Abstract
Heart transplantation (HTx) has become standard treatment for selected patients with end-stage heart failure. Improvements in immunosuppressant, donor procurement, surgical techniques, and post-HTx care have resulted in a substantial decrease in acute allograft rejection, which had previously significantly limited survival of HTx recipients. However, limitations to long-term allograft survival exist, including rejection, infection, coronary allograft vasculopathy, and malignancy. Careful balance of immunosuppressive therapy and vigilant surveillance for complications can further improve long-term outcomes of HTx recipients.
Keywords: Heart transplantation, History, Current practice, Forecasting, Heart failure
HISTORY OF HEART TRANSPLANTATION
Overall history of heart transplantation
The first human heart transplantation (HTx) was performed by Christian Barnard from South Africa in December 1967.1) Right after the event, the first HTx in the United States (US) was performed by Dr. Shumway and colleagues at Stanford University in January 1968. However, initial outcomes of HTx were poor because of the complex postoperative problems, such as graft rejection and infection. As a result, the total number of HTx cases dropped after the initial surgeries. The introduction of cyclosporine-based immunosuppression in 1980 brought an improvement in survival rates2) and HTx subsequently became a standard treatment for end-stage heart failure (HF). Currently, the number of HTx performed worldwide is estimated to be nearly 5,500 procedures annually.3)
History of heart transplantation in Asia and Korea
The first HTx in Asia was performed in 1968 by Dr. Juro Wada at Sapporo Medical University in Japan.4) Similar to the experience in the US, the initial surgeries yielded poor outcomes until the introduction of cyclosporine in 1980. Cultural taboos regarding organ donation along with incomplete legislation regarding brain death delayed the widespread performance of HTx in Asia. Eventually, HTx began to be performed again on the Asian continent in Taiwan5) and Thailand in 19876), in Korea7),8) and Hong Kong in 19929), and in Japan in 1999.10) Currently, most number of HTxs performed in Asia are done in Taiwan and Korea. In Taiwan, 1,354 HTxs were performed between 1987 and 2012,11) and in Korea, 1,513 cases were performed between 1992 and 2017. In other Asian countries, the numbers of cases performed after the initiation of the program were as follows: Japan, 266; Saudi Arabia, 249; Iran, 122; Thailand, 97; Hong Kong, 77; and Singapore, 40.4),12)
CURRENT STATUS OF HEART TRANSPLANTATION
The International Society for Heart and Lung Transplantation Registry
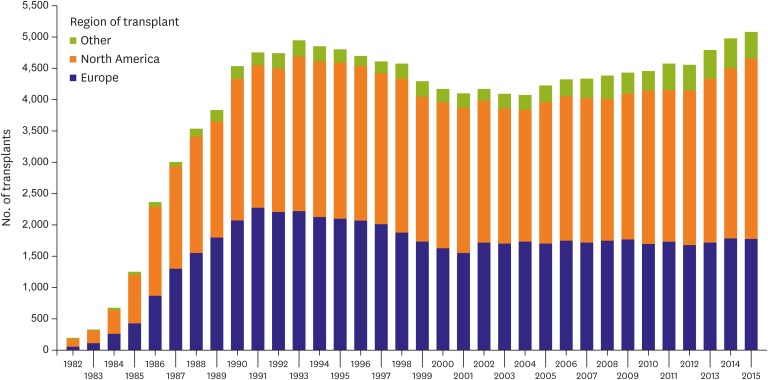
The International Society for Heart and Lung Transplantation (ISHLT) and International Thoracic Transplant Registry reported a continued increase in the annual number of HTxs performed worldwide over the last decade (Figure 1).3) After the initial increase in cases during the 1980s and early 1990s, the numbers of HTxs stabilized because of a limited number of donors. Therefore, the number of candidates on waiting lists exceeds that of available donor organs. In the absence of a reliable prognostic score for stage D HF along with the evolution of mechanical circulatory support (MCS) options, it has been difficult to design an organ allocation system that would reliably prioritize transplantation among patients in need of HTx. Continued efforts are underway to develop ideal allocation systems.13),14)
Figure 1. Number of HTx (adult and pediatric) by year and geographic region.
HTx = heart transplantation.
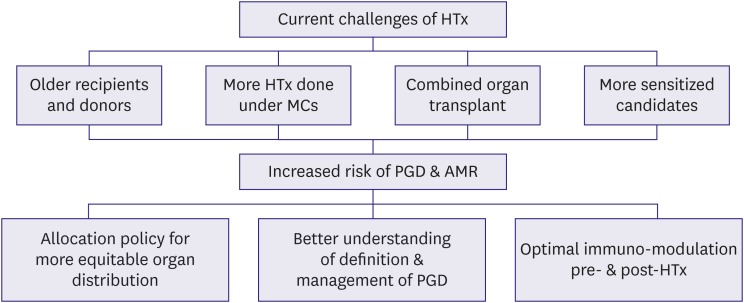
Current challenges of HTx can be characterized by the following parameters: 1) older age of both recipients and donors; 2) increased number of HTx performed with MCS; 3) the growing use of combined organ transplants (comprising more than 4% of the total number of HTx); and 4) a high proportion of sensitized candidates.3),15) The complex HTx candidates are at higher risk of poor outcomes, including primary graft dysfunction (PGD) and antibody-mediated rejection (AMR).3),16),17) To minimize this risk, the following strategies should be considered: 1) proposing changes in the current HTx allocation policy to provide a more equitable organ distribution,18),19) 2) propagating a better understanding of the definition and management of PGD,20) and 3) encouraging advances in the management of sensitized HTx candidates.21),22) Developments in these areas could result in a more equitable distribution of organs, expansion of the donor pool, and improved quality of life and survival for heart transplant recipients (Figure 2).
Figure 2. Current status of HTx and risk modification strategy.
AMR = antibody-mediated rejection; HTx = heart transplantation; MCS = mechanical circulatory support; PGD = primary graft dysfunction.
The Korean Organ Transplant Registry data
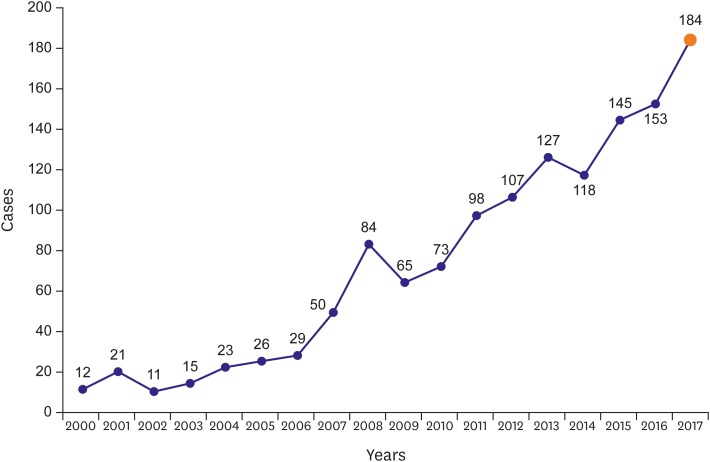
After the first HTx in 1992, the annual number of surgeries performed in Korea has been increasing. The number has increased to more than 50 cases between 2000 and 2007 and reached to 184 cases in 2017 (Figure 3). Recently, Lee et al.23) reported data from the Korean Organ Transplant Registry (KOTRY) on HTx surgery. This registry was established by the Korean Society for Transplantation and the Korean Center for Disease Control in 2013. Data from a total of 183 HTxs were collected from 4 nationally representative hospitals from April 2014 to December 2015.
Figure 3. Temporal trends in HTx in Korea after 2000.
HTx = heart transplantation.
The characteristics of the current state of HTx in Korea compared with ISHLT and the Japan HTx registry were described recently (Table 1). In Korea, HTx data for patients with long-term durable MCS were unavailable because of the current reimbursement system. In contrast, HTx with concurrent inotropic support is the most common strategy in Korea. The 1-year survival rate in Korea (91.6%) was higher than that reported in the ISHLT registry (85.0%).24)
Table 1. Comparison of the KOTRY and a representative ISHLT HTx registry and Japanese HTx registry24) .
| KOTRY (183 patients; Apr 2014–Dec 2015) | ISHLT (30,503 patients; Jan 2009–Jun 2016) | Japan (284 patients; Feb 1999–Jun 2016) | |
|---|---|---|---|
| Number of HTxs (annually, /year) | 131.5 (2014–2015) | ≒2,500 | 33.3 (2010–2015) |
| Mean donor age (years) | 37.6 | 35.0 | NA |
| Mean recipient age (years) | 50.5 | 55.0 | 38.1 |
| Sex (male) | 67.2 | 75.0 | 74.0 |
| Underlying diseases (DCM/ICM) | 69/14 | 50/34 | 66/8 |
| Inotropic support | 93 | 40 | 5 (2010–2015) |
| ECMO | 19 | 1 | 34 (2010–2015) |
| Long-term VAD | 0 | 43 | 61 (2010–2015) |
| One-year survival rate | 91.6 | 85.0 | 98.0 |
Values are presented as numbers or percentages.
DCM = dilated cardiomyopathy; ECMO = extracorporeal membrane oxygenator; HTx = heart transplantation; ICM = ischemic cardiomyopathy; ISHLT = International Society for Heart and Lung Transplantation; KOTRY = Korean Organ Transplant Registry; NA: not available; VAD = ventricular assist device.
