Abstract
Background and Aim:
The optimal management of metastatic hormone-sensitive prostate cancer has been controversial in recent years with introduction of upfront chemohormonal treatment based on results of several Western studies. This changing landscape has renewed interest in the concept “disease volume”, the focus of the present study is the Egyptian patients.
Methods:
Patients with hormone sensitive metastatic prostate cancer presenting at Menoufia University Hospital, Egypt, during the period from June 2013 to May 2016, were enrolled. All received hormonal treatment. Radiologic images were evaluated and patients were stratified according to their disease volume into high or low, other clinical and pathological data that could affect survival also being collected and analyzed.
Results:
A total of 128 patients were included, with a median age of 70 years (53.9% ≥70). About 46% had co-morbidities, 62% having high volume disease. During the median follow up period of 28 months about half of the patients progressed and one third received chemotherapy. On univariate analysis, disease volume, performance status (PS), prostate specific antigen level (PSA) and presence of pain at presentation were identified as factors influencing overall survival. Multivariate analysis revealed the independent predictor factors for survival to be PS, PSA and disease volume. The median overall survival with 27 months was high volume versus 49 with low volume disease (hazard ratio 2.1; 95% CI 1.2 - 4.4; P=0.02). Median progression free survival was 19 months in the high volume, as compared with 48 months in the low volume disease patients (hazard ratio, 2.44; 95% CI, 1.42 – 7.4; P=0.009).
Conclusions:
Disease volume is a reliable predictor of survival which should be incorporated with other important factors as; patient performance status and comorbidities in treatment decision-making.
Keywords: Disease volume, metastatic prostate cancer, survival
Introduction
Prostate cancer is the second most common cancer worldwide among men, the incidence increases with age starting at 50 years to the peak at 70 years of age in most cases. There is significant geographic variations of 50-fold across differences with the highest incidence in the United States and Canada then European countries, the reported incidence is 19% of all male cancers in the USA, prostate cancer is the first in the incidence and the third in mortality (Jemal et al., 2017).
Lower incidence is reported in Asian populations and even less in the Arabic populations (Cooperberg and Chan, 2017); e.g. incidence is only 3.1 and 6.5/100,000 population in Saudi Arabia and Kuwait. (Hanash et al., 2000). In Egypt, similar low incidence ranging from 2.6 to 3.4/100,000 population reported in different cancer registry series (Ibrahim et al., 2014). however, according to Elabbady et al., (2014) Egyptian men with prostate cancer had baseline poor prognostic features as higher prostate specific antigen (PSA), PSA density and higher Gleason grade at initial diagnosis.
The expected survival of metastatic cancer prostate depends on multiple factors; patient performance status (PS), baseline PSA, advanced primary Gleason sum, presence of visceral metastases, baseline hemoglobin, lactate dehydrogenase, albumin, alkaline phosphatase (Halabi et al., 2003). Pretreatment risk stratification is still not well defined, and this changing landscape has renewed the interest of the concept “disease volume”(Sweeney et al., 2015).
Although androgen deprivation treatment (ADT) provides a clinical response in 70% of patients, of them will relapse in approximately 18 months (Gravis et al., 2013). In 2004, based on large multicenter randomized clinical trials, Docetaxel was approved for the treatment of men with metastatic hormone-refractory prostate cancer (HRPC); it resulted in a median survival that was approximately 2.5 months longer than that with mitoxantrone and prednisone (Petrylak et al., 2004). Docetaxel acts by inducing apoptosis in cancer cells through TP53- independent mechanisms that are thought to be a result of the inhibition of microtubule depolymerization and the blockade of antiapoptotic signaling (Darshan et al., 2011).
Following this approval, a relevant question is whether administering chemotherapy to patients who are sensitive to hormone therapy can improve the patient outcomes (Armstrong et al., 2007). Recent western studies reported improved patients survival with up front chemotherapy (Sweeney et al., 2015; James et al., 2015). We aimed to study different patients and disease characteristics including disease volume as prognostic factors affecting survival of newly diagnosed metastatic prostate cancer Egyptian patients.
