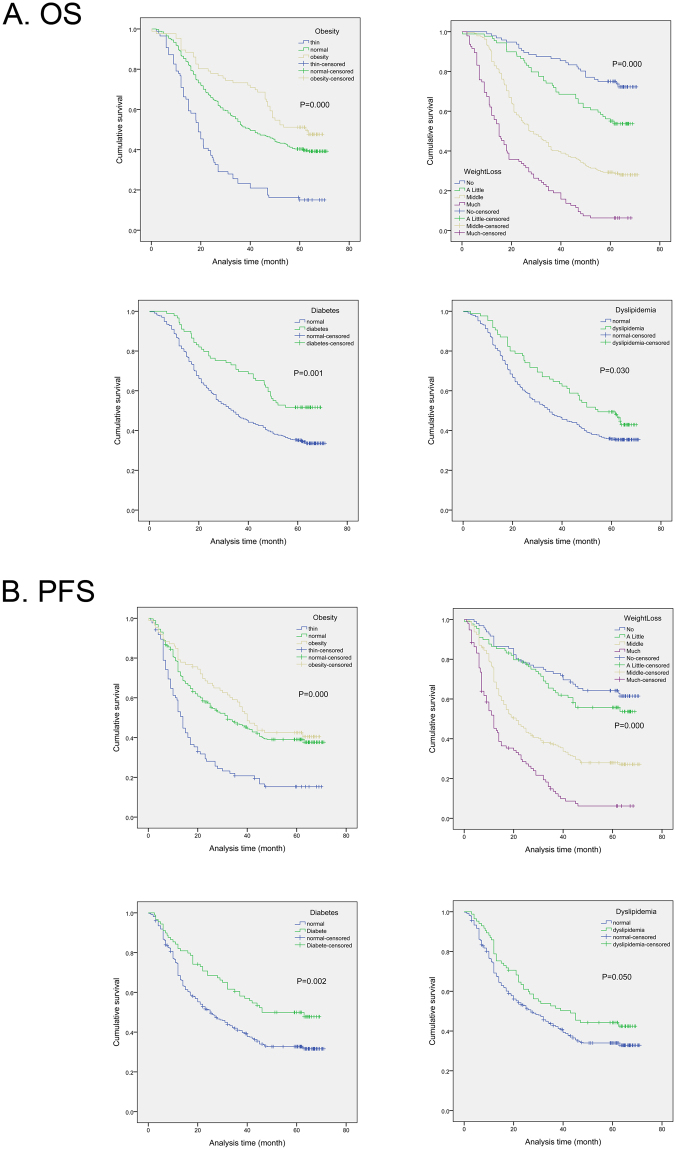
In our research, Kaplan–Meier survival analysis revealed a correlation between BMI, weight loss, diabetes, dyslipidemia and OS, PFS of esophageal cancer. As shown in Fig. 1, Kaplan-Meier curves showed that ESCC patients with obesity had significantly longer OS (P < 0.001) and PFS (P < 0.001). Furthermore, patients with middle or more weight loss, regardless of OS or PFS, were significantly shorter than patients with little body weight changes during treatment (OS, P < 0.001; PFS, P < 0.001). Compared with people in normal blood glucose level, esophageal cancer patients with diabetes showed a better prognosis in OS (P = 0.001), and PFS (P = 0.002). In the same way, dyslipidemia patients had longer survival in OS (P = 0.030) than those in normal blood lipids, although there was no statistical difference in PFS (P = 0.050). However, hypertension had no relationship with the prognosis of patients with esophageal squamous cell carcinoma.
Figure 1.
Kaplan-Meier analysis for overall survival (OS) and progression-free survival (PFS) of esophageal squamous cell carcinoma patients according to metabolic syndrome components, including obesity, weight loss, diabetes and dyslipidemia.