RECIPIENT SELECTION AND MANAGEMENT
General principles (listing criteria)
End-stage HF patients who are truly refractory to maximal medical therapy and who could be benefitted by HTx surgery must be listed. The commonly accepted indications for HTx are presented in Table 2. Generally, the 3 major indications for HTx are severe functional limitations, refractory angina, and ventricular arrhythmias refractory to maximal medical therapy.25) Optimization of medical therapy is a crucial factor for determining whether a patient would benefit from HTx. Patients with HF and reduced ejection fraction (EF) should be prescribed the following medications, when feasible, to improve symptoms and survival: angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker or angiotensin receptor-neprilysin inhibitor, β-receptor antagonist, sinoatrial node modulator (ivabradine), and mineralocorticoid antagonists.8),26),27),28),29) In addition to optimal medical therapy, cardiac implantable electronic devices including cardiac resynchronization therapy with or without implantable defibrillators should be considered to increase survival.30),31),32),33) For patients with ischemic cardiomyopathy (ICM), surgical revascularization may be indicated to improve long-term survival.32)
Table 2. Commonly accepted indications for HTx34) .
| Systolic HF with severe functional limitations or refractory symptoms despite optimal medical and device therapy |
| NYHA functional class IIIb–IV |
| LVEF usually <35%* |
| Maximal oxygen uptake (VO2max) of ≤12–14 mL/kg/min and/or VO2max <50% predicted, and/or VE/VCO2 |
| Slope >35 on cardiopulmonary exercise stress testing† |
| Cardiogenic shock not expected to recover |
| Acute myocardial infarction |
| Acute myocarditis |
| Ischemic heart disease with intractable angina not amenable to surgical or percutaneous revascularization, and refractory to maximal medical therapy |
| Intractable ventricular arrhythmias, uncontrolled with standard antiarrhythmic medication, device, or ablative therapy |
| Severe symptomatic hypertrophic or restrictive cardiomyopathy |
| Congenital heart disease in which severe, fixed pulmonary hypertension is not a complication |
| Cardiac tumors with a low likelihood of metastasis |
HF = heart failure; HTx = heart transplantation; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; VE/VCO2: minute ventilation/carbon dioxide production slope; VO2max = maximal oxygen uptake. *Low LVEF alone is not an adequate indication for HTx; †Abnormal cardiopulmonary exercise testing in the absence of functional limitations is not a sufficient criterion for transplantation.
HTx evaluation aims to determine whether patients meet the following conditions: 1) “sick enough,” such that their his/her cardiac status is sufficiently limited on optimal medical therapy in order to benefit from HTx; 2) are patients “well enough,” such that they do not have comorbidities that would preclude HTx; and 3) “can adapt to new transplant lifestyle,” such that the patient demonstrates compliance with therapy and possesses adequate social support. Although these indications are generally well accepted, it is challenging to list optimal patients who can benefit from HTx (Figure 4).
Figure 4. Balanced HTx listing strategy.
HTx = heart transplantation.
Contraindications
Contraindications for HTx should be considered prior to listing patients to provide the best chance for achieving an optimal outcome (Table 3). Many of these factors are not absolute and must be considered in the context of the severity of the patient's heart disease and associated comorbidities. The degree to which they are interpreted and applied may vary considerably among transplant programs.
Table 3. Contraindications to HTx34) .
| Age | Over 70 is a relative contraindication depending on associated comorbidities |
| Obesity | BMI <30 kg/m2 is recommended; most centers will tolerate BMI <35 kg/m2* |
| Malignancy | Active neoplasm, except nonmelanoma skin cancer, is an absolute contraindication; cancers that are low grade or in remission may be acceptable in consultation with an oncologist |
| Pulmonary hypertension | The inability to achieve PVR <2.5 wood units with vasodilator or inotropic therapy is a contraindication; such patients may benefit from unloading with a VAD |
| Diabetes | Uncontrolled diabetes or that associated with significant end-organ damage is an absolute contraindication |
| Renal dysfunction | If caused by diabetes, may be an absolute contraindication (unless combined heart-kidney transplantation is considered) |
| Cirrhosis | May be secondary to cardiac disease and is an absolute contraindication in most centers (unless combined heart/liver transplant is considered) |
| Peripheral vascular disease | Severe disease not amenable to revascularization is an absolute contraindication, especially if associated with ischemic ulcers |
| Infection | HIV and hepatitis C are absolute contraindications at most centers; with novel hepatitis C therapy, some centers may consider such patients |
| Substance use | 6 months of abstinence from smoking, alcohol, and illicit drugs are required; in critically ill patients, consultation with psychiatry and social work is essential |
| Psychosocial issues | Noncompliance, lack of caregiver support (either by family or agencies), and dementia are absolute contraindications; mental retardation may be a relative contraindication |
BMI = body mass index; HIV = human immunodeficiency virus; HTx = heart transplantation; PVR = pulmonary vascular resistance; VAD = ventricular assist device.
*A higher BMI may be acceptable in certain populations with larger body habitus, such as patients of Pacific Islander descent.
Korean situation
Although similar listing criteria and contraindications are also widely accepted in Korea, patients on waiting lists seem rather different because of the difference in medical situations and the reimbursement system. According to the recent KOTRY report, 93% of HTx was performed with concomitant inotropic support; this value is much higher than that reported in ISHLT (40%) and Japanese (5%) data. Notably, a relatively high proportion of patients undergo HTx surgery with the concomitant use of extracorporeal membrane oxygenator (ECMO) in Korea (19%) than that reported in ISHLT data (only 1%) (Table 1).24) Recently, Korean Network for Organ Sharing status codes for medical urgency were modified to a version similar to that of the United Network for Organ Sharing strategies, which put higher priority on durable ventricular assist device (VAD)-related problems (Table 4).
Table 4. A comparison of UNOS and KONOS status codes for medical urgency.
| UNOS | KONOS | |||
|---|---|---|---|---|
| Status 1A | Inpatient at the listing transplant center | Status 0 | (Re-registration in 8 days) | |
| (a) | MCS for acute hemodynamic decompensation that includes at list one of the following | |||
| (i) LVAD/RVAD for 30 days or less | VT/VF needs VAD | |||
| (ii) TAH | ||||
| (iii) IABP or | VT/VF needs IABP | |||
| (iv) ECMO | V-A ECMO | |||
| (b) | MCS more than 30 days with complication (thromboembolism, device infection, mechanical failure, life threatening ventricular arrhythmia) | VAD with serious complication (thromboembolism, device infection, mechanical failure, recurrent ventricular arrhythmia) who needs admission at the ICU | ||
| (c) | Requiring continuous mechanical ventilation | Requiring continuous mechanical ventilation due to heart failure | ||
| (d) | Continuous infusion of a single high-dose IV inotrope or multiple IV inotropes | External VAD (RVAD, LVAD, Bi-VAD) | ||
| Status 1B | At least one of the following therapy | Status 1 | Inpatient, at least one of the following (Re-registration in 8 days) | |
| (a) | LVAD/RVAD for more than 30 days | Artificial heart, VAD (no need of admission), IABP | ||
| (b) | Continuous infusion of IV inotropes | Continuous infusion of IV inotropes for more than 4 weeks | ||
| Continuous infusion of a single high-dose IV inotrope or multiple moderate dose IV inotropes for 1 week | ||||
| VT/VF for more than 3 times/24 hours (despite the antiarrhythmics or the previous anti-arrhythmic procedure) | ||||
| More than 3 ICD shock events during re-registration period | ||||
| Status 2 | (Re-registration in 1 month) | |||
| Continuous infusion of IV inotropes but not fulfilling criteria for Status 1 | ||||
| VT/VF despite the antiarrhythmics or the previous anti-arrhythmic procedure, ICD shock | ||||
| (but not fulfilling criteria for Status 1) | ||||
| Status 2 | A patient who does not meet the criteria for status 1A or 1B is listed as Status 2 | Status 3 | A patient who does not meet the criteria for Status 0, 1 or 2 | |
| Status 7 | Temporarily unsuitable to receive a thoracic organ transplant | Status 7 | Deferred for heart transplantation listing | |
ECMO = extra-corporeal membrane oxygenation; IABP = intra-aortic balloon pump; ICD = implantable cardioverter-defibrillator; ICU = intensive care unit; IV = intravenous; KONOS = Korean Network for Organ Sharing; LVAD = left ventricular assist device; MCS = mechanical circulatory support; RVAD = right ventricular assist device; Bi-VAD = Biventricular assist device; TAH = total artificial heart; UNOS = United Network for Organ Sharing; VT/VF = ventricular tachycardia/ventricular fibrillation.
DONOR SELECTION AND MANAGEMENT
General principles
The process of donor evaluation starts with detailed history-taking and physical examination, focusing on the cause of death, past medical history, donor height and weight, and clinical course. Basic laboratory studies, including a complete blood count, metabolic panel, ABO blood typing, and viral serologies (hepatitis B and C, human immunodeficiency virus [HIV], human T-cell leukemia virus, Epstein-Barr virus [EBV], and cytomegalovirus [CMV]), are ordered. Additional studies include chest X-ray, 12-lead electrocardiography, and echocardiography.
Brain-dead individuals must meet certain minimum criteria to be considered an HTx donor (Table 5). Most cardiac donors are younger than 55 years, although older donors may be selectively considered in critically ill or older recipients. An initial echocardiogram is obtained to identify significant structural heart disease, such as left ventricular (LV) hypertrophy or dysfunction, occlusive coronary artery disease, valvular dysfunction, and congenital lesions. Generally, echocardiographically normal hearts are considered; however, selected marginal organs may be allocated to higher risk recipients. Angiography is performed in several countries to exclude significant coronary artery disease in male donors older than 45 years and female donors older than 50 years; however, it may also be performed in younger patients with multiple risk factors for coronary artery disease. Patients with active malignancy (excluding nonmelanocytic skin cancers and certain isolated brain tumors) or severe systemic infections are typically excluded. HIV and hepatitis B and C infections would preclude organ donation. However, hepatitis B donors might be carefully allocated in anti-HBs Ab (+) recipients with perioperative hepatitis B immunoglobulin (Ig) prophylaxis,35),36) hepatitis C-positive donors could be considered with the advent of new nucleotide polymerase inhibitors that offer a potential for cure.37)
Table 5. Favorable donor characteristics34).
| Age (<55 years) | |
| Absence of significant structural abnormalities such as: | |
| LV hypertrophy (wall thickness >13 mm by echocardiography) | |
| Significant valvular dysfunction | |
| Significant congenital cardiac abnormality | |
| Significant coronary artery disease | |
| Adequate physiologic function of donor heart | |
| LVEF ≥45% or | |
| Achievement of target hemodynamic criteria after hormonal resuscitation and hemodynamic management | |
| MAP >60 mmHg | |
| PCWP 8–12 mmHg | |
| Cardiac index >2.4 L/min·m2 | |
| CVP 4–12 mmHg | |
| SVR 800–1200 dyne/s·cm5 | |
| No inotrope dependence | |
| Donor-recipient body size match (usually within 20–30% of height and weight) | |
| Negative hepatitis C antibody, hepatitis B surface antigen, and HIV serologies absence of active malignancy (except nonmelanoma skin cancers and certain primary brain tumors) or overwhelming infection | |
CNS = central nervous system; CVP = central venous pressure; LV = left ventricle; LVEF = left ventricle ejection fraction; MAP = mean arterial pressure; pCO2: partial pressure of carbon dioxide.; PCWP: pulmonary capillary wedge pressure; SVR: systemic vascular resistance
*Removal of CNS-depressing drugs may take several days to take effect.
