Abstract
Background:
Hepatocellular carcinoma (HCC) is a high incidence disease in Egypt with a poor prognosis and survival. Biomarkers are important for diagnosis of HCC at an early stage. Osteopontin (OPN), a glycoprotein secreted by macrophages, osteoblasts, and T cells, is also highly expressed in a variety of tumors, such as examples in the breast, colon, and stomach. The present study aimed to correlate the serum level of OPN in HCV-positive hepatocellular carcinoma patients, with OPN expression in tumor and non-tumor liver tissues in order to identify its efficacy as a biomarker for diagnosis.
Material and Methods:
Out of total of 146 patients, 80 were selected for inclusion in the study. Blood samples as well as specimens of tumor and non-tumor liver tissue were collected. In addition, blood samples from 20 healthy volunteers were obtained as controls. Serum OPN and alpha-fetoprotein (AFP) were evaluated by ELISA for HCC and control groups. OPN and AFP gene expression were examined by real-time PCR, after homogenization and DNA extraction from serum samples and liver tissues.
Results:
It was found that serum OPN levels were significantly higher in the HCC group compared to normal group (P=0.009), with a strong positive correlation with AFP expression. However, there was no significant difference between OPN expression in tumor and non-tumor liver tissue.
Conclusion:
Serum OPN is highly suggested to be a professional candidate for HCC early diagnosis, with a diagnostic ability and accuracy equal or higher than for AFP.
Keywords: Osteopontin, alpha-fetoprotein, hepatocellular carcinoma, real-time PCR
Introduction
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver that occurs predominantly in patients with underlying chronic liver disease and cirrhosis (Balogh et al., 2016). The incidence of HCC is highest in Asia and Africa, where the endemic high prevalence of hepatitis B and hepatitis C strongly predisposes to the development of chronic liver disease and subsequent development of HCC (Mohamed et al., 2015).
In Egypt, according to the recent study carried out by the National Population-Based Cancer Registry Program, in 2014, liver cancer was ranked first, among cancers in Egyptian males (33%) and females (13.5%) (Ibrahim et al., 2014).
The main problem physicians are facing in the management of HCC patients is that the available biomarkers are not sufficiently specific and sensitive, at the same time, there is imminent need for novel circulating biomarkers to increase disease-free survival rate (Wen-Hsien et al., 2012). Although liver biopsy is a diagnostic procedure for HCC, its limitations include being invasive, accompanied by pain and high risk of infection and spread of malignant cells. The identification of a biochemical marker with better sensitivity and/or specificity than alpha-fetoprotein could extremely be helpful in improving early diagnosis of HCC (Bruix and Sherman, 2011).
Osteopontin (OPN) is a phosphorylated glycoprotein secreted by macrophages, osteoblasts and T cells. The OPN gene is located on Chromosome 4 region 22 (4q22.1) as shown in (Figure 1) (Nagoshi, 2014). OPN expression has been detected in many different organs and has been attributed to many pathological conditions, including inflammation, angiogenesis, fibrosis, and carcinogenesis (Wen et al., 2016).
Figure 1.
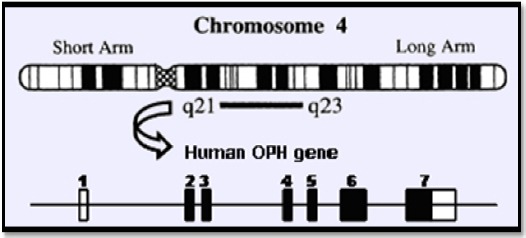
OPN Gene Located on Chromosome 4 Region 22 (4q22.1).
In this study, we aimed to correlate serum level of OPN in HCV- positive hepatocellular carcinoma patients, with OPN gene expression in tumor and non-tumor liver tissue, in order to evaluate its role in HCC development and its use as a biomarker for development of HCC.
Materials and Methods
Patients
After an informed consent, a group of 80 patients diagnosed as HCC undergoing liver resection, were included in this study. They presented to the Outpatient Clinic at the National Hepatology and Tropical Medicine Research Institute and at the National Cancer Institute, Cairo University and also at the outpatient clinic of Theodore Bilharz Research Institute during the period from Jan 2015 to October 2016. A total of 146 patients were examined, out of which 80 were included in the study. Blood samples as well as specimens from tumor and non-tumor liver tissue, were collected.