1-year and 3-year survival of patients with BMI, weight loss, diabetes, dyslipidemia
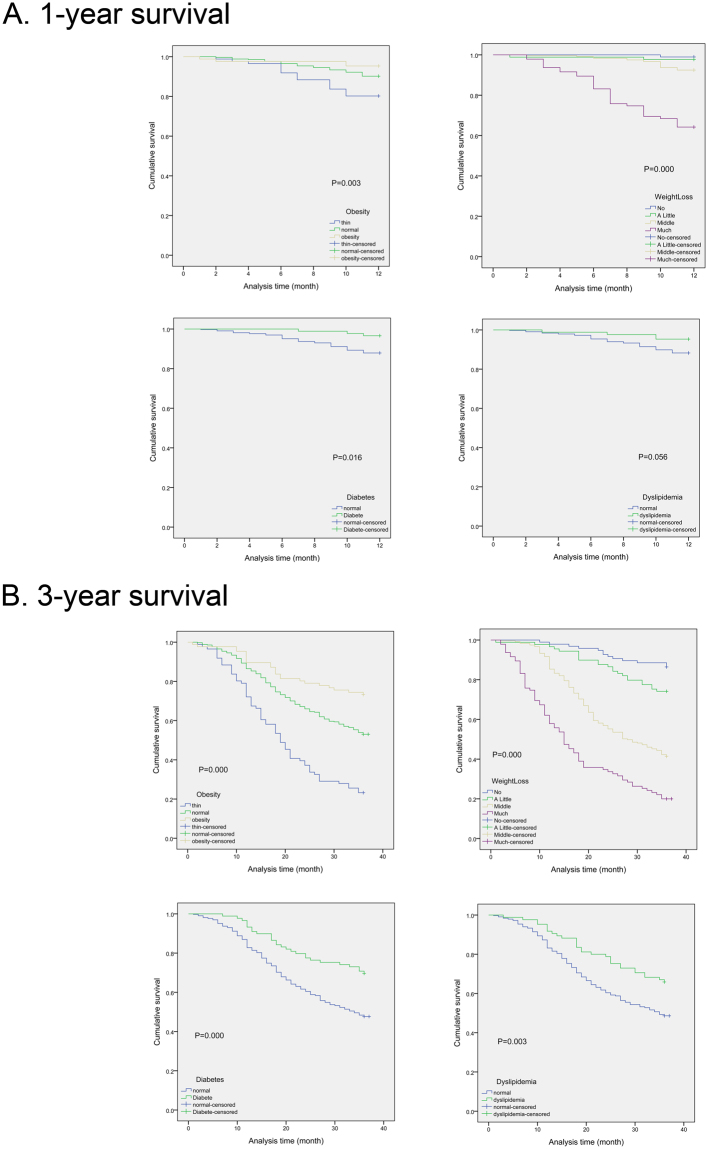
In the research, we detected the effects of BMI, weight loss, diabetes, dyslipidemia on 1-year and 3-year survival rate. It was showed in Fig. 2 that patients with obesity (1-year, P = 0.003; 3-year, P = 0.000), weight loss (1-year, P = 0.000; 3-year, P = 0.000), diabetes (1-year, P = 0.016; 3-year, P = 0.000) and dyslipidemia (1-year, P = 0.056; 3-year, P = 0.003) had better prognosis compared with normal metabolism patients, whether in 1-year OS or in 3-year OS.
Figure 2.
The association between obesity, weight loss, diabetes, dyslipidemia and 1-year, 3-years survival in esophageal cancer patients.
Univariate and multivariate analyses
The univariate analysis of suvival was exhibited in Table 3. There was a significant correlation between OS and gender (P = 0.019), tumor length (P = 0.008), tumor differentiation (P = 0.002), T stage (P = 0.000), LNM (P = 0.000), N stage (P = 0.000), TNM stage (N = 0.000), obesity (P = 0.000), weight loss (P = 0.000), diabetes (P = 0.001) and dyslipidemia (P = 0.032). The tumor location, treatment, hypertension, HDL, TG, MS and other factors did not have statistical significance with OS. In addition, univaviate analysis revealed a relationship between tumor length (P = 0.003), tumor differentiation (P = 0.002), T stage (P = 0.000), LNM (P = 0.000), N stage (P = 0.000), TNM stage (N = 0.000), treatment (P = 0.009), obesity (P = 0.000), weight loss (P = 0.000), diabetes (P = 0.002) and PFS time.
Table 3.
Univariate analysis of prognostic factors in esophageal squamous cell carcinoma with respect to overall survival and progression-free survival.