Materials and Methods
This prospective observational study included 128 patients with hormone sensitive metastatic prostate cancer presented to Menoufia University Hospital, Egypt during the period from June 2013 to May 2016, all provided written informed consent in accordance with our institutional guidelines, this study was approved by ethical committee of Human Rights in Research at Menoufia University. All patients were hormone sensitive metastatic prostatic adenocarcinoma, all patients received hormonal treatment; (ADT) in the form of bilateral orchiectomy or luteinizing hormone-releasing hormone (LHRH) agonists; Goserelin (Zoladex) 3.6 mg SC q28 days alone or with antiandrogen Bicalutamide (Casodex) 50 mg, antiandrogen started at least 7 days before commencing treatment with an LHRH analogue. Patients with incomplete data or images were excluded from analysis. All clinical and pathological data were collected: age, co-morbidities, World Health Organization (WHO) performance status, pathology, baseline PSA levels, initial Gleason score, treatment outcome and survival.
Disease volume was assessed by radiologic revision and evaluation of all patients’ images {contrast enhanced computed tomography (CT), contrast enhanced Magnetic resonance imaging (MRI) and technetium-99m bone scans} for presence, site and number of bony lesions, either they are sclerotic or lytic, presence and size of associated soft tissue components. Bone scan images were revised for presence of remote bony lesions beyond the vertebrae and the pelvis (ribs, skull or long bones). The images were analyzed for presence, size and number of lymph nodes, Presence and size of visceral lesions and assessment of their pattern of enhancement to assure their metastatic nature.
Patients were classified according to the extent of metastases (high volume [defined as the presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis] vs. low volume; all other patients) (Sweeney et al., 2015).
Statistical analysis
The data were collected, tabulated, and analyzed by SPSS (statistical package for social science) version 17.0 on IBM compatible computer (SPSS Inc., Chicago, IL, USA). Chi-square test (χ2) was used to study association between qualitative variables and student t-test was used to compare two groups with normally distributed quantitative variables. Overall survival was defined as the time from diagnosis until death or last contact. Progression free survival was the time from date of diagnosis to date of progression. Survival was analyzed in relation to patient and disease characteristics using Kaplan-Meier curve. Hazard ratios and 95% confidence intervals (95% CI) were derived from multivariate Cox regression models.
Results
A total of 128 patients were included in this study, the median age was 70 years and ranged from 48 – 92 years. 59 of the patients (46.1%) were < 70 years and 69 patients (53.9%) were ≥70 years. 59 patients (46.1%) had comorbidities and 109 patients (85.1%) had performance status 0-2, also 59 patients (46.1%) of the patients had PSA range 100-1,000 ng/mL, and about 37 patients (28.9%) of the patients had Gleason score ≥8, 87 patients (67.9 %) of the studied patients had a metastasis in only bone, bone and soft tissue 26 patients (20.3%) and soft tissue only 15 patients (11.7%). Half of the studied patients had extensive bony lesion with 80 patients (62.5%) of the patients had symptomatic distant metastases (Table 1).
Table 1.