Inclusion criteria, for HCC patients in the study, consisted of a definite diagnosis of HCC that was confirmed pathologically and radio-logically, clinical stage A and B (early stage), according to Barcelona Clinic Liver Cancer Staging, (Llovet et al., 1999), chronic HCV cirrhosis and prior to any therapeutic intervention. Exclusion criteria included negative serum markers for HCV IgG and/or HCV RNA, positive HBsAg and/or HBV DNA, the presence of prolonged obstructive jaundice, Child Pugh C, benign liver tumors, metastatic HCC, signs of liver cell failure and hepatic encephalopathy. The clinical and laboratory data of all patients were collected from the hospitals files.
The control group consisted of twenty apparently healthy volunteers, age and sex matched, who were collected from the staff of the National Cancer Institute, Cairo University and Theodore Bilharz Research Institute (TBRI), Giza. These individuals consisted of healthy subjects without a history of liver disease, coronary artery disease, or other chronic diseases. The study was carried out in full accordance with the Helsinki Declaration of 1975, as revised in 1983, and was approved by the Institutional Ethics Committee. Informed consent for future evaluation of blood samples for scientific purposes was obtained from all subjects at the time of sample collection.
Methods
All the participants were subjected to full history taking, abdominal ultrasonography, and laboratory evaluation mainly Albumin (ALB), Bilirubin, Aspartate aminotransferase (AST), Alanine transaminase (ALT), Urea, HCV-RNA by PCR, HCV antibody determined with an EIA third-generation testing kit (Ortho-Clinical Diagnostics, Tokyo, Japan), serum Alpha- fetoprotein(AFP) and OPN levels.
Ten ml of venous blood sample was collected from each patient and serum was separated by centrifugation for 15 minute at 1,000 × g to determine the liver functions and serum OPN and alpha-fetoprotein then stored at -80°C. Only serum samples were collected from control group.
Liver tissue specimens were collected after liver resection, from tumor and non-tumor tissues. Specimens were divided into 3 parts, one for pathologic examination including tumor and non-tumor tissues, second specimen from the tumor tissue and third one from the non-tumor tissue. The first specimen was immersed in 10% formaldehyde in normal saline, the second and third specimens were processed for RNA extraction.
Assessment of Serum OPN and AFP by ELISA
All serum samples were tested for OPN and AFP by Enzyme-Linked Immunosorbent Assay (ELISA) method according to manufacturer’s instructions using Human Osteopontin assay kit, and Human Alpha-fetoprotein assay kit, (Sunlong Biotech Co., Ltd). Briefly, 100 μl of prepared standards and samples were added to appropriate wells of ELISA plate and then assayed according to the manufacturer’s instructions. The absorbance was measured at 450 nm in a microtest plate spectrophotometer, (Abcamm CA, USA) and OPN and AFP levels were quantified with a calibration curve using human OPN and AFP as a standard. Each standard or sample was assayed in duplicate.
Real-Time PCR for OPN and Alpha-fetoprotein in Serum and HCC liver tissue
From serum samples, RNA was extracted using Abbott msample preparation system kit, cat.no (02K02-96) (Abbott Molecular, Inc., Des Plaines, IL) according to manufacturer’s protocol, and stored at -80°C. However, liquid nitrogen was added firstly to the tumor and non-tumor tissues which were grinded separately, then 1 ml mlysis buffer was added (Abbott Molecular, Inc., Des Plaines, IL), total RNA was extracted from lysed serum and tissue using the magnetic beads technique as described according to Abbott msample preparation system kit.
The reverse transcription step using High-capacity cDNA reverse transcritpion kit (AB Applied Biosystems, Foster City, CA, USA). The RT master mix was performed with 20 µl reaction mixture containing; 2 µl reverse transcription buffer (10x), 0.8 µl dNTPs (25x), 1 µl multiscribe™ reverse transcriptase (50 U/μl), 1 µl RNase inhibitor, 3.2 µl H2O, 2 µl random primers (10x), 10 µl of RNA sample (5µg). The PCR thermal cycler (BioRad-T 100, Singapore) program was as follows: 10 min at 25°C, 120 min at 37°C, and 5 min at 85°C.
