Abstract
This study aimed to evaluate the diagnostic yield of fiberoptic bronchoscopic (FOB) transbronchial biopsy and its relation with quantitative findings of high resolution computerized tomography (HRCT). A total of 83 patients, 19 males and 64 females with a mean age of 45.1 years diagnosed with sarcoidosis with complete records of high resolution computerized tomography were retrospectively recruited during the time period from Feb 2005 to Jan 2015. High resolution computerized tomography scans were retrospectively assessed in random order by an experienced observer without knowledge of the bronchoscopic results or lung function tests. According to the radiological staging with HRCT, 2.4% of the patients (n=2) were stage 0, 19.3% (n=16) were stage 1, 72.3% (n=60) were stage 2 and 6.0% (n=5) were stage 3. This study showed that transbronchial lung biopsy showed positive results in 39.7% of the stage I or II sarcoidosis patients who were diagnosed by bronchoscopy. Different high resolution computerized tomography patterns and different scores of involvement did make a difference in the diagnostic accuracy of transbronchial biopsy (p=0.007).
Keywords: Transbronchial biopsy, high resolution computerized tomography, sarcoidosis, total lob scores
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown etiology. Though involvement occurs in many organs, the lung and intra-thoracic lymph nodes are mainly involved. The diagnosis of sarcoidosis is made by the confirmation of non-caseating granulomas in a setting with proper clinic and the exclusion of other possible etiologies (ATS Board of Directors, 1999).
Fiberoptic bronchoscopy (FOB) is a tolerable, less invasive diagnostical method that allows sampling from various different areas of the lung. It is possible to obtain trans-bronchial biopsy (TBB), endobronchial mucosal biopsy (EBB), trans-bronchial fine-needle aspiration biopsy (TBNA) and bronchoalveolar lavage (BAL) (ATS Board of Directors, 1999; Baughman, 2004) with this technique. TBB is a procedure recommended for patients with a suggestive diagnosis of sarcoidosis but diagnostic yield varies between 40% and 90% with respect to the experience of bronchoscopist, number of the biopsies performed and the stages of the disease (ATS Board of Directors,1999; Fraser, 1999). Although TBNA through endobronchial ultrasonography (EBUS) has shown to yield more diagnostic accuracy than conventional bronchoscopic TBB, it is not widely distributed throughout the world (ATS Board of Directors, 1999; Baughman, 2004; Von Bartheld et al., 2013).
Twenty-five - fifty % of the patients with sarcoidosis have pulmonary parenchymal infiltrates (Lync, 2003). High-resolution computerized tomography (HRCT) is superior to chest radiography for in-detail analysis of parenchymal sarcoidosis and mediastinal lymph nodes. HRCT based scoring system may provide compatible results with disease extension that is correlated with impairment in pulmonary function tests (Ors et al., 2013).
The purpose of this study was to determine the diagnostic yield of TBB and to present if there is any relation between the results and radiological scorings based on HRCT findings and the clinical and laboratory parameters that pertain to sarcoidosis.
Materials and Methods
Patient Selection
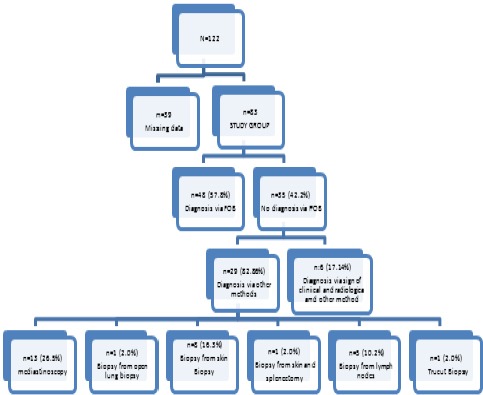
Patients who were admitted to Gazi University, School of Medicine, Department of Pulmonary Medicine between February 2005 and January 2015 and who are diagnosed with sarcoidosis were retrospectively screened through medical files and radiology archives. A total of 122 patients were detected. Since data that pertained to 39 patients were either missing, their HRCT sections were absent or they were not diagnosed by FOB they were excluded from the study. Thus, the study was completed with 83 patients. The study was approved by the Gazi University School of Medicine, Institutional Review Board.