Expanding the donor pool (extended criteria donor)
Currently, fewer than 50% of potential organ donors in the US become actual donors and efforts have been undertaken to place to increase the usage rate.38),39) Transplantation centers now use extended criteria donor (ECD) hearts with acceptable outcomes, which matches higher risk recipients with higher risk donors. The criteria for higher risk recipients are as follows: age >65 years, renal insufficiency, peripheral arterial disease, or poorly controlled diabetes. Considerable evidence shows that ECD hearts that may result in favorable post-HTx survival continue to be discarded. A retrospective review of 1,872 potential organ donors in California from 2001 to 2008 showed predictors of non-usage to be as follows: age >50 years, female sex, death attributable to cerebrovascular accident, hypertension, diabetes mellitus, a positive troponin assay result, LV dysfunction (LVEF of <50%), regional wall motion abnormalities, and LV hypertrophy. These characteristics of ECD hearts, however, seemed to have little effect on recipient outcomes they were transplanted.40) Other ongoing efforts to expand the donor pool include an increase in identification of donors, increase in the consent rate, expansion of donor selection criteria, maximization of donor organ function, and use of mechanical support devices as an adjunctive approach to organ donation. Overcoming cultural issues should be considered to expand the donor pool in Asian countries as well.12)
Donation after circulatory death and the organ care system
Recently, the concept of donation after circulatory death (DCD) has been successfully utilized for HTx in line with an effort to expand the donor pool.41) In DCD, retrieval of organs for the purpose of transplantation occurs from patients whose death is diagnosed and confirmed using cardiorespiratory criteria. Asystole must be confirmed for at least 5 minutes to declare death, but this standoff time varies in different countries. To minimize ischemic injury for DCD organs, efforts are drawn to limit cold ischemic time through the use of an ex vivo heart perfusion platform that maintains the donor heart in a warm, beating state prior to transplantation. In a trial to assess the safety of ex vivo perfusions, 130 patients were randomized to receive donor hearts preserved with either the ex vivo perfusion or standard cold storage. There was no difference in 30-day patient and graft survival rates or serious adverse events.42) An ongoing phase III clinical trial, the International Trial to Evaluate the Safety and Effectiveness of the Portable Organ Care System (OCS™, TransMedics, Andover, MA, USA) Heart for Preserving and Assessing Expanded Criteria Donor Hearts for Transplantation (EXPAND trial; NCT02323321), will offer further insight into the utility of this platform.
POSTOPERATIVE MANAGEMENT AFTER HEART TRANSPLANTATION
Physiology of the transplanted heart
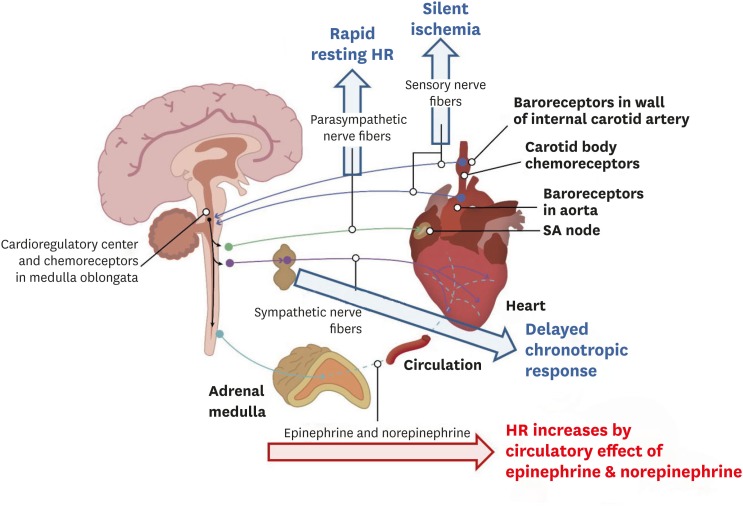
Surgical excision of the heart from the donor results in immediate denervation of both sympathetic and parasympathetic nervous fibers (Figure 5). Cardiac denervation after HTx is clinically important because it explains why recipients are unable to experience angina due to ischemia, but may have bradycardia and hypotension related to inferior wall infarction post-HTx.34),43),44) Cardiac denervation is also responsible for diminished exercise capacity early post-HTx, and is related to a slower increase in heart rate during exercise because the heart relies on non-cardiac circulating catecholamines. Cardiac denervation also explains loss of the nocturnal decline in blood pressure, and the higher resting heart rate early post-HTx. Because cardiac denervation depletes the catecholamine stores in the cardiac muscle, HTx recipients may experience more arrhythmias because of dependence on circulating catecholamines. This can also cause an abnormal response to adrenergic agonist or antagonist medications. To compensate for chronotropic incompetence, the denervated heart must increase its stroke volume to increase cardiac output, even during mild exercise. Heart transplant recipients have a much lower peak VO2 compared with age-adjusted, non-transplant cardiac patients. Peripheral factors, such as damage to the pulmonary capillary bed, play significant roles in reducing the exercise tolerance of transplant patients. Exercise training improves the exercise capacity of heart transplant recipients by improving peripheral factors and the chronotropic response. Beta blockers markedly reduce the exercise tolerance of heart transplant recipients and should be avoided where possible. Atropine and digoxin have no effect on the denervated heart and should not be used to treat arrhythmias in heart transplant patients.34),43),44)
Figure 5. Physiology of the transplanted heart. Blue arrow indicates blockage of the nerve fibers, red arrow indicates remained effect of circulatory catecholamines.
HR = heart rate; SA = sinoatrial.
Immediate postoperative management after heart transplantation
Intraoperative transesophageal echocardiography to assess systolic function is typically recommended. Invasive monitoring of arterial pressure, central venous pressure, pulmonary artery pressure, pulmonary capillary wedge pressure, cardiac output, and oxygen saturation should also be performed immediately following transplantation. Continuous inotropic infusions of isoproterenol, dobutamine, dopamine, and/or milrinone are warranted to avoid early ventricular dysfunction. Alpha-adrenergic agonists (norepinephrine and epinephrine) may also be used to treat persistent systemic hypotension. MCS including intra-aortic balloon pumps (IABPs) or temporary assist devices should be considered if there is a failure to wean from cardiopulmonary bypass or if there is persistent hemodynamic instability despite multiple high-dose inotrope administrations. ECMO is an option in severe graft dysfunction with cardiogenic shock that is unresponsive to pharmacological treatment. Causes of early hemodynamic instability include hyperacute rejection, cardiac tamponade, PGD, and elevated pulmonary vascular resistance (causing right ventricular [RV] dysfunction). RV dysfunction may be treated with pulmonary vasodilators such as inhaled nitric oxide, sildenafil, and prostacyclin analogues, to lower pulmonary vascular resistance. Sinus node dysfunction is common post-transplant, resulting in post-transplantation bradycardia that may be treated with chronotropic agents or temporary pacing. Rarely, permanent pacing may be required. Tachyarrhythmias would need prompt investigation for rejection and may be treated with rate-controlling agents, such as diltiazem or amiodarone. Digoxin is not effective for the rate control of atrial fibrillation in the denervated heart as it requires an intact vagus nerve to lower the heart rate. Renal dysfunction is common in the first 24–48 hours post-transplant. Therefore, continuous assessment of urine output in the early postoperative period is crucial. Immunosuppression and anti-microbial prophylaxis should be initiated perioperatively. Early ambulation and physical therapy is important, and subsequent cardiac rehabilitation has been demonstrated to be beneficial.34),43)
Primary graft dysfunction
Early allograft dysfunction can be apparent in the intraoperative period or can develop within 24 hours of transplantation. It can manifest as LV dysfunction, isolated RV dysfunction, or biventricular dysfunction, and is associated with significantly increased 30-day and 1-year mortality.20) Early graft dysfunction is classified as primary or secondary graft dysfunction according to the suspected etiology. Secondary graft dysfunction can occur as a result of recipient and procedural factors. The causes include hyperacute rejection and excessive volume or pressure load on the right ventricle. Unrecognized pulmonary hypertension in the recipient can result in RV failure immediately after allograft implantation. Extensive intraoperative bleeding can result in massive blood product transfusion requirements and can result in RV volume overload and dysfunction.
PGD is currently defined as LV, RV, or biventricular dysfunction that occurs within 24 hours after surgery and is not associated with a discernible cause, such as hyperacute rejection, pulmonary hypertension, or uncontrolled intraoperative bleeding requiring massive blood product transfusions and prolonged graft ischemic time.20),34),43) A survey of 47 international heart transplant centers found that the incidence of PGD was 7.4%. Mortality among patients reported in this survey was 30% at 30 days and 35% at 1 year. The most common causes of 30-day mortality were multiorgan failure in 70% of patients, graft failure in 20%, and sepsis in 10%. Although the etiology of PGD is poorly understood, it is felt that preexisting donor heart disease, injury to the donor heart during brain death, ischemia during the organ recovery process, preservation, and re-implantation, and reperfusion injury immediately after re-implantation of the allograft and release of the aortic cross-clamp may contribute to the development of PGD. Total ischemic time greater than 4 hours has been associated with an increased risk of PGD45) and hearts from older donors are more susceptible to ischemic injury.46) Injury to the allograft may be transient (myocardial stunning), lasting for 12 to 24 hours after transplantation in some cases. However, contraction band necrosis and other evidence of ischemic injury can be seen in biopsy specimens from persistently dysfunctional hearts as well as in those experiencing functional recovery. Treatment options for PGD include the use of high-dose inotropic agents to support LV and RV function and the use of nitric oxide for RV dysfunction. If medical management does not improve the patient's hemodynamics, early initiation of MCS with an IABP, ECMO, or a temporary VAD is recommended before the development of severe end-organ dysfunction. Graft function will recover in many patients after a few weeks, but re-transplantation is an option for selected patients with persistent graft dysfunction and reasonable end-organ function.20),34),43)
IMMUNOSUPPRESSION STRATEGIES IN HEART TRANSPLANTATION
Current immunosuppression strategies in HTx follow several general principles.34),43) The first is that immune reactions leading to graft rejection are the highest early (within the first 3–6 months) after graft implantation and gradually decrease thereafter. Thus, most regimens use the highest levels of immunosuppression immediately after surgery and decrease those levels over the first year, eventually settling on the lowest maintenance levels of immune suppression that are compatible with preventing graft rejection and minimizing drug toxicities. The second general principle is to use low doses of several drugs without overlapping toxicities over the use of higher doses of fewer drugs whenever feasible. The third principle is that excessive immunosuppression is undesirable because it leads to a myriad of undesirable effects, such as susceptibility to infection and malignancy. Finding the right balance between over- and under-immunosuppression in an individual patient is truly an art that uses science.34) Immunosuppressive regimens can be classified as induction and maintenance. Induction regimens provide intense early postoperative immune suppression while maintenance regimens are used throughout the patient's life to prevent rejection.