Real-time PCR amplification was performed at a final volume of 25 µl containing 15 µl of TaqMan universal master mix II, no UNG (cat. no. 4440043) (AB Applied Biosystems, Foster City, CA, USA), 5 µM of each primer, 0.5 unit of AmpliTaq DNA polymerase (Invitrogen). Primers used for OPN and GAPDH are shown in Table (1). The real time PCR (AB Applied Biosystems, Foster City, CA, USA) program was initiated by 10 min at 95°C, followed by 15 sec at 95°C, then 60 sec at 60°C, and the last two steps were repeated 40 times. All Real time PCR results were analyzed using relative quantification method with the formula 2-∆∆Ct (Akin et al., 2012).
Table 1.
Primers and Probes Sequence Used in the Study
| Primer | Nucleotide position* |
|---|---|
| OPN forward primer | 5′-CAA ATACCCAGATGCTGTGGC-3′ |
| OPN reverse primer | 5′-TCCTGGCTGTCCACATGGTC-3′ |
| AFP forward primer | 5′AGCGAGGAG AAATGGTCCGG-3′ |
| AFP reverse primer | 5′GGACATCTTCACCATGTGG-3′ |
| GAPDH forward primer | 5′-CTTAGCACCCCTCCCCAAG-3′ |
| GAPDH reverse primer | 5′- GATGTTCTGGAGAGCCCCG-3′ |
| OPN TaqMan probe | 5′-GACCCTACTCAGAAGCAGAATCTCCTAGCC-3′ |
| AFP TaqMan probe | 5′-TGGGACCCGAACTTTCCAAGCCATC- 3′. |
| GAPDH TaqMan probe | 5′-CATGCCATCACTGCCACCCAGAAGA-3′ |
OPN, Osteopontin; AFP, alpha fetoprotein; GAPDH, Human glyceraldehyde-3-phosphate dehydrogenase (housekeeping gene);
Ref. Huang et al., (2012)
Histopathological examination Histological Study
Paraffin blocks were made and 4 microns thick sections were stained with hematoxylin and eosin stain (H and E stain) for routine histopathological examination, grading and staging of hepatitis activity and tumor grade. Sections were also stained by Masson’s trichrome stain for assessment of fibrosis stage using METAVIR scoring system (Bedossa et al., 1994).
Grade of hepatitis activity; based on amount of inflammation
A1:- mild activity, A2:- moderate activity, A3:- severe activity.
Stage of fibrosis; representing amount of fibrosis or scarring
F1:-portal fibrosis without septa, F2: portal fibrosis with few septa, F3: numerous septa without cirrhosis, F4: cirrhosis.
HCC grade was done according to the WHO classification of tumors of the liver and intrahepatic bile ducts (Bosman, 2010) into
Grade 1: (well differentiated)
Grade 2: (Moderately differentiated)
Grade 3: (Poorly differentiated)
Statistical analysis
Analysis of the data was carried out with SPSS 18 statistical software (SPSS Inc., Chicago, IL, USA). Data were presented as the mean ± standard deviation (± SD). Comparisons of quantitative variables between two groups were analyzed using Mann Whitney U test and Chi-square test. P value <0.05 was considered statistically significant, and highly significant at p<0.001. Sensitivity and specificity of the test was determined using the receiver operating characteristic (ROC) curve to determine the optimum cut-off value for the studied diagnostic biomarker. In addition, the accuracy was measured by the area under curve (AUC).
Results
Eighty patients with HCC were included in this study, 52 males (65%) and 28 females (35%). Twenty age and sex matched, healthy volunteers were included as the control group. There were no statistical differences between patients and controls in respect to age and gender. HCC patients were divided according to tumor grade as follows: 39 in grade I, 22 in grade II and 19 patients in grade III. The clinico-pathological and laboratory parameters of the patients and control group are given in Tables (2 and 3). Liver functions tests showed significant differences between patients and controls as the mean Albumin, Bilirubin, AST and ALT levels (P value=0.0195, 0.0286, 0.007 and 0.004 respectively). All patients were positive for HCV IgG Abs (100%).
Table 2.