Data Search
Demographic data, smoking histories, symptoms, extra-pulmonary involvement, chest radiography, the laboratory data, pulmonary function tests (PFTs), FOB and HRCT findings of the patients were retrospectively recorded from medical database. HRCT findings of patients were prospectively scored (Boer et al., 2009). According to the study protocol, cases with compatible clinical and radiological findings and with histologically proven non-caseating granulomas were accepted as sarcoidosis after the exclusion of other causes of granulomas. The detection rates of the correct diagnosis via TBB were determined. The relationship between the diagnostic rate by TBB and HRCT scores, demographic data and PFT findings were analyzed. Patients were staged both according to Siltzbach classification with direct chest radiography (ATS Board of Directors, 1999) and with HRCT findings. We used HRCT scans for the radiological staging.
Fiberoptic Bronchoscopy
FOB was applied via fiber-optic bronchoscope (FB) (Olympus BF type, 30, Tokyo, Japan) after premedication with local anesthesia (with 4% lidocaine spray). FB 15C (Olympus, Japan) spoon forceps were used for TBB. The number of TBBs and the lobes to which procedures were applied were recorded. TBB sample were obtained three times via FOB in all the patients by an experienced performer.
HRCT Scoring
HRCT scans were retrospectively assessed in random order by an experienced observer without knowledge of the bronchoscopic results or lung function tests and were scored in accordance with the definitions below (Boer et al., 2009).
HRCT patterns were classified as below
1) Nodules about 0 – 1 cm diameters were classified as micronodules, 2) 1 – 3 cm diameters as macronodules, 3) > 3cm as bulk, 4) reticular pattern accompanied by interlobular septa thickening or honeycomb appearance, 5) ground-glass, 6) consolidation.
The extension of each pattern within lobes was scored in the following manner
1) 1 = ≤25% of one lobe, 2) 2 = 26-50% of one lobe, 3) 3 = 51-75% of one lobe, 4) 4 = >75% of one lobe.
Scoring was conducted in accordance with the dominant HRCT pattern of patients. Total extension of each pattern was scored over 6 lobes (upper-left lobe lingular segments were evaluated as a separate lobe). Thus, lobe scores for each lobe and a total lung score through summing of these separate scores were obtained for every patient (Boer et al., 2009).
Statistical Analyses
SPSS (Statistical Package for Social Sciences) version 17 was used in order to assess the data obtained through the study and to create tables. Continuous variables (quantitative variables) obtained through measurement are presented together with means and standard deviations as well as medians, when necessary. For the presentation of (qualitative variables) frequency and percentage values were used. Chi-Square (X2) test and Fisher exact test were used for the comparison of categorical variables. For the comparison of quantitative variables, firstly the presence of parametric test conditions (number of test subjects and analysis of conformity with normal distribution) was sought. In order to compare two groups, Student’s t test was used for variables for which parametric test conditions are ensured; and when parametric test conditions were not provided Mann-Whitney U test was used. In the comparison of three or more groups, on the other hand, Kruskal Wallis H test was used. In all statistical analyses, the significance level was determined to be p<0.05 and p<0.01 values.
Results
83 patients [77.1% female (n=64), 22.9% male (n=19)] who were diagnosed with sarcoidosis were included in the study. The mean age was 45.1 years ± 11.7 (min-max: 21-79). Active smoking rate was 18.1%. Twenty five% of the patients (n=17) were asymptomatic. The most common pulmonary symptom was dyspnea (44.6%), followed by coughing (43.4%), chest pain (25.3%) and sputum (9.6 %). The most common extrapulmonary symptoms were both erythema nodosum and joint pain (15.7% and 15.7%), followed by weakness (8.4%), weight loss (4.8%) and fever (3.6%).
Extra-pulmonary involvement was detected in 12.4% (n=15) of the patients. The most common extra-pulmonary involvement was skin (10.8%, n=9), followed by peripheral lymph nodes (9.6%, n=8), muscle-skeletal system (8.5%, n=7), eyes (8.4%, n=7), liver (1.2%, n=1) and spleen (1.2%, n=1).