Induction therapy
Approximately 50 percent of heart transplant programs currently employ a strategy of augmented immunosuppression, or induction therapy, during the early postoperative period.3) The goal of induction therapy is to provide intense immunosuppression when the risk of allograft rejection is highest. From a clinical perspective, the main advantages of induction therapy are to allow delayed initiation of nephrotoxic immunosuppressive drugs in patients with compromised renal function prior to or following surgery. This also provides some flexibility with respect to early glucocorticoid weaning or the administration of glucocorticoid-sparing maintenance regimens after transplantation.34),43) However, the overall utility of induction is uncertain and data comparing induction protocols are limited. Patients at highest risk for fatal rejection include patients with panel reactive antibodies (PRA) >10% or a high number of human leukocyte antigen (HLA) mismatches. Younger patients with ‘memory’ feature (prior history of pregnancy, multiple transfusion, and MCS) may derive a benefit from induction therapy. Agents used for induction therapy include polyclonal anti-thymocyte antibodies derived from the immunization of horses (ATGAM®; Pfizer Inc., New York, USA) or rabbits (Thymoglobulin; Genzyme Corporation Indication, Cambridge, MA, USA) with human thymocytes (antithymocyte globulin [ATG]). These agents may reduce the risk of early rejection, but have also been associated with an increased risk of infection.34) Anti-interleukin-2 receptor antagonists (IL2RA) result in reduced rates of moderate or severe cellular rejection among heart transplant patients treated with standard immunosuppression, without increasing the incidence of opportunistic infections or cancer at 1 year. However, among patients receiving IL2RA induction, there was an increased risk of fatal infections when cytolytic therapy was concomitantly used. Although induction therapy is commonly used, there have been no large randomized trials demonstrating the benefit of induction therapy versus no induction therapy.34) Recent systemic reviews and meta-analyses revealed that the use of induction therapy was not associated with improved survival or rejection rates compared with no induction therapy. Moderate-to-severe rejection may be reduced by the administration of ATG compared to IL2RA, especially in patients at higher risk of rejection.47)
Maintenance immunosuppressive regimens
Most maintenance immunosuppressive protocols employ a three-drug regimen consisting of a calcineurin inhibitor (CNI, cyclosporine or tacrolimus), an antimetabolite agent (mycophenolate mofetil [MMF] or less commonly azathioprine), and tapering doses of glucocorticoids over the first year post-transplantation.34),43) The commonly used drugs in HTx and their toxicities are outlined in Table 6.
Table 6. Immunosuppressive agents used in HTx.
| Drug | Dosing | Target levels | Major toxicities | |
|---|---|---|---|---|
| CNIs | ||||
| Cyclosporine | 4–8 mg/kg/day in 2 divided doses, titrated to keep target 12-hour trough levels | 0–6 months: 250–350 ng/mL | Renal insufficiency, hypertension, dyslipidemia, hypokalemia and hypomagnesemia, hyperuricemia, neurotoxicity (encephalopathy, seizures, tremors, neuropathy), gingival hyperplasia, and hirsutism | |
| 6–12 months: 200–250 ng/mL | ||||
| >12 months: 100–200 ng/mL | ||||
| Tacrolimus | 0.05–0.1 mg/kg/day in 2 divided doses, titrated to keep target 12-hour trough levels | 0–6 months: 10–15 ng/mL | Renal dysfunction, hypertension, hyperglycemia and diabetes mellitus, dyslipidemia, hyperkalemia, hypomagnesemia, neurotoxicity (tremors, headaches) | |
| 6–12 months: 8–12 ng/mL | ||||
| >12 months: 5–10 ng/mL | ||||
| Cell cycle agents | ||||
| Azathioprine | 1.5–3.0 mg/kg/day, titrated to keep WBC up to 3K | None | Bone marrow suppression, hepatitis (rare), pancreatitis, malignancy | |
| MMF | 2,000–3,000 mg/day in 2 divided doses | MPA: 2–5 mcg/mL | Gastrointestinal disturbances (nausea, gastritis, and diarrhea), leukopenia | |
| Mycophenolate sodium | 1,440–2,160 mg/day in 2 divided doses | None | Fewer gastrointestinal disturbances compared with MMF | |
| Leukopenia | ||||
| PSIs | ||||
| Sirolimus | 1–3 mg/day, titrated to keep therapeutic 24-hour trough levels | 5–10 ng/mL | Oral ulcerations, hypercholesterolemia and hypertriglyceridemia, poor wound healing, lower extremity edema, pulmonary toxicities (pneumonitis, alveolar hemorrhage), leukopenia, anemia, and thrombocytopenia, potentiation of CNI nephrotoxicity | |
| Everolimus | 1.5 mg/day in 2 divided doses | 3–8 ng/mL | Similar to sirolimus | |
| Corticosteroids | ||||
| Prednisone | 1 mg/kg/day in 2 divided doses, tapered to 0.05 mg/kg/day by 6–12 months. | None | Weight gain, hypertension, hyperlipidemia, osteopenia, hyperglycemia, poor wound healing, salt and water retention, proximal myopathy, cataracts, peptic ulcer disease, growth retardation | |
CNI = calcineurin inhibitor; HTx = heart transplantation; MMF = mycophenolate mofetil; MPA = mycophenolic acid; PSI = proliferation signal inhibitor; WBC = white blood cell.
Calcineurin inhibitors
Since the introduction of cyclosporine in the early 1980s, CNIs have remained the cornerstone of maintenance immunosuppressive therapy in heart and other solid organ transplantation.34),43) These drugs exert their immunosuppressive effects by inhibiting calcineurin, which is normally responsible for the transcription of interleukin-2 and several other cytokines, including tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma. The end result is a blunting of T-lymphocyte activation and proliferation in response to alloantigens. The 2 available CNIs, cyclosporine and tacrolimus, form complexes with different intracellular binding proteins. These drug-protein complexes subsequently bind to and inhibit calcineurin. Generally, tacrolimus is favored over cyclosporine based on evidence from clinical trials suggesting that tacrolimus-based immunosuppression is associated with decreased rates of acute rejection.48),49) Although both agents can result in nephrotoxicity, more cases of hypertension and dyslipidemia were reported after cyclosporine use, and a higher incidence of new-onset insulin-requiring diabetes was observed after tacrolimus use.50),51)
Antimetabolite agents
Antimetabolites, or antiproliferative agents, interfere with the synthesis of nucleic acids and exert their immunosuppressive effects by inhibiting the proliferation of both T and B lymphocytes.34) MMF has replaced azathioprine as the preferred antimetabolite agent in recent years on the basis of a clinical trial demonstrating a significant reduction in both mortality and the incidence of treatable rejection at 1 year with MMF versus azathioprine.52) Mycophenolate sodium is an enteric-coated, delayed-release formulation developed to improve upper gastrointestinal tolerability of MMF. Because of this coating, the tablet should not be crushed. It is therapeutically similar to MMF with respect to the prevention of biopsy-proven and treated acute rejection episodes, graft loss, or death.53) The following conversions between MMF and mycophenolate sodium should provide equimolar amounts of mycophenolic acid (MPA) (1,000 mg MMF = 720 mg mycophenolate sodium and 1,500 mg MMF = 1,080 mg mycophenolate sodium, respectively).