Base Line Characteristics of the Studied Patients (Low Volume, High Volume Disease):
| The studied patients | P value | ||||
|---|---|---|---|---|---|
| Low volume N = (49) | High volume N = (79) | ||||
| Age | |||||
| X ±SD | 71.96±8.41 | 69.37±9.14 | 0.11 | ||
| No | % | No | % | ||
| Age groups | |||||
| <70 years | 16 | 32.7 | 43 | 54.4 | 0.02 |
| ≥70 years | 33 | 67.3 | 36 | 45.6 | |
| PSA range: | |||||
| < 20 | 15 | 30.6 | 6 | 7.6 | |
| 20 – 100 | 13 | 26.5 | 22 | 27.8 | 0.004 |
| 100 – 1,000 | 19 | 38.8 | 40 | 50.6 | |
| > 1,000 | 2 | 4.1 | 11 | 13.9 | |
| Gleason score: | |||||
| Unknown | 5 | 10.2 | 27 | 34.2 | |
| < 8 | 29 | 59.2 | 30 | 30 | 0.007 |
| ≥ 8 | 15 | 30.6 | 22 | 32 | |
| Metastatic sites: | |||||
| Bone only | 46 | 93.9 | 42 | 53.2 | |
| Bone and Soft tissue | 1 | 2 | 23 | 29.1 | <0.001 |
| Soft tissue only | 2 | 4.1 | 14 | 17.7 | |
| Bone lesion | |||||
| No and Scanty | 49 | 100 | 15 | 19 | <0.001 |
| Extensive | 0 | 0 | 64 | 81 | |
| Metastatic symptoms: | |||||
| Asymptomatic | 29 | 59.2 | 19 | 24.1 | |
| Symptomatic | 20 | 40.8 | 60 | 75.9 | <0.001 |
| Skeletal related events | |||||
| No | 34 | 69.4 | 27 | 34.2 | <0.001 |
| Yes | 15 | 30.6 | 52 | 65.8 | |
Seventy-nine of the studied patients (61.7%) had high volume disease while forty nine patients (38.3%) had low volume disease. All patients received hormonal treatment; about half the patients had surgical and the other half had medical orchiectomy. Antiandrogen therapy; Bicalutamide (complete androgen blockade) received in 84 patients (65.6%). Bisphosphonates and palliative radiotherapy received according to indication. After median follow up duration 28 months about half of the patients progressed and about one third of the patients received chemotherapy at progression, 35 patients (27.3 %) received docetaxel prednisone protocol and 7 patients (5.4 %) received mitoxantrone.
Overall survival was significantly affected by disease volume, performance status, PSA level range, metastatic sites; presence of visceral and non regional nodal metastasis, burden of bony metastases, metastasis symptoms (asymptomatic or symptomatic) and presence of skeletal related events. On Cox regression, disease volume and performance status were independent predictors of patients’ overall survival with hazard ratio (2.1 and 2.52) respectively (Table 2, Table 3 and Figure 1).
Table 2.
Significant Prognostic Factors for Overall Survival and PFS
| Overall Survival | Progression Free Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | SE | 95%CI | Log rank | P value | Median | SE | 95%CI | Log rank | P value | |
| Disease volume | ||||||||||
| Low | 49 | 7.8 | 33.8-64.2 | 9.6 | 0.002 | 48 | 8.4 | 31.6 – 64.4 | 21 | <0.001 |
| High | 27 | 4.7 | 17.8-36.2 | 19 | 2.9 | 13.4 – 24.6 | ||||
| PSA range | ||||||||||
| < 20 | --- | --- | ----- | 13.3 | 0.004 | 36 | 8.7 | 19-53 | 29.3 | <0.001 |
| 20 – 100 | 38 | 2.8 | 32.6-43.4 | 35 | 4.2 | 26.8-43.2 | ||||
| 100 – 1,000 | 49 | 14.3 | 21-77 | 21 | 7.3 | 6.8-35.2 | ||||
| > 1,000 | 15 | 7.6 | 0.04-30 | 6 | 1.8 | 2.5-9.5 | ||||
| Metastatic sites | ||||||||||
| Bone only | 40 | 5.1 | 30.1-49.9 | 9.4 | 0.002 | 36 | 2.6 | 30.8-41.2 | 19.1 | <0.001 |
| bone and | 24 | 3.4 | 17.3-30.7 | 12 | 1.9 | 8.2-15.8 | ||||
| Soft tissue | 19 | 5.6 | 8.0-30 | |||||||
| Visceral metastases | 36 | 3.5 | 29.1-42.9 | 17.8 | <0.001 | |||||
| present | 39 | 5.7 | 27.8-50.2 | 8.7 | 0.003 | 12 | 2 | 8.1-15.9 | ||
| absent | 24 | 5.6 | 12.9-35.1 | |||||||
| Bony metastases | ||||||||||
| No and Scanty | 40 | 1.9 | 36.3-43.7 | 7.4 | 0.007 | ---- | --- | ----- | 17.7 | <0.001 |
| Extensive | 24 | 6.8 | 10.7-37.3 | 41.1 | 4.2 | 27.7-44.3 | ||||
| 25.1 | 4.6 | 7.0-25.0 | ||||||||
| Skeletal related event | ||||||||||
| absent | 52 | 7.9 | 37.8-65.7 | 7.5 | 0.006 | 48 | 3.5 | 21-64.3 | 21.8 | <0.001 |
| present | 32 | 5.4 | 25.6-43.8 | 19 | 4.2 | 10.9-27.1 | ||||
Table 3.