| Characteristics | Classification | OS | PFS | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age | <60 years | 1.000 | Ref. | ||||
| ≥60 years | 1.141 | 0.911–1.430 | 0.250 | 1.194 | 0.953–1.495 | 0.123 | |
| Gender | Male | 1.000 | Ref. | ||||
| Female | 0.695 | 0.513–0.941 | 0.019 | 0.769 | 0.574–1.029 | 0.078 | |
| Smoking | Ever | 1.000 | Ref. | ||||
| Never | 0.869 | 0.699–1.079 | 0.203 | 0.939 | 0.757–1.165 | 0.566 | |
| Drinking | Ever | 1.000 | Ref. | ||||
| Never | 0.908 | 0.730–1.130 | 0.386 | 1.055 | 0.847–1.314 | 0.632 | |
| Family history | No | 1.000 | Ref. | ||||
| Yes | 1.081 | 0.701–1.667 | 0.723 | 1.056 | 0.685–1.627 | 0.805 | |
| Location | Cervical | 1.000 | Ref. | ||||
| Upper | 0.699 | 0.395–1.236 | 0.218 | 0.735 | 0.410–1.317 | 0.301 | |
| Middle | 0.756 | 0.503–1.137 | 0.179 | 0.892 | 0.591–1.347 | 0.587 | |
| Lower | 0.812 | 0.534–1.234 | 0.328 | 0.831 | 0.542–1.275 | 0.397 | |
| Length | <4 cm | 1.000 | Ref. | ||||
| ≥4 cm | 1.341 | 1.079–1.666 | 0.008 | 1.388 | 1.118–1.722 | 0.003 | |
| Differentiation | GX | 1.000 | Ref. | ||||
| G1 | 3.309 | 1.033–10.605 | 0.044 | 2.422 | 0.874–6.711 | 0.089 | |
| G2 | 4.368 | 1.390–13.728 | 0.012 | 3.361 | 1.242–9.094 | 0.017 | |
| G3 | 6.268 | 1.997–19.674 | 0.002 | 4.788 | 1.772–12.934 | 0.002 | |
| T stage | Tis | 1.000 | Ref. | ||||
| T1 | 1.602 | 0.467–5.498 | 0.454 | 2.326 | 0.696–7.773 | 0.170 | |
| T2 | 4.298 | 1.360–13.581 | 0.013 | 4.935 | 1.564–15.573 | 0.006 | |
| T3 | 6.530 | 2.088–20.423 | 0.001 | 6.283 | 2.008–63.112 | 0.002 | |
| T4 | 14.681 | 3.888–55.439 | 0.000 | 17.038 | 4.600–63.112 | 0.000 | |
| LNM | No | 1.000 | Ref. | ||||
| Yes | 2.901 | 2.322–3.626 | 0.000 | 2.584 | 2.074–3.220 | 0.000 | |
| N stage | N0 | 1.000 | Ref. | ||||
| N1 | 2.548 | 1.973–3.291 | 0.000 | 2.220 | 1.717–2.869 | 0.000 | |
| N2 | 3.188 | 2.338–4.348 | 0.000 | 2.880 | 2.115–3.922 | 0.000 | |
| N3 | 4.621 | 3.045–7.012 | 0.000 | 4.311 | 2.863–6.490 | 0.000 | |
| TNM | I | 1.000 | Ref. | ||||
| II | 2.992 | 1.807–4.955 | 0.000 | 2.437 | 1.556–3.816 | 0.000 | |
| III | 8.180 | 4.952–13.509 | 0.000 | 5.681 | 3.626–8.900 | 0.000 | |
| Treatment | S | 1.000 | Ref. | ||||
| S + RT | 1.063 | 0.688–1.643 | 0.783 | 1.248 | 0.788–1.976 | 0.345 | |
| S + CT | 0.858 | 0.396–1.856 | 0.697 | 1.144 | 0.525–2.493 | 0.735 | |
| S + RCT | 1.288 | 0.797–2.082 | 0.302 | 1.607 | 1.010–2.604 | 0.009 | |
| BMI | <18.5 | 1.000 | Ref. | ||||