PFTs revealed that mean (SD) FEV1% was 88.6% (20.4) and DLCO Adj was 90.7% (113.2). Results are shown in Table 1.
Table 1.
Pulmonary Function Test, Laboratory Data Variables and Tuberculin Skin Test Results in Study Patients
| Variables | Mean ± SD | Median | (min – max) |
|---|---|---|---|
| Pulmonary Function Test | |||
| FEV1 (ml) | 2446.6 ± 743.5 | 2320 | (540.0 - 4640.0) |
| FEV1 (% predicted) | 88.6 ± 20.4 | 90 | (32.0 - 140.0) |
| FVC (ml) | 3080.4 ± 989.7 | 2995 | (102.0 - 6330.0) |
| FVC (% predicted) | 92.9 ± 18.0 | 97 | (51.0 - 136.0) |
| FEV1/FVC | 78.6 ± 9.5 | 81 | (42.0 - 96.0) |
| DLCOADJ (% predicted) | 90.7 ± 113.2 | 75 | (12.0 - 1040.0) |
| DLCOVA (% predicted) | 90.9 ± 20.3 | 89 | (43.0 - 154.0) |
| Laboratory Data | |||
| ACE U/L | 71.9 ± 59.3 | 54 | (0.0 - 289.0) |
| Blood Ca level (mg/dl) | 9.3 ± 0.5 | 9.1 | (8.2 - 12.0) |
| Urine Ca Level (mg/dl) | 183.2 ± 164.7 | 127.5 | (6.4 - 1011.0) |
| ESR (mm/h) | 34.4 ± 26.6 | 27 | (3.0 - 144.0) |
| Clinical Data | |||
| Tuberculin Skin Test (mm) | 1.3 ± 5.2 | 0 | (0.0 - 30.0) |
ACE, Angiotensin converting enzyme; Ca, Calcium; ESR, Erythrocyte sedimentation rate
The mean (SD) plasma Angiotensin Converting Enzyme (ACE) level was determined as 71.9 (59.3) U/L, erythrocyte sedimentation rate (ESR) as 34.4 (26.6) mm/h, blood calcium level as 9.3 (0.5) mg/dl, urine calcium level as 183.2 (164.7) mg/dl and, tuberculin test as 1.3 (5.2) mm (Table 1).
According to the radiological staging with chest radiography, 31.3% of the patients (n=26) were stage 1, 54.2% (n=45) were stage 2 and 14.5% (n=12) were stage 3. Hensley, according to the radiological staging with HRCT, 2.4% of the patients (n=2) were stage 0, 19.3% (n=16) were stage 1, 72.3% (n=60) were stage 2 and 6.0% (n=5) were stage 3. We used HRCT scans for the radiological staging.
Complete data was obtained in 83 patients. 57.8 % of 83 patients included in the study (n=48) were diagnosed via FOB.
In approximately 75.9% (n=63) of subjects TBB was performed due to parenchyma findings in the CT.39.7 % of 63 patients who underwent TBB via FOB (n=25) displayed histopathological diagnosis (granulomatous inflammation without necrosis). TBB was positive in 20.0% of the patients in Stage 1 and 68.0% patients in Stage 2 and 12.0% patients in Stage 3.
EBB was performed in 90.4% (n=75) of patients and in 20.0% (n=15) mucosal abnormality was observed. 20% of 75 patients who underwent EBB via FOB (n=15) displayed histopathological diagnosis.
TBNA was performed in 67.5% (n=56) of the patients with hilar and mediastinal lymph nodes. TBNA was positive in 25% (n=14) out of 56 subjects (Table 2).
Table 2.