Proliferation signal inhibitors
A new class of drugs known as proliferation signal inhibitors (PSIs), or mammalian target of rapamycin (mTOR) inhibitors, has been used in selected patients with renal insufficiency, cardiac allograft vasculopathy (CAV), or malignancies in an attempt to reverse or slow progression of these conditions. The 2 agents in this class, sirolimus and everolimus, inhibit the proliferation of T cells, B cells, and vascular smooth muscle cells in response to growth factor and cytokine signals.34) Compared to azathioprine, both sirolimus and everolimus reduce the incidence of acute rejection and slow the development of CAV when used in conjunction with cyclosporine and prednisone in de novo heart transplant recipients.54),55) The main difference between sirolimus and everolimus is that the half-life of everolimus (30 hours) is approximately half that of sirolimus (60 hours). Compared to MMF, everolimus is not inferior with respect to the combined end point of rejection, graft loss, and death.56) MMF demonstrates less CAV progression as measured by intravascular ultrasound (IVUS) at 1 year.57) Other benefits of sirolimus include slowing of CAV progression and reduction in the incidence of clinically significant cardiac events.58) Also, when used in heart transplant recipients with significant renal impairment, sirolimus permits the minimization or complete withdrawal of the CNIs, resulting in notable improvements in renal function without an associated increased risk of rejection.59),60) Sirolimus is generally not initiated de novo after transplantation because of an increased risk of sternal wound dehiscence.61) However, on the basis of the benefits observed in clinical trials, sirolimus can be initiated later after transplantation for specific indications. The PSIs are often used in place of MMF in patients with rejection, allograft vasculopathy, malignancy, and viral infections such as CMV62) to prevent recurrence or progression. When used in place of CNI, the PSIs may prevent progression of renal dysfunction. In the Scandinavian Heart Transplant Everolimus De Novo Study with Early Calcineurin Inhibitor Avoidance (SCHEDULE) trial, everolimus was associated with significant improvement in renal function for de novo heart transplant recipients compared to cyclosporine. This was accompanied by a significant reduction in CAV progression and its incidence at 12 months post-transplant. However, there was significantly more acute rejection which could potentially counteract the aforementioned observed benefits.63)
Glucocorticoids
Glucocorticoids are non-specific anti-inflammatory agents that interrupt multiple steps in immune system activation, including antigen presentation, cytokine production, and proliferation of lymphocytes. Although steroids are highly effective for the prevention and treatment of acute rejection, their long-term use is associated with a number of adverse effects, including new-onset or worsening diabetes mellitus, hyperlipidemia, hypertension, fluid retention, myopathy, osteoporosis, and a predisposition toward opportunistic infections.34),43) While most programs employ glucocorticoids as one of the three maintenance immunosuppressive agents, they are used in relatively high doses in the early postoperative period but then tapered to low doses or discontinued altogether after the first 6 to 12 months.34)
DIAGNOSIS AND TREATMENT OF REJECTION
Diagnosis
Transplant rejection remains one of the major causes of death after HTx3) and is classified as hyperacute rejection, acute cellular rejection (ACR), or AMR. Because symptoms are often vague, routine testing for rejection is standard practice. Unlike renal or liver transplantation, there are no laboratory markers for rejection in HTx, and the endomyocardial biopsy (EMB) remains the cornerstone of rejection surveillance. Despite its limitations (sampling error, interobserver interpretation variability among pathologists, and invasiveness64)), EMB has remained the gold standard for the diagnosis of acute allograft rejection. It is performed via the right internal jugular vein or femoral vein by introducing a bioptome into the right ventricle and obtaining three to five pieces of endomyocardium, typically from the RV septum.34)
A new frontier in cardiac allograft rejection screening includes gene expression profiling (GEP).65) The AlloMap (CareDx, Brisbane, CA, USA) is a gene expression profile of peripheral blood that has been incorporated into the ISHLT guidelines.66) The test screens for a 20-gene panel with 11 rejection-related genes and 9 genes for normalization and quality control. The result is reported as a single score on a scale of 0 to 40, and indicates the probability of moderate or severe ACR based on findings from the Cardiac Allograft Rejection Gene Expression Observational (CARGO) study. The 11 genes were identified based on the highest and most statistically significant expression differences.67) In a randomized trial, GEP was shown to be non-inferior to biopsy in the diagnosis of ACR,68) and it was also useful early after transplantation.69) One role of GEP is to screen low-risk patients at predetermined intervals, with biopsies performed only if the GEP score is abnormal. However, patients with a history of, or risk factors for, AMR are not candidates for GEP screening, as the test has only been validated for ACR.34)
Hyperacute rejection
Although now uncommon, the development of hyperacute rejection was the most feared complication prior to the advent of prospective cross-matching and effective immunosuppressive therapy.43) Hyperacute rejection is mediated by preformed antibodies to the allograft in the recipient. It typically presents following surgical engraftment and restoration of native circulation as an almost immediate, aggressive and inevitably lethal immune attack on the organ. Hyperacute rejection manifests as severe graft failure within the first few minutes to hours after transplantation. Without inotropic agents and MCS, plasmapheresis, and intense immunosuppression, the recipient usually does not survive.34)
Acute cellular rejection
ACR, the most common form of rejection in heart transplant, is characterized by a predominantly T-cell mediated response with infiltration of macrophages and lymphocytes, which in turn can lead to myocyte necrosis. Histologically, ACR is defined by inflammatory infiltrates, which are typically lymphocyte-predominant with associated evidence of myocyte injury (Table 7).43),66),70)
Table 7. ISHLT standardized cardiac biopsy grading: ACR70) .
| Grade | Description | Prior classification |
|---|---|---|
| 0R | No rejection | 0 |
| 1R, mild | Interstitial and/or perivascular infiltrate with up to one focus of myocyte damage | 1A, 1B, 2 |
| 2R, moderate | Two or more foci of infiltrate with associated myocyte damage | 3A |
| 3R, severe | Diffuse infiltrate with multifocal myocyte damage ± edema ± hemorrhage ± vasculitis | 3B, 4 |
ACR = acute cellular rejection; ISHLT = International Society for Heart and Lung Transplantation; R = revised.
The diagnosis of ACR is made from EMB. The most recent ACR grading scale, which classifies rejection into mild (1R), moderate (2R) or severe (3R) grades has allowed standardization of reporting, although variability of interpretation and discordance between pathologists remains, particularly at higher grades of rejection. The main benefit of the new grading scale is that it allows improved guidance toward appropriate therapy, in conjunction with clinical assessments. Generally speaking, mild grades of rejection (ISHLT grade 1R) do not require augmentation of immunosuppressive therapy as the vast majority of these episodes resolve spontaneously, without any increased risk of subsequent poor outcomes. However, higher grades (≥2R) invariably require aggressive supplemental immunosuppression. ACR may occur at any time after HTx, but is most frequently seen in the first 6 months post-transplant. The initial risk of allograft rejection increases in the first 1–3 months after transplantation, and then rapidly decreases thereafter, merging with a low constant risk of rejection after 1 year. A number of risk factors have been identified for ACR: younger age of recipients, female sex (donors and recipients), higher number of HLA mismatches, black recipients, and use of induction therapy. The development of acute rejection requiring augmentation of immunosuppression leads to a higher incidence of CAV and mortality.3),43),66)
Antibody-mediated rejection
AMR develops when recipient antibodies are directed against donor-HLA antigens (and less so non-HLA antigens) on allograft endothelium, which initiates the complement cascade and causes tissue injury via inflammatory pathways. Complement and are deposited within the allograft microvasculature, resulting in an inflammatory process characterized by endothelial cell activation, macrophage infiltration, cytokine upregulation, increased vascular permeability, and microvascular thrombosis.43),71) The diagnosis of AMR remains technically challenging. A consensus on its definition and management has only recently been reached. AMR is divided into 3 grades of severity based on immunologic and histopathologic criteria: pAMR1(H+) or pAMR1(I+), pAMR2, and pAMR3 (Table 8).72)
Table 8. The 2013 ISHLT working formulation for the pathologic diagnosis of cardiac AMR.
| Grade | Definition | Substrates |
|---|---|---|
| pAMR 0 | Negative for pathologic AMR | Histologic and immunopathologic studies are both negative |
| pAMR 1 (H+) | Histopathologic AMR alone | Histologic findings are present and immunopathologic findings are negative |
| pAMR 1 (I+) | Immunopathologic AMR alone | Histologic findings are negative and immunopathologic findings are positive (CD68+ and/or C4d+) |
| pAMR 2 | Pathologic AMR | Histologic and immunopathologic findings are both present |
| pAMR 3 | Severe pathologic AMR | Interstitial hemorrhage, capillary fragmentation, mixed inflammatory infiltrates, endothelial cell pyknosis, and/or karyorrhexis, and marked edema and immunopathologic findings are present. These cases may be associated with profound hemodynamic dysfunction and poor clinical outcomes |
AMR = antibody-medicated rejection; ISHLT = International Society for Heart and Lung Transplantation.
Clinically, AMR most frequently presents during the first 1–2 months after transplantation, and is accompanied by a rise in donor-specific antibodies.43),73) In cases where AMR occurs within the first week post-transplant, the recipient usually has evidence of pre-sensitization to donor HLA antigens. In these early cases of AMR, the patient typically has accompanying graft dysfunction. When AMR occurs 1 year after transplantation, it is typically due to de novo donor-specific antibodies and the prognosis is poor, with increased mortality and associated CAV in these cases.43),74) Risk factors associated with the development of AMR include elevated pre-transplant PRAs, positive donor-specific crossmatch, development of de novo donor-specific antibodies post-transplant, female sex, CMV seropositivity, prior implantation of a VAD, and/or re-transplantation.43)
Treatment
The management of rejection proceeds in a stepwise fashion based on the severity of rejection detected on biopsy and the patient's clinical presentation (Table 9).34)
Table 9. Treatment of ACR and AMR.
| Asymptomatic | Reduced EF | HF/shock | |
|---|---|---|---|
| ACR grade ≥2R | - Target higher CNI levels | - Oral steroid bolus/taper | Treat based on clinical presentation; do not await biopsy findings |
| - Oral steroid bolus + taper | or | ||
| - MMF → PSI | - IV pulse steroids | ||
| AMR grade ≥2 with no/↓ DSA | - Target higher CNI levels | - IV pulse steroids | - IV pulse steroids |
| - MMF → PSI | - Consider IV Ig | - Cytolytic therapy | |
| - Plasmapheresis | |||
| AMR grade ≥2 with ↑ DSA | - Oral steroid bolus + taper | - IV pulse steroids | - IV immune globulin |
| - MMF → PSI | - IV Ig | - Inotropic therapy | |
| - Consider ATG, rituximab, bortezomib | - IV heparin | ||
| - IABP or ECMO support |
AMR = antibody-mediated rejection; ATG = anti-thymocyte globulin; CNI = calcineurin inhibitor; DSA = donor-specific antibody; ECMO = extra-corporeal membrane oxygenation; HF = heart failure; IABP = intra-aortic balloon pump; Ig = immunoglobulin; IV = intravenous; MMF = mycophenolate mofetil; PSI = proliferation signal inhibitor; R = revised.
Biopsies with grade 1R or AMR 1, in the absence of clinical or hemodynamic compromise, generally merit no intervention. More serious findings on biopsy, including grade 2R or higher and AMR 2 or higher warrant treatment. As shown in Table 9, the intensity of treatment depends on the patient's clinical presentation. If the patient is asymptomatic (no HF symptoms and normal LVEF), treatment options include: oral pulse steroids, targeting higher levels of immunosuppressive medications, switching from cyclosporine to tacrolimus or switching from MMF to a PSI. Given the equivalent success of intravenous (IV) and oral corticosteroid therapy for the treatment of asymptomatic ACR,75) an outpatient course of oral corticosteroids is often the first-line of treatment. For patients with HF symptoms or reduced EF, treatment is more aggressive, comprising IV corticosteroids and anti-thymocyte globulin cytolytic therapy. If there is evidence of grade AMR 2 or higher, patients will also often receive IV Ig. If donor-specific anti-HLA antibodies are present in the setting of AMR, patients may receive more intensive therapy with rituximab or bortezomib. Plasmapheresis may also be used in this setting. Finally, in patients presenting with cardiogenic shock, empiric aggressive treatment includes IV corticosteroids, cytolytic therapy, plasmapheresis, IV Ig, heparin, and hemodynamic support with IABP or even ECMO.34) Any rejection episode should prompt an investigation for precipitating causes such as infection, noncompliance, or drug interactions resulting in subtherapeutic immunosuppressive drug levels. A biopsy should be repeated 2 weeks after completion of treatment to document improvement or resolution of the rejection episode.