| 18.5~25 | 0.455 | 0.348–0.595 | 0.000 | 0.489 | 0.373–0.641 | 0.000 | |
| >25 | 0.328 | 0.225–0.478 | 0.000 | 0.421 | 0.292–0.606 | 0.000 | |
| Weight loss | No | 1.000 | Ref. | ||||
| A little | 1.905 | 1.165–3.114 | 0.010 | 1.271 | 0.808–2.000 | 0.299 | |
| Middle | 4.234 | 2.798–6.406 | 0.000 | 2.857 | 1.991–4.099 | 0.000 | |
| Much | 9.749 | 6.263–15.174 | 0.000 | 5.693 | 3.833–8.455 | 0.000 | |
| Hypertension | No | 1.000 | Ref. | ||||
| Yes | 0.845 | 0.643–1.111 | 0.227 | 0.828 | 0.630–1.087 | 0.174 | |
| Diabetes | No | 1.000 | Ref. | ||||
| Yes | 0.579 | 0.420–0.799 | 0.001 | 0.611 | 0.446–0.837 | 0.002 | |
| Dyslipidemia | No | 1.000 | Ref. | ||||
| Yes | 0.713 | 0.523–0.971 | 0.032 | 0.740 | 0.545–1.005 | 0.054 | |
| HDL | <0.9 | 1.000 | Ref. | ||||
| ≥0.9 | 1.384 | 0.934–2.053 | 0.106 | 1.081 | 0.747–1.564 | 0.681 | |
| TG | <1.7 | 1.000 | Ref. | ||||
| ≥1.7 | 0.756 | 0.499–1.145 | 0.187 | 0.700 | 0.458–1.069 | 0.099 | |
| MS | No | 1.000 | Ref. | ||||
| Yes | 0.687 | 0.463–1.018 | 0.062 | 0.738 | 0.501–1.088 | 0.125 | |
The multivariate analysis was shown in Table 4. The results indicated that advanced TNM staging (P = 0.007, HR: 1.760, 95% CI: 1.167–2.654) and more weight loss (P = 0.000, HR: 1.961, 95% CI: 1.697–2.267) were independent factors for adverse prognosis of esophageal squamous carcinoma patients. In contrast, diabetes was a protective factor in the prognosis of patients with esophageal cancer (P = 0.018, HR: 0.668, 95% CI: 0.478–0.933). It was also shown that TNM stage (P = 0.008, HR: 1.643, 95% CI: 1.140–2.369), weight loss (P = 0.000, HR: 1.674, 95% CI: 1.462–1.917) and dibetes (P = 0.027, HR: 0.691, 95% CI: 0.499–0.958) were independently associated with PFS.
Table 4.
Multivariate analysis of prognostic factors in esophageal squamous cell carcinoma with respect to overall survival and progression-free survival.
| Characteristics | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | 0.760 | 0.554–1.040 | 0.087 | |||
| Length | 0.955 | 0.764–1.194 | 0.685 | 0.875 | 0.701–1.093 | 0.240 |
| Differentiation | 1.137 | 0.973–1.328 | 0.105 | 1.141 | 0.980–1.329 | 0.090 |
| T stage | 1.211 | 0.954–1.536 | 0.116 | 1.119 | 0.901–1.390 | 0.311 |
| LNM | 1.095 | 0.676–1.773 | 0.713 | 0.998 | 0.638–1.563 | 0.994 |
| N stage | 1.093 | 0.884–1.352 | 0.410 | 1.114 | 0.901–1.377 | 0.318 |
| TNM | 1.760 | 1.167–2.654 | 0.007 | 1.643 | 1.140–2.369 | 0.008 |
| Treatment | 1.097 | 0.997–1.206 | 0.058 | |||