COX Regression of Independent Predictor of Patient Overall Survival and PFS
| B | SE | Wald X2 | P Value | Hazard ratio | 95.0% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Overall Survival | Lower | Upper | |||||
| Disease volume | 0.88 | 0.22 | 5.44 | 0.02 | 2.1 | 1.2 | 4.44 |
| PSA range | 0.36 | 0.19 | 3.5 | 0.06 | 1.43 | 0.98 | 2.08 |
| Metastatic sites | 0.48 | 0.75 | 0.41 | 0.52 | 1.62 | 0.37 | 7.04 |
| Visceral metastases | -0.19 | 0.76 | 0.06 | 0.81 | 0.83 | 0.19 | 3.72 |
| Bone metastases (extensive or scanty) | 0.39 | 0.32 | 1.46 | 0.23 | 1.48 | 0.79 | 2.78 |
| Skeletal related events | -0.38 | 0.33 | 1.31 | 0.25 | 0.69 | 0.36 | 1.31 |
| Progression Free Survival | Lower | Upper | |||||
| Disease volume | 0.12 | 0.51 | 0.06 | 0.82 | 1.13 | 0.42 | 3.04 |
| PSA range | 1.33 | 0.17 | 3.8 | 0.05 | 2.39 | 1 | 3.93 |
| Metastasis sites | -0.54 | 0.44 | 1.51 | 0.22 | 0.58 | 0.25 | 1.38 |
| Visceral metastases | 1.69 | 0.86 | 3.83 | 0.05 | 5.43 | 1 | 29.5 |
| Bone metastases (extensive or scanty) | 0.42 | 0.38 | 1.23 | 0.27 | 1.52 | 0.72 | 3.19 |
| Skeletal related events | 0.53 | 0.33 | 2.53 | 0.11 | 1.7 | 0.88 | 3.27 |
Figure 1.
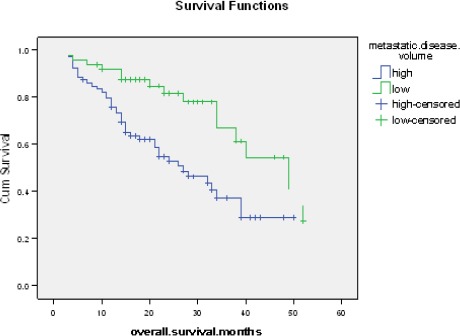
Kaplan-Meier Curve for Overall Survival among Low and High Volume Disease Patients. Kaplan-Meier curve for overall survival among, the median overall survival is 27 months in high volume disease versus 49 months in low volume disease; hazard ratio 2.1; [95% CI] was 1.2 - 4.4, P=0.002)
Progression free survival was significantly related to disease volume, PSA level range, metastatic sites; presence of visceral and non regional nodal metastasis, burden of bony metastases and presence of skeletal related events. Cox regression of progression free survival revealed that PSA range and presence of visceral metastasis were independent predictor for it with hazard ratio (2.93 and 5.43) respectively. (Table 2, Table 3 and Figure 2).
Figure 2.
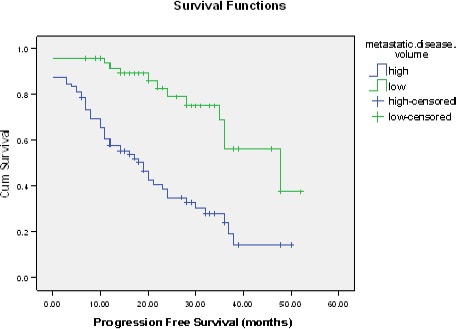
Kaplan-Meier Curve for PFS among Low and High Volume Disease Patients. Kaplan-Meier curve for progression free survival. The median PFS was 19 months in the high volume, as compared with 48 months in low volume disease patients (hazard ratio, 2.44; 95% CI, 1.42 – 7.4; P=0.009)
Figure 3.