OUTPATIENT MANAGEMENT AND LONG-TERM COMPLICATIONS IN HEART TRANSPLANTATION
Cardiac allograft vasculopathy
CAV, best characterized as a diffuse immune-mediated pan-arteritis with concentric, longitudinal intimal thickening of the coronary arteries, remains a major cause of long-term morbidity and mortality after transplantation.43) The incidence of CAV varies widely as a result of differences in the definition of the disease and in patient populations. However, by various estimates, the CAV incidence ranges from 42% at 5 years to 50% at 10 years.34) CAV can occur as early as 1 year after transplantation, and this accelerated form of disease is more aggressive and associated with a worse prognosis. Even in patients without apparent angiographic epicardial disease, microvascular abnormalities may be present and are associated with adverse outcomes.76) Despite improvements in immunosuppression over the past three decades, the incidence of CAV has not significantly decreased, and its development continues to limit long-term survival in patients undergoing cardiac transplantation. Risk factors for CAV include a history of rejection, older age of the donor, the presence of donor-specific anti-HLA antibodies, ischemia-reperfusion injury, and traditional risk factors for coronary atherosclerosis. CAV often remains asymptomatic due to denervation of the donor heart, which blunts anginal pain. Symptomatic CAV may present with dyspnea, LV dysfunction, restrictive physiology, or even sudden cardiac death. The gold standard for diagnosis of CAV is the coronary angiogram, which can be performed annually. The maximal intimal thickness measured by IVUS at 1-year compared to baseline is a predictor for subsequent angiographic development of CAV and other poor long-term outcomes. Clinically apparent CAV is associated with a poor prognosis and, therefore, prevention is an important strategy. Medical strategies to abrogate CAV include the administration of statins77) and targeted use of PSIs such as sirolimus/everolimus. Interventional options for CAV include the placement of drug-eluting stents, but these are usually temporary measures. The only definitive solution is re-transplantation.
Infection
Infections are the major cause of death during the first postoperative year and remain a threat throughout the life of a chronically immunosuppressed patient. Infections in the first postoperative month are commonly bacterial and typically related to indwelling catheters and wound infections.34) A significant source of these infections are nosocomial organisms such as Legionella, Staphylococcus, Pseudomonas, Proteus, Klebsiella, and Escherichia coli. These infections typically present in the form of pneumonias, urinary tract infections, sternal wound infections and mediastinitis, and bacteremia. Late infections (those that occur 2 months to 1 year after transplantation) are more diverse. In addition to typical pathogens, transplant recipients are susceptible to viruses (particularly CMV78)), fungi79) (Aspergillus, Candida, and Pneumocystis spp.), and Mycobacterium, Nocardia, and Toxoplasma species. Effective therapy requires an extremely aggressive approach to obtaining a specific diagnosis. A background of experience in recognizing the more common clinical presentations of CMV, Aspergillus species, and other opportunistic infectious agents is also important. Infection surveillance is mainly clinical, but routine chest radiography often detects infections, especially fungal and mycobacterial pulmonary infections, that may be at an early and asymptomatic stage.
Balancing the risks of infection and rejection with immunosuppression relies on an understanding of the patient's global immune state. An immune-monitoring assay (ImmuKnow®; Viracor-IBT, Lee's Summit, MO, USA) performed on peripheral blood, which measures adenosine triphosphate (ATP) release from activated lymphocytes, may offer some guidance in profoundly immunosuppressed patients.80) Given the degree of immunosuppression, all transplant recipients receive antimicrobial prophylaxis for oral candidiasis, toxoplasmosis and pneumocystis, and CMV over the first post-transplant year. The use of vaccines in heart transplant recipients remains controversial. Live vaccines are definitely contraindicated because of the patients' immunosuppressed states. Even dead vaccines may pose a risk because they can promote activation of the immune system and cause rejection.81) At some centers, dead vaccines such as the influenza or pneumococcal vaccines are recommended only to patients more than 6 months after transplant and those with no history of rejection within the previous 6 months.
Post-heart transplantation malignancy
Malignancy is one of the most common causes of late mortality in heart transplant recipients.3) Malignancies are approximately 2- to 4-fold more common in heart transplant compared to renal transplant recipients. The enhanced risk of cancer among cardiac transplant recipients is thought to reflect the greater degree of immunosuppression that heart transplant recipients receive, possibly because of inherent immunologic requirements.34),82) All immunosuppressive agents are believed to contribute to the cumulative risk of malignancy, with the possible exception of corticosteroids. However, the PSIs may be associated with a decreased incidence and progression of malignancy compared to CNIs and antimetabolites.83),84) Cancer in solid organ transplant recipients usually presents at least 3 to 5 years after transplantation.34) Cutaneous malignancies are the most common type after HTx, and include mainly squamous cell and basal cell carcinomas. Risk factors common to the general population for the development of skin cancers include fair skin, previous history of skin cancer, and geographic location (in areas of high sun exposure). Specifically, in heart transplant recipients, more than 10% of adult heart transplant recipients developed a de novo malignancy between years 1 and 5 after transplantation, which was associated with an increase in mortality.85) The incidence of post-transplant de novo solid malignancy increased temporally, with the largest increase seen in the skin cancers. Long-term voriconazole use for treatment of fungal infections such as aspergillosis is known to be associated with a more increased risk of developing skin cancer.86)
Post-transplant lymphoproliferative disorder (PTLD), most commonly a B-cell lymphoma related to EBV infection, may occur after transplantation.87) More than 50% of patients with PTLD present with extranodal masses involving the gastrointestinal tract, lungs, skin, liver, central nervous system, and the allograft itself. Risk factors for the development of PTLD include the use of cytolytic therapy for induction88) and EBV serostatus (with EBV-seronegative recipients of EBV-seropositive donors being at the highest risk). Neoplasms common in the general population also occur in heart transplant recipients, including breast, lung, and prostate. Lung cancer is more common in heart and lung transplant recipients than in recipients of other solid organs, likely because smoking, a strong risk factor for lung cancer, may also contribute to end-stage heart and lung disease requiring transplantation.34)
The most critical factor in the treatment of malignancies is prevention. Heart transplant recipients should undergo routine health maintenance screenings with their primary care physicians, including mammograms, pap smears, prostate exams, and colonoscopies as indicated for non-transplant patients. In addition, patients are instructed to use sun protection and to establish care with a dermatologist for routine skin exams. Individualized immunosuppression strategies and enhanced cancer screening should be studied to determine whether they can reduce the adverse outcomes of post-transplantation malignancy.
FUTURE OF HEART TRANSPLANTATION
Rejection diagnostic methods
Early detection of rejection is crucial for post-transplant care. Despite progress in immunosuppression, ACR and AMR remain serious complications during and after the first post-transplant year.65) The current gold standard for diagnosis is EMB, although this is not ideal due to its invasiveness and associated risk, sampling error, and inter-reader variability. Therefore, there is currently an unmet need to establish an objective diagnostic test for cardiac allograft rejection.65)
Recently, cardiac magnetic resonance (CMR) has been investigated, and shows promise for the detection of allograft rejection with high sensitivity.89),90) CMR lacks ionizing radiation and can survey the entire myocardium, thus decreasing the possibility of false negatives due to sampling error as observed in EMB. In a study evaluating the diagnostic accuracy of CMR versus EMB for acute rejection, CMR had a high sensitivity (93%) and high negative predictive value (98%), highlighting that CMR could be utilized as a screening test before routine EMB.90) The presence of late gadolinium enhancement (LGE) has also been investigated in small cohorts,89) but its role in transplant screening is not clear in the absence of prospective data with larger sample sizes from multiple centers. Overall, CMR has the potential to detect early changes accompanying allograft rejection, and may be helpful in cases in which the biopsy is negative. However, much larger studies are needed for validation.43)
Another emerging technology in the noninvasive diagnosis of rejection involves cell-free DNA technology. Donor derived cell-free DNA (dd-cfDNA) is detectable in both the urine and blood of transplant recipients.91) After transplantation and organ engraftment, dd-cfDNA will reach its highest value, up to >5% of total cfDNA, with a rapid decrease to <0.5% within 1 week. During an episode of rejection, dd-cfDNA are shed into the blood and can be accompanied by a 5-fold increase that can be measured by current assays.65) The release of dd-cfDNA occurs earlier than other clinical markers of rejection and could potentially be used to monitor transplant patients at regular intervals to allow early diagnosis and treatment.92) This dd-cfDNA may be a candidate marker for the noninvasive diagnosis of graft injury, because increased levels of donor-derived DNA correlate with ACR events, as determined by EMB in early studies.93)
Immune tolerance
Immune tolerance would lessen the need for immunosuppression, and thus reduce immunosuppressant-related complications. Chimerism, defined as the existence of 2 allogeneic cell lines, remains the ultimate goal and would enable specific tolerance to donor antigens while simultaneously retaining the ability to fight infection and prevent malignancy.43) In recent years, researchers have attempted to establish central tolerance via transplantation of donor bone marrow. One study reported the findings of 6 human kidney transplant patients who were appropriately conditioned with non-myeloablative therapy including cyclophosphamide, ATG, and thymic irradiation. This study showed that bone marrow transplantation could induce tolerance without requiring immunosuppression. However, all reports of this method have resulted in a loss of mixed chimerism within months of transplantation,94) possibly due to the inflammatory response. A newer approach using bioengineered mobilized cellular products enriched for hematopoietic stem cells and tolerogenic graft facilitating cells combined with non-myeloablative conditioning, was employed in a recent study involving 19 kidney allograft recipients with highly mismatched donors.95) Thus far, 12 of the 19 patients have been effectively weaned off immunosuppression, with intact grafts and maintenance of stable mixed chimerism. In the future, this early success of induction of stable mixed chimerism across HLA barriers may be achievable in the regular clinical practice of HTx. Future tolerance induction research will depend on further investigation of the mechanism of tolerance, and further studies to increase safety and broaden the applicability of initial studies using enhanced stem cell transplantation.43)
Xenotransplantation
Xenotransplantation explores transplantation of organs between different species. Leonard Bailey at Loma Linda University performed the first cardiac xenotransplantation in 1984, transplanting a baboon's heart into an infant with hypoplastic left heart syndrome. The baby survived only 12 days, and ultimately died of multi-organ failure. Initially, the function of the transplanted organs was limited to only days or weeks, due to the powerful response of the human immune system against nonhuman donor antigens that could not be overcome by standard immunosuppression methods.96) However, with increasing numbers of patients in end-stage HF and the shortage of donors, there has been a renewed interest in the possibility of cardiac xenotransplantation. Advantages include an unlimited supply and elective availability of donor organs, expansion of the candidate pool, and avoidance of the detrimental effects of brain death on donor organ function.65),97),98) Although monkeys and baboons are most phylogenetically like humans, given the ecological and ethical restrictions, the pig is the only animal available to replace human tissues. The pig is physiologically similar to humans and its organ sizes are comparable. Pigs can be raised in controlled environments and, more importantly, can be genetically modified. The main limitations to xenotransplantation are rejection, including hyperacute, acute, and chronic vascular forms, and the risk of transmitting zoonoses. The development of genetically-modified pigs has allowed for the expression of human antigens that can reduce the risk of rejection and provide hope for future feasibility. With the introduction of galactose-α-1,3-galactose (Gal)-knockout pigs, prolonged survival especially in heart and kidney xenotransplantation, was recorded. However, remaining antibody barriers to non-Gal antigens continue to be the hurdle to overcome. The production of genetically engineered pigs was difficult and required a significant amount of time. However, advances in gene editing, such as zinc finger nucleases, transcription activator-like effector nucleases, and most recently, clustered regularly interspaced short palindromic repeats (CRISPR) technology have made the production of genetically engineered pigs easier and available to more researchers. Today, the survival of pig-to-nonhuman primate heterotopic heart xenotransplantation has reached 945 days.99) This required higher-than-standard immunosuppression therapy, including maintenance with anti-CD40 antibody. However, survival of orthotopic pig to nonhuman primate HTx grafts has so far been limited to less than 2 months, mostly as a result of perioperative cardiac xenograft dysfunction, which is believed to be distinct from acute rejection. Another concern in xenotransplantation is the risk of transmission of infection. Although numerous biological and logistical issues must be resolved before pig to human HTx can be undertaken, recent progress in the clinical testing of pig to human kidney and islet transplantation highlight future possibilities.96)
CONCLUSION
HTx has become the preferred standard therapy for patients with end-stage HF. Improvements in immunosuppressant therapy, donor procurement, surgical techniques, and post-HTx care have resulted in a substantial decrease in acute allograft rejection, which had previously significantly limited the survival of transplant recipients. However, limitations to long-term allograft survival exist, including rejection, infection, CAV, and malignancy. Nevertheless, with a careful balance of immunosuppressive therapy and vigilant surveillance for complications, we can expect further advances in the long-term outcomes of heart transplant recipients over the decades to come.