Clinico-Pathological Characteristics of HCC Patients
| Item | No | % |
|---|---|---|
| Total No of Patients | 80 | 100 |
| Child-Pugh grade | ||
| A | 45 | 56.25 |
| B | 26 | 32.5 |
| C | 9 | 11.25 |
| Tumor Stage (BCLC) | ||
| Stage 0 | 0 | 0 |
| Stage 1 | 2 | 2.5 |
| Stage 2 | 67 | 83.75 |
| Stage 3 | 6 | 7.5 |
| Stage 4 | 5 | 6.25 |
| Multiplicity of focal lesions | ||
| Single | 48 | 60 |
| Multiple | 32 | 40 |
| Portal Vein Thrombosis | ||
| Yes | 18 | 22.5 |
| No | 62 | 77.5 |
| Ascites | ||
| Yes | 16 | 20 |
| No | 64 | 80 |
Table 3.
Clinical and Laboratory Findings of HCC Patients and Control Group
| parameters | HCC Patients n=80 | Healthy Volunteers n=20 | P value |
|---|---|---|---|
| Age (years), ±SD | 57.71± 3.03 | 40.66± 8.02 | NS |
| Sex (percentage) | 65% male: 35% female | 70% male: 30% female | NS |
| ALB g/dL | 1.98± 0.22 | 3.7 ± 0.64 | 0.0195* |
| Total Bilirubin mg/dl | 8.97± 1.01 | 3.62± 0.22 | 0.0286* |
| AST IU/L | 71.36± 3.13 | 23.56± 2.55 | 0.007* |
| ALT IU/L | 66.42± 5.01 | 22.27± 2.51 | 0.004* |
| HCV (percentage) | 80 (100%) | 0 | |
| Tumor grade I/II/III | 39/22/19 | - |
Values are represented as mean ± S.D, (n); number of patients, HCC , hepatocellular carcinoma; ALB, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HCV, hepatitis C; NS, non-significant;
significant at p<0.05
Osteopontin concentration in HCC serum samples
The serum levels in the HCC group and healthy control group measured by ELISA test are displayed in Figure 2. Serum OPN levels were significantly higher in HCC group compared to control group (P≤0.0096). The mean value of serum OPN concentration expressed in (ng/ml) was 42.74± 3.56 SD.
Figure 2.
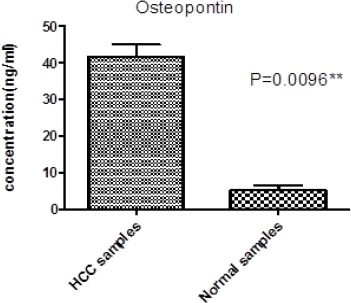
Represents Osteopontin Concentration in HCC Serum and Normal Samples, * significant at p<0.05, ** highly significant at p≤0.001 by Mann-Whitney U test.
Osteopontin expression in HCC and control (serum and tissue) samples
The expression of OPN in the serum samples of HCC, and the control group are displayed in Table 4, in addition to the tissue samples of HCC group. The expression of OPN in the serum of HCC group was significantly higher than control group (P value=0.0350). However, there was no significant difference between OPN expression in tumor tissue and non-tumor tissue.
Table 4.
Relative Expression of OPN in Serum and Tissue
| RQ=2^-∆ Ct | HCC samples | Control samples | P value |
|---|---|---|---|
| Osteopontin Gene Expression in serum | 76.70 ± 5.24 | 23.03 ± 2.54 | 0.035* |
| Osteopontin Gene Expression in tissue | 31.89 ± 2.78 | 22.16 ± 1.57# | NS |
Values are showed as mean ± S.D; Data are presented as mean of relative quantitation (RQ = 2^ -ΔCt);
significant at p≤0.05 by Mann-Whitney U test; # non-tumor liver tissue
Alpha-fetoprotein expression in HCC and control samples
The AFP expression levels in the HCC group and healthy normal group are displayed in Figure 3. Serum AFP expression levels were significantly higher in HCC group compared to normal group (p=0.004).
Figure 3.