| Obesity | 0.823 | 0.667–1.014 | 0.067 | 0.891 | 0.724–1.097 | 0.277 |
| Weight loss | 1.961 | 1.697–2.267 | 0.000 | 1.674 | 1.462–1.917 | 0.000 |
| Diabetes | 0.668 | 0.478–0.933 | 0.018 | 0.691 | 0.499–0.958 | 0.027 |
| Dyslipidemia | 0.969 | 0.706–1.331 | 0.847 | |||
Subgroup analysis
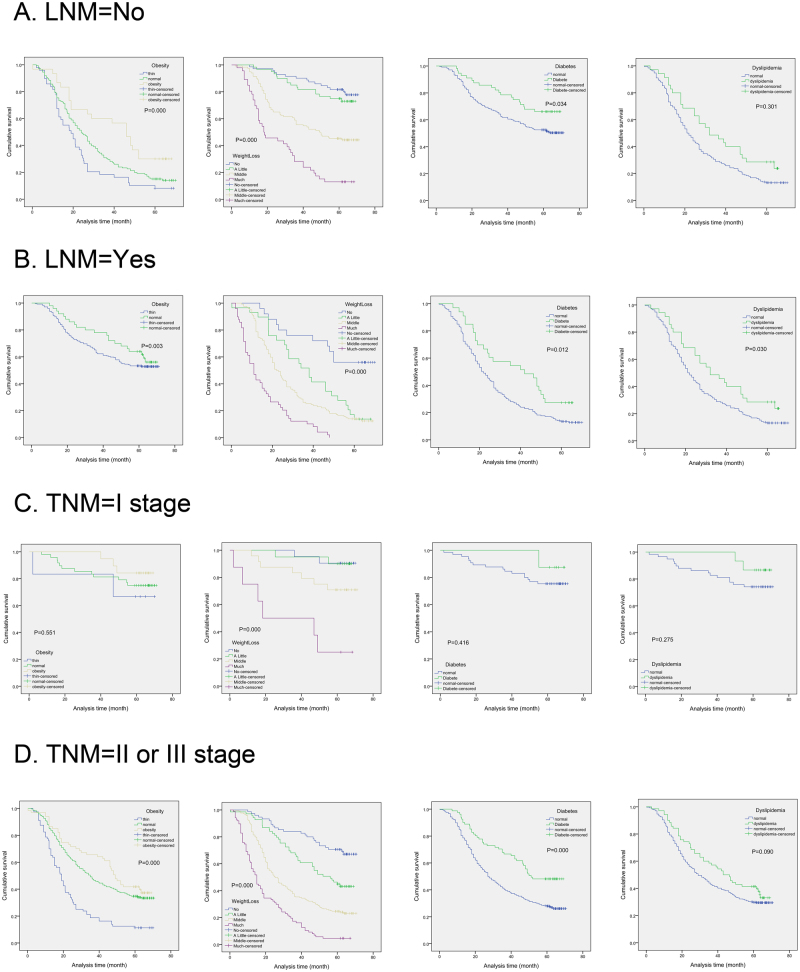
To detect the effects of BMI, weight loss, diabetes and dyslipidemia on survival in different level of lymph node metastases, we divided the patients into two subgroups (N0 vs N1–3). In N0 subgroup, obesity (P = 0.000), little weight loss (P = 0.000) and diabetes (P = 0.034) showed association with longer survival time, while dyslipidemia (P = 0.301) had no statistical significance (Fig. 3A and B). At the same time, it was indicated that BMI (P = 0.003), weight loss (P = 0.000), diabetes (P = 0.012) and dyslipidemia (P = 0.030) were all associated with survival in N1–3 subgroup.
Figure 3.
The effects of MS on survival in different subgroups. The effects of obesity, weight loss, diabetes and dyslipidemia on survival in different level of lymph node metastases (N0 vs N1–3) (A,B).The effects of obesity, weight loss, diabetes and dyslipidemia on survival in different TNM stages (TNM I stage vs TNM II-III stage) (C,D).
As shown in Fig. 3C and D, we divided the patients into two subgroups (TNM I stage vs TNM II-III stage). It was revealed that BMI (P = 0.000), weight loss (P = 0.000) and diabetes (P = 0.000) showed association with longer OS in TNM II-III subgroup, while weight loss (P = 0.000) was related to survival time in subgroup TNM I stage.
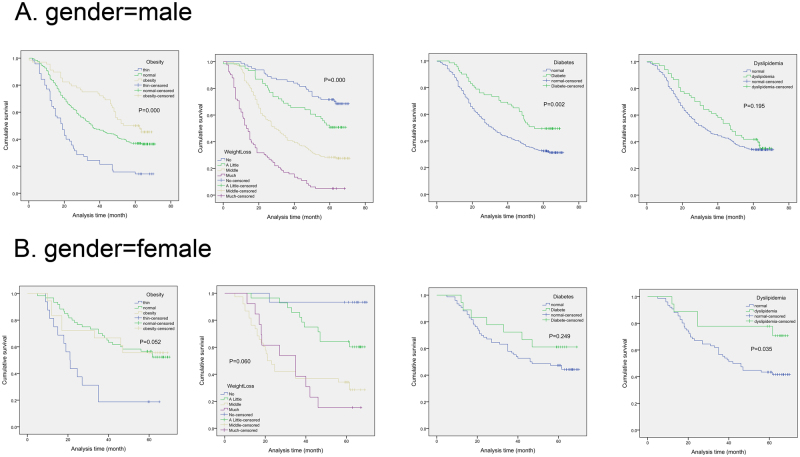
In male subgroup, obesity (P = 0.000), little weight loss (P = 0.000) and diabetes (P = 0.002) showed better survival compared with normal group in Fig. 4. Dislipidemia in female group showed better OS than normal patients (P = 0.035). Conversely, there were no significance in OS between patients accompanied by obesity (P = 0.052), little weight loss (P = 0.060), diabetes (P = 0.249) and ESCC patients with normal metabolic state in female subgroup. MS group showed better survival compared with normal group (P = 0.041) in males, while there was no significance between MS patients and normal ESCC patients in female subgroup (P = 0.846).
Figure 4.
Kaplan–Meier analysis for the effects of obesity, weight loss, diabetes and dyslipidemia on survival in different gender categories (male vs female).