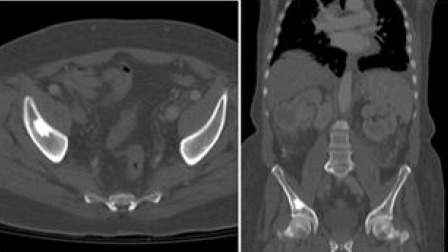
CT Scan Demonstrating Patient with Low Volume Bony Disease. Low volume disease: CT scan with I.V contrast axial and sagittal bone window. Single sclerotic bone lesion involving right iliac bone.
Figure 4.
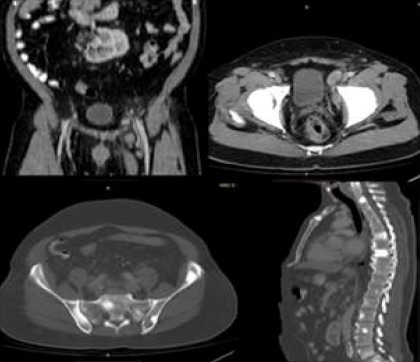
CT Scan Demonstrating Patient with High Volume Bony Disease. High volume disease: CT scan with oral and I.V contrast Coronal and axial soft tissue window, axial and sagittal bone window; Left internal iliac lymph nodes and multiple sclerotic bony lesions involving cervical, dorsal, lumbar spines and pelvic bones.
PS was prognostic factor of overall survival (p=0.03), also presence of symptomatic metastatic disease at presentation (p=0.04) but not for PFS (p= 0.42) and (p=0.12) respectively. Gleason score was predictor of PFS but, didn’t reach the statistical significance (p= 0.07)
Discussion
Recently, there has been a demonstration of a survival benefit with the addition of docetaxel to ADT, Shenoy and Kohil, (2016) reported that the addition of chemotherapy in combination with ADT taken in men with hormone sensitive metastatic prostate cancer resulted in improvements in survival if it is administered before the disease progresses, in particular, patients with high volume disease.
Two randomized clinical trials; CHAARTED and STAMPEDE trials compared ADT (as LHRH analogues or antagonists) plus Docetaxel (at a dose of 75 mg per square meter of body-surface area every 3 weeks for six cycles with premedication with 8 mg of oral dexamethasone at 12 hours, 3 hours, and 1 hour before Docetaxel infusion, daily prednisone was not required) or ADT alone (Sweeney et al., 2015; James et al., 2015).
CHAARTED and STAMPEDE trials found that; compared with ADT alone, docetaxel combined with ADT statistically significantly improved overall survival by around 10–15 months in this population. In STAMPEDE, in the docetaxel plus ADT group compared with the ADT alone group, median overall survival was 10 months longer in the total population of men with and without metastases (n=1776; 81 months compared with 71 months; hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.66 to 0.93, p=0.006) and 15 months longer in the subgroup of men with metastases (n=1086; 60 months compared with 45 months; HR 0.76, 95% CI 0.62 to 0.92, p=0.005) (James et al., 2015).
In men using docetaxel plus ADT compared with ADT alone, median overall survival was 13.6 months longer in CHAARTED (n=790; 57.6 months compared with 44.0 months; HR 0.61, 95% CI 0.47 to 0.80, p<0.001) (Sweeney et al., 2015). Prior studies of chemotherapy plus ADT, which did not show a benefit, were small studies that involved primarily patients with a relatively low tumor burden (Millikan et al., 2008).
In this study, we stratified patients with hormone sensitive metastatic prostate cancer into two risk groups; high and low volume disease according to their disease burden to study the impact of disease volume and other risk factors in Egyptian patients being a different ethnic and geographical population to define the poor prognostic factors that affect survival to help us to select the patient who may benefit from early addition of chemotherapy to the standard hormonal treatment to improve their survival and we studied other patients parameters as performance status, presence of co-morbidities and age as these factors make the patient less fit to receive chemotherapy.