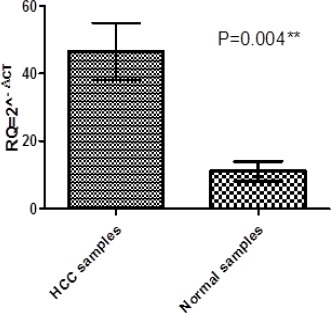
Represents Alpha-Fetoprotein Expression in Serum of Patients and Healthy Volunteers, each Column Represents the Relative Amount of Alpha-Fetoprotein, data are presented as mean of relative quantitation (RQ = 2^ (−ΔCt)), * significant at p<0.05, ** highly significant at p≤0.001 by Mann-Whitney U test.
Correlation between Osteopontin and Alpha-fetoprotein expression
The correlation analysis between the expression of OPN and AFP is presented in Figure 4. There was a strong positive correlation between OPN expression and AFP expression where spearman’s rank= 0.754 and p=0.0072.
Figure 4.
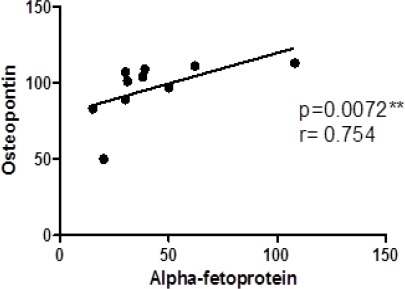
Correlation Analysis between Osteopontin and Alpha-Fetoprotein. Data are presented as mean of relative quantitation (RQ = 2(−ΔΔCt), r; Spearman’s rank, * significant at p<0.05, ** highly significant at p≤0.001.
Figure 5.
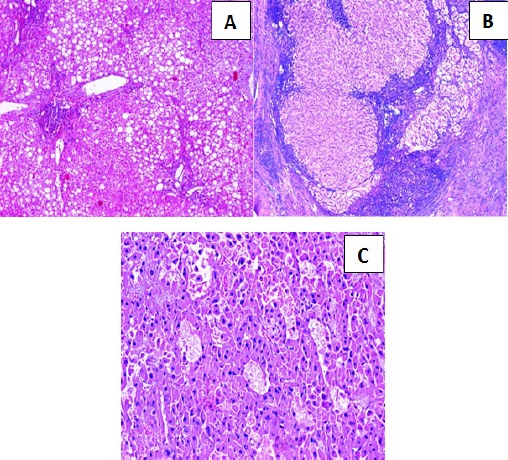
A- Section in chronic hepatitis C with Grade II activity and Stage 3 fibrosis, showing marked steatosis (Hematoxylin and eosin stain, X100). B- Section in chronic hepatitis C with Grade III activity and Stage 4 fibrosis (cirrhosis), showing well defined regenerating nodules surrounded by fibrous tissue (Hematoxylin and eosin stain, X100). C- Section in post-HCV hepatocellular carcinoma of moderate differentiation, showing solid and acinar patterns. (Hematoxylin and eosin stain, X200).
Correlation between Osteopontin and Alpha-fetoprotein expressions in the serum of HCC patients and tumor grade
The correlation between OPN and AFP expression levels and tumor grade in HCC patients presented in Table 5. Patients were grouped in two subjects below and above the mean expression levels of Osteopontin and Alpha-fetoprotein of the 80 cases as cut-off value. Tumor grade was positively correlated with OPN (p= 0.0392) and AFP (p= 0.0371).
Table 5.
Correlation between OPN and AFP and Tumor Grade in HCC on-top of HCV
| RQ= 2^-∆∆ CT | Tumor Grade 1 | Tumor Grade 2 | Tumor Grade 3 | P value |
|---|---|---|---|---|
| Osteopontin <109.01 | 24 | 10 | 5 | 0.0392* |
| Osteopontin >109.01 | 15 | 12 | 14 | |
| Alpha-Feto protein <18.51 | 22 | 9 | 4 | 0.0371* |
| Alpha-feto protein >18.51 | 17 | 13 | 15 |
Chi-square test between Osteopontin and Alpha-fetoprotein expressions and tumor grade; RQ, the relative quantification measurements according to 2^-∆∆ CT method;
significant at p<0.05
Efficacy of Osteopontin and Alpha-fetoprotein as diagnostic biomarkers
Sensitivity and specificity of serum OPN and AFP at selected cut-off values are summarized in Table 6. The sensitivity and specificity of serum OPN expression in HCC patients relative to the control group were 83.16% and 80.22%, respectively, at a cut-off value of >116.01 compared to 85.55% sensitivity and 72.98% specificity at a cut-off value of >19.55 ng/ml for serum OPN concentration. Although the sensitivity and specificity of serum AFP expression in HCC patients relative to the control group were 85.46% and 81.97%, respectively, at a cut-off value of >18.51. The accuracy of serum Osteopontin expression by real time PCR and concentration by ELISA was measured by the area under the ROC curve (AUC) which was 0.821 and 0.853 sequentially and 0.867 for AFP.