ACKNOWLEDGEMENTS
The authors express sincere gratitude to Korean representative transplant cardiologists (Dr. Jae-Joong Kim, Dr. Eun-Seok Jeon, Dr. Seok-Min Kang, Dr. Hae-Young Lee, Dr. Jin-Oh Choi and Dr. Hyun-Jai Cho) who are the founders of HTx in Korea.
Footnotes
Funding: This study was supported by the fund from the Korean Society of Cardiology (201703-03), by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6005448) and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0575).
Conflict of Interest: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist.
- Conceptualization: Kim IC, Youn JC, Kobashigawa JA.
- Data curation: Kim IC, Youn JC, Kobashigawa JA.
- Formal analysis: Kim IC, Youn JC, Kobashigawa JA.
- Funding acquisition: Kim IC, Kobashigawa JA.
- Investigation: Kim IC, Youn JC, Kobashigawa JA.
- Methodology: Kim IC, Youn JC, Kobashigawa JA.
- Project administration: Kim IC, Youn JC, Kobashigawa JA.
- Resources: Kim IC, Youn JC, Kobashigawa JA.
- Software: Kim IC, Youn JC, Kobashigawa JA.
- Supervision: Kobashigawa JA.
- Validation: Kim IC, Youn JC, Kobashigawa JA.
- Visualization: Kim IC, Youn JC.
- Writing - original draft: Kim IC, Youn JC.
- Writing - review & editing: Kim IC, Youn JC, Kobashigawa JA.
References
- 1.Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271–1274. [PubMed] [Google Scholar]
- 2.Reitz BA, Bieber CP, Raney AA, et al. Orthotopic heart and combined heart and lung transplantation with cyclosporin-A immune suppression. Transplant Proc. 1981;13:393–396. [PubMed] [Google Scholar]
- 3.Lund LH, Khush KK, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth Adult Heart Transplantation Report - 2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017;36:1037–1046. doi: 10.1016/j.healun.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Krittayaphong R, Ariyachaipanich A. Heart transplant in Asia. Heart Fail Clin. 2015;11:563–572. doi: 10.1016/j.hfc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang SS, Chu SH, Ko WJ. Clinical outcome of heart transplantation: experience at the National Taiwan University Hospital. Transplant Proc. 1996;28:1733–1734. [PubMed] [Google Scholar]
- 6.Chawalit O, Meunmai S, Kittichai L, et al. The first successful heart transplantation in South East Asia. Rinsho Kyobu Geka. 1988;8:480–483. [PubMed] [Google Scholar]
- 7.Jung SH, Kim JJ, Choo SJ, Yun TJ, Chung CH, Lee JW. Long-term mortality in adult orthotopic heart transplant recipients. J Korean Med Sci. 2011;26:599–603. doi: 10.3346/jkms.2011.26.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017;47:16–24. doi: 10.4070/kcj.2016.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng AK, Jim MH, Yip GW, Ng PY, Fan K. Long term survival and prevalence of cardiac allograft vasculopathy in Chinese adults after heart transplantation - a retrospective study in Hong Kong. Int J Cardiol. 2016;220:787–788. doi: 10.1016/j.ijcard.2016.06.319. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima N, Ono M, Saiki Y, Sawa Y, Nunoda S, Isobe M. Registry report on heart transplantation in Japan (June 2016) Circ J. 2017;81:298–303. doi: 10.1253/circj.CJ-16-0976. [DOI] [PubMed] [Google Scholar]
- 11.Lee KF, Lin CY, Tsai YT, et al. The status of heart transplantation in Taiwan, 2005–2010. Transplant Proc. 2014;46:934–936. doi: 10.1016/j.transproceed.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Oh BH. Heart transplantation in Asia. Circ J. 2017;81:617–621. doi: 10.1253/circj.CJ-17-0162. [DOI] [PubMed] [Google Scholar]
- 13.Smits JM, de Vries E, De Pauw M, et al. Is it time for a cardiac allocation score? First results from the Eurotransplant pilot study on a survival benefit-based heart allocation. J Heart Lung Transplant. 2013;32:873–880. doi: 10.1016/j.healun.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Davies RR, Farr M, Silvestry S, et al. The new United States heart allocation policy: progress through collaborative revision. J Heart Lung Transplant. 2017;36:595–596. doi: 10.1016/j.healun.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Kittleson MM. Changing role of heart transplantation. Heart Fail Clin. 2016;12:411–421. doi: 10.1016/j.hfc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant. 2012;12:3213–3234. doi: 10.1111/j.1600-6143.2012.04258.x. [DOI] [PubMed] [Google Scholar]
- 17.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007;84:1556–1562. doi: 10.1016/j.athoracsur.2007.05.095. [DOI] [PubMed] [Google Scholar]
- 18.Meyer DM, Rogers JG, Edwards LB, et al. The future direction of the adult heart allocation system in the United States. Am J Transplant. 2015;15:44–54. doi: 10.1111/ajt.13030. [DOI] [PubMed] [Google Scholar]
- 19.Kobashigawa JA, Johnson M, Rogers J, et al. Report from a forum on US heart allocation policy. Am J Transplant. 2015;15:55–63. doi: 10.1111/ajt.13033. [DOI] [PubMed] [Google Scholar]
- 20.Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014;33:327–340. doi: 10.1016/j.healun.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant. 2009;28:213–225. doi: 10.1016/j.healun.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobashigawa J, Crespo-Leiro MG, Ensminger SM, et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2011;30:252–269. doi: 10.1016/j.healun.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Jeon ES, Kang SM, Kim JJ. Initial report of the Korean Organ Transplant Registry (KOTRY): heart transplantation. Korean Circ J. 2017;47:868–876. doi: 10.4070/kcj.2016.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IC, Youn JC. Understanding the current status of Korean heart transplantation based on initial KOTRY report. Korean Circ J. 2017;47:858–860. doi: 10.4070/kcj.2017.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 28.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017;47:555–643. doi: 10.4070/kcj.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 31.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 32.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 34.Kittleson MM, Patel JK, Kobashigawa JA. Chapter 72: cardiac transplantation. In: Fuster V, Harrington RA, Narula J, Eapen ZJ, editors. Hurst's the Heart. 14th ed. New York (NY): McGraw-Hill; 2017. [Google Scholar]
- 35.Huprikar S, Danziger-Isakov L, Ahn J, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant. 2015;15:1162–1172. doi: 10.1111/ajt.13187. [DOI] [PubMed] [Google Scholar]
- 36.Shin HS, Cho HJ, Jeon ES, et al. The impact of hepatitis B on heart transplantation: 19 years of national experience in Korea. Ann Transplant. 2014;19:182–187. doi: 10.12659/AOT.889680. [DOI] [PubMed] [Google Scholar]
- 37.Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 38.Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am J Transplant. 2015;15:642–649. doi: 10.1111/ajt.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobashigawa J, Khush K, Colvin M, et al. Report from the American Society of Transplantation conference on donor heart selection in adult cardiac transplantation in the United States. Am J Transplant. 2017;17:2559–2566. doi: 10.1111/ajt.14354. [DOI] [PubMed] [Google Scholar]
- 40.Zaroff JG, Rosengard BR, Armstrong WF, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28–29, 2001, Crystal City, Va. Circulation. 2002;106:836–841. doi: 10.1161/01.cir.0000025587.40373.75. [DOI] [PubMed] [Google Scholar]
- 41.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385:2585–2591. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 42.Ardehali A, Esmailian F, Deng M, et al. PROCEED II trial investigators Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385:2577–2584. doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 43.Kobashigawa J. Clinical Guide to Heart Transplantation. Los Angeles (CA): Springer; 2017. [Google Scholar]
- 44.Awad M, Czer LS, Hou M, et al. Early denervation and later reinnervation of the heart following cardiac transplantation: a review. J Am Heart Assoc. 2016;5:e004070. doi: 10.1161/JAHA.116.004070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo MJ, Iribarne A, Hong KN, et al. Factors associated with primary graft failure after heart transplantation. Transplantation. 2010;90:444–450. doi: 10.1097/TP.0b013e3181e6f1eb. [DOI] [PubMed] [Google Scholar]
- 46.Russo MJ, Chen JM, Sorabella RA, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2007;133:554–559. doi: 10.1016/j.jtcvs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Briasoulis A, Inampudi C, Pala M, Asleh R, Alvarez P, Bhama J. Induction immunosuppressive therapy in cardiac transplantation: a systematic review and meta-analysis. Heart Fail Rev. 2018 doi: 10.1007/s10741-018-9691-2. [DOI] [PubMed] [Google Scholar]
- 48.Grimm M, Rinaldi M, Yonan NA, et al. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. Am J Transplant. 2006;6:1387–1397. doi: 10.1111/j.1600-6143.2006.01300.x. [DOI] [PubMed] [Google Scholar]