In our study, the median age was 70 age and patient age subgroups classified as (46%) of the patients were <70 year and (54%) of the patients were ≥ 70 year; our patients had older median than reported in CHAARTED trial; 63 year in ADT arm and 64 year in ADT plus docetaxel; also older when compared with age subgroups of the patients of STAMPEDE trial in which (68%) of the patients ≥70 year and only (32) % were > 70 year (Sweeney et al., 2015; James et al., 2015).
In this study, 79 patients had high volume disease (62%) while 49 had low volume disease represented (38%). Similar results reported in CHAARTED trial in which about two thirds of the patients had high volume disease, (Sweeney et al., 2015) the most common metastatic sites were bone only 68%, bone and soft tissue 20% and soft tissue only 12%. Similar results were reported in STAMPEDE trial in which bone only 62%, bone and soft tissue 26% and soft tissue only 12% (James et al., 2015).
We reported chronic co-morbidities in (46%) of the studied patients. Performance status 0-2 were documented in 85 % of the patients with performance status zero only in 41% of the patients; both CHAARTED and STAMPEDE trials included only patients with no significant co morbidity and good performance status; 69% of the patients of CHAARTED trial had zero performance status and 72% in STAMPEDE trial (Sweeney et al., 2015; James et al., 2015).
In this study 46% of the patients had Gleason score < 8 and 29% had Gleason score ≥ 8 and 25% of the patients had unknown Gleason score compared with CHAARTED trial patients with about 28% of the patients had Gleason score < 8 and 61% had Gleason score ≥ 8 only 10 % unknown Gleason score, also STAMPEDE trial patients with about 17 % of the patients had Gleason score < 8 and 64 % had Gleason score ≥ 8 and 19 % unknown Gleason score, in this study the noticed higher unknown Gleason score and lower Gleason score than the European studies may be attributed to their better use of prostatic needle biopsy sampling with more adequate number of cores that may help in upgrading the Gleason score and reduction of unknown cases.
Patients with Gleason score < 8 were (59%) of low volume disease patients vs. only (30%) in high volume disease patients, the difference was statistically significant (p=0.007), indicating a more aggressive disease and a worse prognosis. Although a higher Gleason grade is associated with worse prognosis, it is not used alone for risk prediction (Millikan et al., 2008). This agrees with our results as in multivariate analysis, baseline Gleason score was not an independent predictor factors for survival.
In this study, PSA was <20 ng/ml in about (16 %), 20 – 100 ng/ml in about (27%) and 100-1000 ng/ml in about (46%) and >1000 ng/ml in about (10%). Of those patients with PSA>1000 ng/ml, 14% had high volume disease vs. only 4% in low volume diseases, patients with high volume disease showed higher level of PSA level. The multivariate analysis discriminated baseline PSA as an independent predictor factors for survival. This agrees with Koo et al., (2015) in which PSA was <20 ng/ml in about (16 %), 20 – 100 ng/ml in about (36%) and 100-1000 ng/ml in about (36%) and >1000 ng/ml in about (12%) and the overall survival was significant low in patients with PSA range > 1000 ng/ml (Median 15 m).
In this study, about (76%) of our patients with high volume disease had symptomatic metastases vs. (41%) for low volume disease patients. In the studied patients also, about (52%) had therapy for skeletal-related events at time of starting ADT (skeletal related events; bone pain, fractures, spinal cord compression, and frequently hypercalcemia), this number is lower in CHAARTED trial in which (44%) of the studied patients needed therapy for skeletal-related events at time of starting ADT which may give idea that our studied patients had more risk for development of skeletal related events.
Regarding survival, the median overall survival was 34 months for the overall study population, this agrees with a meta-analysis by Tangen et al., revealed resistance to ADT occurs in most patients, with the result that the median survival among patients with metastatic prostate cancer is approximately 3 years (Tangen et al., 2012).
The impact of disease volume on patient survival was significant; the median overall survival is 27 months in high volume disease versus 49 months in low volume disease; hazard ratio 2.1; [95% CI] was 1.2 - 4.4, P=0.002) so, disease volume is reliable predictor of survival and patients with high volume disease have relatively poor survival and short time to progression.