Table 6.
Roc Curve Analysis for OPN and AFP in HCC Patients
| Serum | Cut off value | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| OPN expression levels | >116.01 | 83.16% | 80.22% | 0.821 |
| OPN concentration (ng/ml) | >19.55 | 85.55% | 72.98% | 0.853 |
| Alpha-fetoprotein expression levels | >18.51 | 85.46% | 81.97% | 0.867 |
ROC analysis of Osteopontin and Alpha-fetoprotein in serum; AUC, area under curve.
Discussion
HCC is one of the most common malignant tumors. In most cases, HCC is diagnosed at late stage; therefore, the prognosis of HCC is generally poor and has a <5% of 5-year survival rate (Abu El Makarem et al., 2011).
Early detection of patients with HCC is an attractive goal because it gives better prognosis as HCC tends to grow slowly and stay confined to the liver. Early detection is possible with ultrasound scanning and AFP monitoring, although the use of AFP as a screening test is complicated by frequent false positive and false negative results, so early diagnosis of HCC would not be difficult if tumor markers and medical imaging were combined (Salema et al., 2013).
In our research, we studied the value of serum Osteopontin in patients with HCC on top of HCV cirrhosis. After obtaining liver tissue specimens from HCC patients, we examined OPN gene expression in tumor and non-tumor tissues by Real time PCR and compared all the results with Alpha fetoprotein for evaluation.
Our results proved that, serum OPN levels were significantly higher in patients with HCC on top of HCV cirrhosis compared to healthy controls (P≤0.009). with an AUC of 0.853, (95% confidence interval with 85.55% sensitivity and 72.98% specificity) at cut-off level >19.55(ng/ml) showing significance as a diagnostic marker for the tumor. We compared the results for serum OPN and AFP in both groups. AFP showed an AUC of 0.867, (95% confidence interval with 85.46% sensitivity and 81.97% specificity) at cut-off level >18.51(ng/ml). Chang et al., (2012) reported that OPN plasma levels were significantly elevated in HCC patients, compared to cirrhosis. OPN overall performance remained higher than AFP in comparing cirrhosis and the following HCC groups: HCV-related HCC, HBV-associated HCC, and early HCC. OPN also had a good sensitivity in AFP-negative HCC. However, Chang et al. (2012), study was carried on plasma assessment of OPN and AFP, while our study confirmed these results by PCR evaluation of both markers in tissue extract.
Cabiati et al., (2017) results were also in agreement with ours, confirming that plasma and PCR assessment are considered a useful starting point to validate OPN as a prognostic and diagnostic marker of HCC. The difference is that we carried our study on Egyptian patients with HCC on top of HCV of genotype 4, which is the prevalent type in our country.
As Salema et al., (2013), reported that the sensitivity and specificity of AFP has been shown to vary with the different cut-off values used. According to their results, at a cut-off 10.4 ng/ml, the sensitivity was 90% and the specificity was 77%. These results were comparable to those of Taketa et al., (2002) who reported that sensitivity 95% and specificity 66% with the cut-off value 10 ng/ml, which is the cut-off level of healthy subjects. It has been reported Gad et al., (2005) a significant higher sensitivity of AFP in Egyptian patients in comparison with Japanese patients for HCC diagnosis (99 % versus 67% P < 0.001) for AFP level greater than 10 ng/ml, with comparable specificity (75% versus 82%).
This is in accordance to the study done by Kim et al., (2006) who found that OPN’s diagnostic sensitivity and specificity for HCC was 87% and 82%, respectively, suggesting diagnostic accuracy of OPN.