Discussion
In this study, we investigated the prognostic value of MS in patients with resectable esophageal squamous cell carcinoma, and found significant correlation between OS and gender, tumor length, tumor differentiation, T stage, LNM, N stage, TNM stage, obesity, weight loss, diabetes and dyslipidemia. In addition, analysis revealed a relationship between tumor length, tumor differentiation, T stage, LNM, N stage, TNM stage, treatment, obesity, weight loss, diabetes and PFS time. The results indicated that advanced TNM staging and more weight loss were independent indicators for adverse prognosis of esophageal squamous carcinoma patients. In contrast, diabetes was a protective factor in the prognosis of patients with ESCC. It was also shown that TNM stage, weight loss and diabetes were independently associated with PFS time.
The metabolic syndrome, comprising obesity, dyslipidemia, hypertension, and hyperglycaemia, is a cluster of risk factors for cardiovascular disease and type 2 diabetes6,19. There is some evidence that MS may be associated with the risk of some common cancers7–9, including liver, colorectal, bladder, pancreatic, breast and esophageal cancer10–12. Some prospective cohort studies investigated the association between MS and the risk of the two dominating types of esophageal cancer, EAC and ESCC. Study found that obesity was associated with an increased risk of EAC12 and a decreased risk of ESCC13. In accordance with above study, Corley et al. detected that increasing abdominal diameter was strongly associated with an increased risk of esophageal adenocarcinoma but not esophageal squamous cell carcinoma20. The Me-Can project in Sweden conducted a prospective study and revealed an association between high blood pressure and risk of ESCC without adjusting alcohol consumption21. They also observed that MS was associated with EAC but not ESCC21. The evidence for the positive correlation between lipid metabolism and risk of esophageal cancer was presented16. Several studies indicated an association between high blood glucose and an increased risk of cancer overall22,23.
Although the relationship between MS and tumor risk was well studied, there is little research on the asscioation of metabolic factors and tumor mortality. It was indicated that MS might be an important prognostic factor for colorectal cancer. Patrizia et al. showed that metabolic syndrome at baseline emerged as an important prognostic factor for breast cancer recurrences24. However, there were different conclusions regarding the prediction value of MS in tumor prognosis. An analysis revealed no association between MS and prostate cancer risk. Moreover, it was proposed that the metabolic syndrome be considered as a high-risk state for certain types of cancer, such as colon cancer, and this relationship should be systematically explored across cancer types25,26.
Studies linking the metabolic syndrome to esophageal cancer are scarce. However, several studies had investigated the association between metabolic biomarkers and overall survival of esophageal cancer patients. A retrospective review was performed to show that MS was a significant and independent predictor for better survival in patients with resectable ESCC27. There was no apparent influence of any single component of MetS on OS27. Another study reported that low BMI and low fasting blood glucose were related to poor survival and BMI came to be stronger prognostic factors on lymph node-negative patients28. Our results were consistent with the above two researches. In our study, survival analysis revealed a correlation between BMI (P < 0.001), weight loss (P < 0.001), diabetes (P = 0.001), dyslipidemia (P = 0.030) and OS of esophageal cancer. In addition, our research also found that ESCC patients with obesity or little weight loss or diabetes had significantly longer PFS (obesity, P˂0.001; weight loss, P < 0.001; diabetes, P = 0.002). The multivariate analysis indicated that advanced TNM staging (P = 0.007, HR: 1.760, 95% CI: 1.167–2.654) and more weight loss (P = 0.000, HR: 1.961, 95% CI: 1.697–2.267) were independent factors for adverse prognosis of patients with ESCC. In contrast, diabetes was a protective factor in the prognosis of patients with esophageal cancer (P = 0.018, HR: 0.668, 95% CI: 0.478–0.933). It was also shown that TNM stage (P = 0.008, HR: 1.643, 95% CI: 1.140–2.369), weight loss (P = 0.000, HR: 1.674, 95% CI: 1.462–1.917) and dibetes (P = 0.027, HR: 0.691, 95% CI: 0.499–0.958) were independently associated with PFS. Nevertheless, there were conflicting results in the studies that had investigated whether MS influenced survival among esophageal cancer patients13,17,18,29,30. It was reported that BMI one year prior to diagnosis was not associated with esophageal adenocarcinoma survival in a study conducted in Australia31. Besides, a nationwide study in Sweden found that obese patients had a favourable prognosis in EAC, while lean patients had a better prognosis in ESCC, compared with normal weight patients32. The underlying mechanisms of the inverse relationship between MS and ESCC remained unclear.