Similar results reported by Sweeney et al., (2015) for high-volume disease patients receiving ADT alone with median overall survival 32 months. Sweeney et al; reported that, the median overall survival was 13.6 months longer with the addition of early docetaxel to ADT than with ADT alone (57.6 months vs. 44.0 months; hazard ratio for death in the combination group, 0.61; 95% confidence interval [CI], 0.47 to 0.80; P<0.001). In CHAARTED trial analysis, the survival benefit was more apparent in the subgroup with high-volume disease than in the overall studied patients, with a median overall survival that was 17 months longer in the combination group than in the ADT alone group (49.2 months vs. 32.2 months; hazard ratio for death, 0.60; 95% CI, 0.45 to 0.81; P<0.001).
The median progression free survival was 19 months in the high volume, as compared with 48 months in low volume disease patients (hazard ratio, 2.44; 95% CI, 1.42 – 7.4; P=0.009) so, patients with high volume disease have relatively shorter time to progression and earlier to develop castration resistance and need of chemotherapy.
In univariate analysis, disease volume, performance status, initial PSA, Gleason score and presence of pain at presentation were identified to affect survival outcome; high volume disease, poor performance status, high initial PSA level and Gleason score are bad prognostic factor. According to James et al., (2015) factors prognostic of worsened outcome included presence of bone metastases with or without soft tissue metastases, worse performance status and higher or unknown initial Gleason sum score category.
The multivariate analysis discriminated the independent predictor factors for survival: disease volume, performance status and initial PSA, According to Smaletz et al., (2002) consistently predictive variables (by both univariate and multivariate analysis) of survival in this state include performance status, PSA, serum lactate dehydrogenase, serum alkaline phosphatase and hemoglobin.
Limitation
There are certain limitations in this study regarding the total number of patients and use of only of hormonal treatment arm, so we recommend inclusion larger population number addition of another chemohormonal arm in large multicentre prospective phase III study with longer duration of enrollment and follow up to compare upfront chemohormonal treatment arm versus hormonal treatment only in Egyptian patients being different ethnic and geographical population.
In conclusion, this study identified several factors that affect survival in Egyptian men with hormone sensitive metastatic prostate cancer. Disease volume is a reliable predictor of survival, patients with high volume disease had relatively poor survival and short time to progression.
Conflict of interest
No conflict of interest.
Funding Statement
None.
References
- 1.Armstrong AJ, Garrett-Mayer ES, Yang YC, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer:a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Chan JM. Epidemiology of prostate cancer. World J Urol. 2017;35:849. doi: 10.1007/s00345-017-2038-0. [DOI] [PubMed] [Google Scholar]
- 3.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elabbady A, Eid A, Fahmy A, et al. Pattern of prostate cancer presentation among the Egyptian population:A study in a single tertiary care center. Cent European J Urol. 2014;67:351–6. doi: 10.5173/ceju.2014.04.art7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15):a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 6.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–7. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 7.Hanash KA, Al-Othaimeen A, Kattan S, et al. Prostatic carcinoma:a nutritional disease?Conflicting data from the Kingdom of Saudi Arabia. J Urol. 2000;164:1570–2. [PubMed] [Google Scholar]
- 8.Ibrahim AS, Khaled HM, Mikhail NN, et al. Cancer incidence in egypt:results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “Docetaxel Era” data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019. Eur Urol. 2015;67:1028–38. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109:4–16. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo KC, Park SU, Kim KH, et al. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int. 2015;3:10–5. doi: 10.1016/j.prnil.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millikan RE, Wen S, Pagliaro LC, et al. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol. 2008;26:5936–42. doi: 10.1200/JCO.2007.15.9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 14.Shenoy N, Kohli M. Role of systemic chemotherapy in metastatic hormone-sensitive prostate cancer. Indian J Urol. 2016;32:257–61. doi: 10.4103/0970-1591.191234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smaletz O, Scher HI, Small EJ, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002;20:3972–82. doi: 10.1200/JCO.2002.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tangen CM, Hussain MH, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer:a SWOG phase III trial experience (S8494, S8894 and S9346) J Urol. 2012;188:1164–9. doi: 10.1016/j.juro.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