OPN plasma levels were significantly elevated in HCC patients, compared to cirrhosis, chronic hepatitis C, chronic hepatitis B, or healthy controls (Salema et al., 2013).
In most published studies OPN showed an advantage over AFP in the diagnosis of HCC in patients with cirrhosis due to HBV or HCV infections, with the best performance obtained by combining OPN and AFP (Shang et al., 2012; Said et al., 2013).
Although currently recommended as a fundamental parameter for HCC screening in patients with cirrhosis, the clinical use of AFP has been shown to present some important limitations in sensitivity and specificity (Chen et al., 1984). In fact, low AFP levels have been described in HCC patients, while high levels might be detected in hepatic cirrhosis without HCC (Gogel et al., 2000). Thus, the evaluation of a more accurate biomarker as OPN will improve the prognosis in patients with early-diagnosed HCC.
In the present study, there was a significant correlation between serum OPN and AFP levels with the tumor grade. The tumor grade was positively correlated with OPN (p= 0.0392) and AFP (p= 0.0371).
Zhang et al., (2006) and Salema et al., (2013) were in accordance to our findings, they found a positive correlation between OPN and AFP levels.
In HCC patients, higher OPN positively correlated with reduced liver function, as defined by increasing tumor stage, suggesting use of plasma OPN as a prognostic factor (Kim et al., 2006). Our results showed that, elevated OPN levels were detected in higher BCLC Stages (2, 3 and 4) of HCC which comprised 97.5% of HCC cases.
In patients with HCC, both OPN and AFP levels were significantly higher in patients with Child–Pugh class B compared to class A. In addition, patients with HCC, plasma OPN levels negatively correlated with serum albumin and positively correlated with total and direct bilirubin levels (data not shown). The previous results suggest that a rise in the levels of biomarkers is associated with the deterioration of liver functions and development of HCC.
In agreement to our findings, many studies have established a significant correlation between OPN overexpression and clinico-pathological features of HCC, including the severity of liver damage according to Child-Pugh class, high grade, late stage, LN/vascular/bile duct/capsular invasion, and intrahepatic or distant metastasis (Pan et al., 2003; Chen et al., 2010; Weber et al., 2010; Xie et al., 2007)
We addressed the expression of OPN gene by Real-time PCR in serum samples of HCC, and healthy volunteers as control group, in addition to the tissue samples of HCC group. The expression of OPN in the serum of the HCC group was significantly higher than control group (p value=0.035). However, there was no significant difference between OPN gene expression in tumor tissue and non-tumor tissue.
We found that Real-time PCR can be used to assess the gene expression of OPN in serum, in case OPN protein levels are intractable by ELISA. In this study, there was a strong positive correlation between OPN expression and AFP expression where spearman’s rank= 0.754 and p=0.0072.
The accuracy of serum Osteopontin expression by real time PCR and concentration by ELISA was measured by the area under the ROC curve (AUC) which was 0.821 and 0.853 sequentially and 0.867 for AFP.
In conclusion, the results of the current study indicate that OPN is a promising tumor marker that could be added to the current standard tests for the diagnosis of HCC in patients with liver cirrhosis, due to Chronic HCV infection, in order to detect the disease at an early stage and, hence, improve the prognosis and survival rates of these patients. A combination assay comprising at least two or three markers in combination with imaging techniques is recommended for a more sensitive and specific diagnosis for HCC. In addition, a greater understanding of the patho-physiologic role of OPN in hepatic inflammation and cancer may enable the development of novel inflammation and cancer treatment strategies.