In order to analyze the relationship between MS-related composition and survival at different levels, we carried out the subgroup analysis based on gender, lymph node metastasis and TNM stage. A study showed that HR of metabolic syndrome for ESCC mortality was statistically significant in male (HR: 1.45, 95% CI: 1.14–1.83, P:0.002), but not in female (HR: 1.46, 95% CI: 0.92–2.31, P: 0.107)33. Likewise, our research found that MS group showed better survival compared with normal group (P = 0.041) in males, while there was no significance between MS patients and normal ESCC patients in female subgroup (P = 0.846). In addition, we conducted subgroup analysis in accordance with lymph node metastasis and TNM staging. It was indicated that obesity (P = 0.000), little weight loss (P = 0.000) and diabetes (P = 0.034) showed association with survival time in N0 subgroup, while BMI (P = 0.003), weight loss (P = 0.000), diabetes (P = 0.012) and dyslipidemia (P = 0.030) were all associated with survival in N1–3 subgroup. In TNM II-III subgroup, it was revealed that BMI (P = 0.000), weight loss (P = 0.000) and diabetes (P = 0.000) showed association with longer OS, while weight loss (P = 0.000) was related to survival time in subgroup TNM I stage. To our knowledge, this is the first time that a study has grouped the clinicopathological characteristics to further investigate the effect of MS on ESCC survival.
All in all, our study confirmed that MS patients had a better prognosis in male esophageal cancer patients, not in female. In addition, TNM staging, weight changes and diabetes were also independent predictors for the prognosis of ESCC. The biological mechanisms that could explain the inverse association remain unclear. In the early stage of esophageal cancer, the good control of the blood glucose, lipids, weight and other factors of metabolic sydrome will greatly affect the prognosis.
Methods
Patients
A retrospective study was carried out, which included 519 patients with esophageal squamous cell carcinoma who had received esophagetomy and lymphnode dissections in the Department of Thoracic Surgery, Qilu Hospital of Shandong University between January 2007 and December 2011. The study was approved by the Ethical Committee of Qilu Hospital of Shandong University. Informed consent was obtained from all the patients. All data had been anonymized and deidentified. Moreover, the patient data collection methods were carried out in accordance with the Declaration of Helsinki. The patient’s exclusion criteria were as follows: non-primary esophageal cancer, non-first-time diagnosed esophageal cancer, non-squamous cell carcinoma, combined with other malignancies, incomplete clinical pathological information, received neoadjuvant chemoradiotherapy, palliative or R1/R2 resections, lost to follow-up.
Metabolic syndrome evaluation
There is no consensus on the diagnostic criteria of metabolic syndrome in various groups. In this study, we conducted the diagnosis of MS by the CDS standard proposed by the Chinese medical association diabetes branch.
MS is defined as including three or four of the following criteria: overweight and/or obesity, BMI (Body Mass Index) is greater than 25.0 kg/m2; high blood glucose, FPG is greater than 6.1 mmol/L(110 mg/dl) and (or) 2hPG is greater than 7.8 mmol/L (140 mg/dl), and/or has been diagnosed with diabetes; hypertension, SBP/DBP >140/90 mmHg, and/or has been diagnosed with hypertension; dyslipidemia, blood TG is more than 1.7 mmol/L(110 mg/dl), and (or) fasting blood HDL-C <0.9 mmol/L(35 mg/dl) in males, <1.0 mmol/L (39 mg/dl) in females. Little weight loss refers to weight loss within 5%, middle weight loss is 5% to 10%, much weight loss is greater than 10%.
Follow up
All patients were followed up, including postoperative recovery, general health, necessary psychological support and rehabilitation guidance through telephone, SMS, email, outpatient visit, etc. The information were obtained every 3 months in the first two years, followed by every 6 months. Recurrent, metastatic, lost and dead patients were noted during the follow-up. The time was cut-off until December 2016.
Statistical analysis
SPSS Version 19.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The relationship between MS and various clinical pathological characteristics was tested by the Pearson’s chi square test. OS was the endpoint of the clinical trial, which was defined as the time from the date of the operation to the end of the patient’s death or follow-up. PFS was also one of the endpoints of this study, which was defined as the time from the date of surgery to the progression of the tumor or the end of the patient’s death or follow-up. Kaplan-meier was used to draw the survival curve and the log-rank method was used to test its significance. COX regression included univariate and multivariate analyses. Univariate analysis detected the element related to esophageal cancer survival time, while the independent prognostic factors affecting OS and PFS were determined by multivariate analysis. P < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81572958).