- 49.Kobashigawa JA, Miller LW, Russell SD, et al. Study Investigators Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6:1377–1386. doi: 10.1111/j.1600-6143.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 50.Taylor DO, Barr ML, Radovancevic B, et al. A randomized, multicenter comparison of tacrolimus and cyclosporine immunosuppressive regimens in cardiac transplantation: decreased hyperlipidemia and hypertension with tacrolimus. J Heart Lung Transplant. 1999;18:336–345. doi: 10.1016/s1053-2498(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 51.Ye F, Ying-Bin X, Yu-Guo W, Hetzer R. Tacrolimus versus cyclosporine microemulsion for heart transplant recipients: a meta-analysis. J Heart Lung Transplant. 2009;28:58–66. doi: 10.1016/j.healun.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Kobashigawa J, Miller L, Renlund D, et al. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Transplantation. 1998;66:507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 53.Kobashigawa JA, Renlund DG, Gerosa G, et al. Similar efficacy and safety of enteric-coated mycophenolate sodium (EC-MPS, myfortic) compared with mycophenolate mofetil (MMF) in de novo heart transplant recipients: results of a 12-month, single-blind, randomized, parallel-group, multicenter study. J Heart Lung Transplant. 2006;25:935–941. doi: 10.1016/j.healun.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 55.Keogh A, Richardson M, Ruygrok P, et al. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694–2700. doi: 10.1161/01.CIR.0000136812.90177.94. [DOI] [PubMed] [Google Scholar]
- 56.Eisen HJ, Kobashigawa J, Starling RC, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1203–1216. doi: 10.1111/ajt.12181. [DOI] [PubMed] [Google Scholar]
- 57.Kobashigawa JA, Pauly DF, Starling RC, et al. A2310 IVUS Substudy Investigators Cardiac allograft vasculopathy by intravascular ultrasound in heart transplant patients: substudy from the Everolimus versus mycophenolate mofetil randomized, multicenter trial. JACC Heart Fail. 2013;1:389–399. doi: 10.1016/j.jchf.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Mancini D, Pinney S, Burkhoff D, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 59.Cabezón S, Lage E, Hinojosa R, Ordóñez A, Campos A. Sirolimus improves renal function in cardiac transplantation. Transplant Proc. 2005;37:1546–1547. doi: 10.1016/j.transproceed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Bestetti R, Theodoropoulos TA, Burdmann EA, Filho MA, Cordeiro JA, Villafanha D. Switch from calcineurin inhibitors to sirolimus-induced renal recovery in heart transplant recipients in the midterm follow-up. Transplantation. 2006;81:692–696. doi: 10.1097/01.tp.0000177644.45192.a3. [DOI] [PubMed] [Google Scholar]
- 61.Kuppahally S, Al-Khaldi A, Weisshaar D, et al. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant. 2006;6:986–992. doi: 10.1111/j.1600-6143.2006.01282.x. [DOI] [PubMed] [Google Scholar]
- 62.Kobashigawa J, Ross H, Bara C, et al. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis. 2013;15:150–162. doi: 10.1111/tid.12007. [DOI] [PubMed] [Google Scholar]
- 63.Andreassen AK, Andersson B, Gustafsson F, et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: a randomized trial. Am J Transplant. 2014;14:1828–1838. doi: 10.1111/ajt.12809. [DOI] [PubMed] [Google Scholar]
- 64.Kim IC, Oh J, Lee CJ, Kim JY, Youn YN, Kang SM. Bioptome perforation at superior vena cava anastomosis site in transplanted heart. Korean Circ J. 2017;47:538–539. doi: 10.4070/kcj.2017.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber BN, Kobashigawa JA, Givertz MM. Evolving areas in heart transplantation. JACC Heart Fail. 2017;5:869–878. doi: 10.1016/j.jchf.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 67.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–160. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 68.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 69.Kobashigawa J, Patel J, Azarbal B, et al. Randomized pilot trial of gene expression profiling versus heart biopsy in the first year after heart transplant: early invasive monitoring attenuation through gene expression trial. Circ Heart Fail. 2015;8:557–564. doi: 10.1161/CIRCHEARTFAILURE.114.001658. [DOI] [PubMed] [Google Scholar]
- 70.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Colvin MM, Cook JL, Chang P, et al. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2015;131:1608–1639. doi: 10.1161/CIR.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 72.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2013;32:1147–1162. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Tambur AR, Pamboukian SV, Costanzo MR, et al. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–1025. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]
- 74.Coutance G, Ouldamar S, Rouvier P, et al. Late antibody-mediated rejection after heart transplantation: mortality, graft function, and fulminant cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34:1050–1057. doi: 10.1016/j.healun.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Kobashigawa JA, Stevenson LW, Moriguchi JD, et al. Is intravenous glucocorticoid therapy better than an oral regimen for asymptomatic cardiac rejection? A randomized trial. J Am Coll Cardiol. 1993;21:1142–1144. doi: 10.1016/0735-1097(93)90237-u. [DOI] [PubMed] [Google Scholar]
- 76.Kobashigawa JA, Tobis JM, Starling RC, et al. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 77.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 78.Youn JC, Nahm JH, Kang SM. Duodenal cancer after cardiac transplantation? Heart. 2013;99:1304. doi: 10.1136/heartjnl-2013-303983. [DOI] [PubMed] [Google Scholar]
- 79.Kim SH, Ha YE, Youn JC, et al. Fatal scedosporiosis in multiple solid organ allografts transmitted from a nearly-drowned donor. Am J Transplant. 2015;15:833–840. doi: 10.1111/ajt.13008. [DOI] [PubMed] [Google Scholar]
- 80.Kobashigawa JA, Kiyosaki KK, Patel JK, et al. Benefit of immune monitoring in heart transplant patients using ATP production in activated lymphocytes. J Heart Lung Transplant. 2010;29:504–508. doi: 10.1016/j.healun.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 81.Schaffer SA, Husain S, Delgado DH, Kavanaugh L, Ross HJ. Impact of adjuvanted H1N1 vaccine on cell-mediated rejection in heart transplant recipients. Am J Transplant. 2011;11:2751–2754. doi: 10.1111/j.1600-6143.2011.03743.x. [DOI] [PubMed] [Google Scholar]
- 82.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371–1373. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 83.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 84.Salgo R, Gossmann J, Schöfer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10:1385–1393. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 85.Youn JC, Stehlik J, Wilk AR, et al. Temporal trends of de novo malignancy development after heart transplantation. J Am Coll Cardiol. 2018;71:40–49. doi: 10.1016/j.jacc.2017.10.077. [DOI] [PubMed] [Google Scholar]
- 86.Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014;58:997–1002. doi: 10.1093/cid/cit940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342:1514–1516. doi: 10.1016/s0140-6736(05)80084-4. [DOI] [PubMed] [Google Scholar]
- 88.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323:1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 89.Taylor AJ, Vaddadi G, Pfluger H, et al. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur J Heart Fail. 2010;12:45–51. doi: 10.1093/eurjhf/hfp174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butler CR, Savu A, Bakal JA, et al. Correlation of cardiovascular magnetic resonance imaging findings and endomyocardial biopsy results in patients undergoing screening for heart transplant rejection. J Heart Lung Transplant. 2015;34:643–650. doi: 10.1016/j.healun.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 91.Lo YM, Tein MS, Pang CC, Yeung CK, Tong KL, Hjelm NM. Presence of donor-specific DNA in plasma of kidney and liver-transplant recipients. Lancet. 1998;351:1329–1330. doi: 10.1016/s0140-6736(05)79055-3. [DOI] [PubMed] [Google Scholar]
- 92.Beck J, Oellerich M, Schulz U, et al. Donor-derived cell-free DNA is a novel universal biomarker for allograft rejection in solid organ transplantation. Transplant Proc. 2015;47:2400–2403. doi: 10.1016/j.transproceed.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 93.De Vlaminck I, Valantine HA, Snyder TM, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra77. doi: 10.1126/scitranslmed.3007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl Int. 2008;21:1118–1135. doi: 10.1111/j.1432-2277.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 95.Leventhal JR, Elliott MJ, Yolcu ES, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99:288–298. doi: 10.1097/TP.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 96.Stehlik J, Kobashigawa J, Hunt SA, Reichenspurner H, Kirklin JK. Honoring 50 years of clinical heart transplantation in Circulation: in-depth state-of-the-art review. Circulation. 2018;137:71–87. doi: 10.1161/CIRCULATIONAHA.117.029753. [DOI] [PubMed] [Google Scholar]
- 97.Murthy R, Bajona P, Bhama JK, Cooper DK. Heart xenotransplantation: historical background, experimental progress, and clinical prospects. Ann Thorac Surg. 2016;101:1605–1613. doi: 10.1016/j.athoracsur.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 98.Kobashigawa JA. The future of heart transplantation. Am J Transplant. 2012;12:2875–2891. doi: 10.1111/j.1600-6143.2012.04223.x. [DOI] [PubMed] [Google Scholar]
- 99.Ekser B, Li P, Cooper DK. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant. 2017;22:513–521. doi: 10.1097/MOT.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]