References
- 1.Abu El Makarem MA, Abdel-Aleem A, Ahmed Ali A, et al. Diagnostic significance of plasma osteopontin in hepatitis C vírus-related hepatocellular carcinoma. Ann Hepatol. 2011;10:296–305. [PubMed] [Google Scholar]
- 2.Akin Y, Hacer I, Ebru A, Sevda M. Hernandez P, Gomez A, editors. Real-time PCR for gene expression analysis polymerase chain reaction. In Tech Journals. 2012;(Chapter 12):229–54. [Google Scholar]
- 3.Balogh J, Victor D, Asham E, et al. Hepatocellular carcinoma:a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedossa P, Bioulac-Sage P, Callard P, et al. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 5.Bosman F. In pathology and genetics of tumors of the digestive system. Vol. 4. Lyon, France: IARC Press; 2010. Tumors of the liver and intrahepatic bile ducts; pp. 322–6. [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma:an update. J Hepatol. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabiati M, Gaggini M, Cesare MM, et al. Osteopontin in hepatocellular carcinoma:A possible biomarker for diagnosis and follow-up. Cytokine. 2017;99:59–65. doi: 10.1016/j.cyto.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen HJ, Xiao JR, Yuan W. Loss of p16INK4, alone and with overexpression of osteopontin, correlates with survival of patients with spinal metastasis from hepatocellular carcinoma. Med Oncol. 2010;27:1005–9. doi: 10.1007/s12032-009-9324-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen DS, Sung JL, Sheu JC, et al. Serum α-fetoprotein in the early stage of human hepatocellular carcinoma. Am J Gastroenterol. 1984;86:1404–9. [PubMed] [Google Scholar]
- 10.Gad A, Tanaka E, Matsumoto A. Ethnicity affects the diagnostic validity of alpha-fetoprotein in hepatocellular carcinoma. Asia Pac J Clin Oncol. 2005;1:64–70. [Google Scholar]
- 11.Gogel BM, Goldstein RM, Kuhn JA, et al. Diagnostic evaluation of hepatocellular carcinoma in a cirrhotic liver. Oncology. 2000;14:15–20. [PubMed] [Google Scholar]
- 12.Huang J, Pan C, Hu H, Zheng S, Ding L. Osteopontin-enhanced hepatic metastasis of colorectal cancer cells. PLoS One. 2012;7:e47901. doi: 10.1371/journal.pone.0047901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim A, Khaled H, Mikhail N, Baraka H, Kamel H. Cancer incidence in Egypt:results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:345–9. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Ki SS, Lee SD, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:2051–9. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma:the BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed A, Yassin A, Elhusseiny E, et al. Human aldehyde dehydrogenase (ALDH) in cirrhotic and hepatocellular carcinoma patients related to hepatitis C virus. J Gastroenterol Hepatol Res. 2015;4:1838–43. [Google Scholar]
- 17.Nagoshi S. Osteopontin: Versatile modulator of liver diseases. Hepatol Res. 2014;44:22–30. doi: 10.1111/hepr.12166. [DOI] [PubMed] [Google Scholar]
- 18.Pan HW, Ou YH, Peng SY, et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–27. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 19.Said S, Ahmed A, Mohamed M, et al. Novel markers for the diagnosis of hepatocellular carcinoma. J Am Sci. 2013;9:322–8. [Google Scholar]
- 20.Salema M, Abdel Atti S, El Raziky M, Darweesh S, El Sharkawya M. Clinical significance of plasma osteopontin level as a biomarker of hepatocellular carcinoma. Gastroenterol Res. 2013;6:191–9. doi: 10.4021/gr499w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang S, Plymoth A, Ge S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. J Hepatol. 2012;55:483–90. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taketa K, Okada S, Win N, Hlaing NK, Wind KM. Evaluation of tumor markers for the detection of hepatocellular carcinoma in Yangon General Hospital, Myanmar. Acta Med Okayama. 2002;56:317–20. doi: 10.18926/AMO/31689. [DOI] [PubMed] [Google Scholar]
- 23.Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010;103:861–9. doi: 10.1038/sj.bjc.6605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen Y, Jeong S, Xia Q, Kong X. Role of osteopontin in liver diseases. Int J Biol Sci. 2016;12:1121–8. doi: 10.7150/ijbs.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen-Hsien H, King-Teh L, Hong-Yaw C, Te-Wei H, Herng-Chia C. Disease-free survival after hepatic resection in hepatocellular carcinoma patients:A prediction approach using artificial neural network. PLoS One. 2012;7:29–79. doi: 10.1371/journal.pone.0029179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie H, Song J, Du R, et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig Liver Dis. 2007;39:167–72. doi: 10.1016/j.dld.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Ye QH, Ren N, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709–17. doi: 10.1007/s00432-006-0119-3. [DOI] [PubMed] [Google Scholar]