Author Contributions
All authors contributed significantly to this work. B.L., B.C. and Y.C. designed the study; B.L., B.C., C.W. and P.C. performed the study and collected the data; B.L. and C.W. analyzed the data; B.L. wrote the first draft of the manuscript; B.L. and Y.C. revised the manuscript. All authors reviewed, edited and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28268-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen W, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, et al. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Rustgi AK, El-Serag HB. Esophageal carcinoma. The New England journal of medicine. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 5.Koshy M, Esiashvilli N, Landry JC, Thomas CR, Jr., Matthews RH. Multiple management modalities in esophageal cancer: combined modality management approaches. The oncologist. 2004;9:147–159. doi: 10.1634/theoncologist.9-2-147. [DOI] [PubMed] [Google Scholar]
- 6.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 7.Harvie MN, Howell A, Thatcher N, Baildam A, Campbell I. Energy balance in patients with advanced NSCLC, metastatic melanoma and metastatic breast cancer receiving chemotherapy–a longitudinal study. British journal of cancer. 2005;92:673–680. doi: 10.1038/sj.bjc.6602357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head & neck. 2007;29:893–900. doi: 10.1002/hed.20607. [DOI] [PubMed] [Google Scholar]
- 9.Sogabe M, et al. Influence of metabolic syndrome on upper gastrointestinal disease. Clinical journal of gastroenterology. 2016;9:191–202. doi: 10.1007/s12328-016-0668-1. [DOI] [PubMed] [Google Scholar]
- 10.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandari R, Kelley GA, Hartley TA, Rockett IR. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. International journal of breast cancer. 2014;2014:189384. doi: 10.1155/2014/189384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Smith M, et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. International journal of cancer. 2008;122:1604–1610. doi: 10.1002/ijc.23198. [DOI] [PubMed] [Google Scholar]
- 14.van der Schaaf MK, et al. The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. The Journal of thoracic and cardiovascular surgery. 2014;147:490–495. doi: 10.1016/j.jtcvs.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 15.Martin L, Lagergren P. Risk factors for weight loss among patients surviving 5 years after esophageal cancer surgery. Annals of surgical oncology. 2015;22:610–616. doi: 10.1245/s10434-014-3973-2. [DOI] [PubMed] [Google Scholar]
- 16.Wulaningsih W, et al. Serum Lipids and the Risk of Gastrointestinal Malignancies in the Swedish AMORIS Study. Journal of cancer epidemiology. 2012;2012:792034. doi: 10.1155/2012/792034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi Y, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer. 2010;116:5619–5627. doi: 10.1002/cncr.25745. [DOI] [PubMed] [Google Scholar]
- 18.Yoon HH, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:4561–4567. doi: 10.1200/JCO.2011.37.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2008;17:352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindkvist B, et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC cancer. 2014;14:103. doi: 10.1186/1471-2407-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stocks T, et al. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. Plos medicine. 2009;6:e1000201. doi: 10.1371/journal.pmed.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jee SH, et al. Fasting serum glucose level and cancer risk in Korean men and women. Jama. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 24.Pasanisi P, et al. Metabolic syndrome as a prognostic factor for breast cancer recurrences. International journal of cancer. 2006;119:236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 25.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? The American journal of pathology. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishino K, Mutoh M, Totsuka Y, Nakagama H. Metabolic syndrome: a novel high-risk state for colorectal cancer. Cancer letters. 2013;334:56–61. doi: 10.1016/j.canlet.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Wen YS, et al. Impact of metabolic syndrome on the survival of Chinese patients with resectable esophageal squamous cell carcinoma. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2016;29:607–613. doi: 10.1111/dote.12376. [DOI] [PubMed] [Google Scholar]
- 28.Wu N, et al. The prognostic influence of body mass index, resting energy expenditure and fasting blood glucose on postoperative patients with esophageal cancer. BMC gastroenterology. 2016;16:142. doi: 10.1186/s12876-016-0549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grotenhuis BA, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World journal of surgery. 2010;34:2621–2627. doi: 10.1007/s00268-010-0697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan MA, et al. Prognostic significance of body mass indices for patients undergoing esophagectomy for cancer. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus. 2007;20:29–35. doi: 10.1111/j.1442-2050.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- 31.Pischon T, Nimptsch K. Obesity and Risk of Cancer: An Introductory Overview. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2016;208:1–15. doi: 10.1007/978-3-319-42542-9_1. [DOI] [PubMed] [Google Scholar]
- 32.Sundelof M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. European journal of cancer. 2008;44:1566–1571. doi: 10.1016/j.ejca.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Peng F, et al. Analysis of Preoperative Metabolic Risk Factors Affecting the Prognosis of Patients with Esophageal Squamous Cell Carcinoma: The Fujian Prospective Investigation of Cancer (FIESTA) Study. EbioMedicine. 2017;16:115–123. doi: 10.1016/j.ebiom.2017.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.