The Diagnostic Efficiency of Vary Methods of Fiberoptic Bronchoscopy
| FOB biopsy methods | Positive | Negative | Total | Diagnostic Efficiency (%) |
|---|---|---|---|---|
| TBB* | 25 | 38 | 63 | 39.7 |
| EBB* | 15 | 60 | 75 | 20 |
| TBNA* | 14 | 42 | 56 | 25 |
| TBB+EBB | 39 | 30 | 69 | 56.5 |
| TBB+TBNA | 35 | 22 | 57 | 61.4 |
| EBB+ TBNA | 28 | 32 | 60 | 46.7 |
| TBB+EBB+ TBNA | 47 | 18 | 65 | 72.3 |
FOB, Fiberoptic bronchoscopy; TBB, Transbronchial lung biopsy; EBB, Endobronchialbiopsy; TBNA, Transbronchial Needle Aspiration
No results were obtained in 42.2% (n=35) of patients whose biopsy was taken by FOB. In 29 (82.2%) of these patients other biopsy methods were used for diagnosis (mediastinoscopy, lymph node biopsy, open lung biopsy, skin biopsy) whereas in 6 (17.14%) patients clinical and radiological findings were used (Figure 1).
Figure 1.
The Distribution of Diagnosis Method in the Study Group
No association was found between diagnostic efficiency of TBB and age, gender, smoking history, blood ACE and calcium levels, urinary calcium levels, ESR and PFTs (Table 3).
Table 3.
Demographic, Pulmonary Function Test, Laboratory Variables and Tuberculin Skin Test in TBB Positive and Negative Groups
| Variables | Positive Biopsy (n: 25) | Negative Biopsy (n: 38) | P-value |
|---|---|---|---|
| Demographic | |||
| Age (years) | 44,0 ± 11,7 | 46,6 ± 12,2 | 0.39 |
| Female, n (%) | 20 -40.8 | 29 -59.2 | 0.731 |
| Smoker, n (%) | 9 -60 | 6 -40 | 0.059 |
| Pulmonary Function Test | |||
| FEV1 (ml) | 2310.4 ± 787.7 | 2448.2 ± 777.0 | 0.641 |
| FEV1 (% predicted) | 83.8 ± 21.9 | 89.1 ± 22.2 | 0.371 |
| FVC (ml) | 2991.7 ± 944.0 | 3006.3 ± 1033.3 | 0.931 |
| FVC (% predicted) | 88.3 ± 18.8 | 93.8 ± 20.1 | 0.29 |
| FEV1/FVC | 77 ± 10.9 | 79.2 ± 9.9 | 0.219 |
| DLCOADJ (% predicted) | 74.9 ± 26.9 | 108.8 ± 168.4 | 0.422 |
| DLCOVA (% predicted) | 94 ± 17.5 | 88.9 ± 22.0 | 0,584 |
| Laboratory Data | |||
| ACE U/L | 78.2 ± 59.9 | 76.7 ± 68.1 | 0.479 |
| Blood Ca level (mg/dl) | 9.3 ± 0.4 | 9.3 ± 0.6 | 0.211 |
| Urine Ca Level (mg/dl) | 205.3 ± 133.0 | 184.4 ± 200.8 | 0.721 |
| ESR (mm/h) | 35.1 ± 20.5 | 35.8 ± 27.8 | 0.628 |
| Clinical Data | |||
| Tuberculin Skin Test (mm) | 0.1 ± 0.3 | 2.2 ± 6.9 | 0.348 |
Data were presented as mean± SD. SD, Standard deviation
Total pulmonary scoring was made according to the Fleischner scoring systems in the study groups (Hansell, 2008). Parenchymal involvement in HRCT was observed in 78.3% of patients (n=65). Macro-nodule was the most prominent findings in 25 (30.1%) of the patients followed by micro-nodule 18 (21.7%), ground-glass appearance 11 (13.3%) and reticular pattern in 8 (9.6%), respectively (Table 4). The involvement patterns in HRCT did differ statistically in patients with TBB positive and negative (p<0.05). Seven (28.0%) of TBB positive patients displayed micronodular pattern just similar to general population.
Table 4.
The Distribution of Radiological Findings in HRCT (n:83)
| Pattern | n | % |
|---|---|---|
| Macro-nodule | 25 | 30.1 |
| Micro-nodule | 18 | 21.7 |
| Ground-glass | 11 | 13.3 |
| Reticular | 8 | 9.6 |
| Consolidation | 2 | 2.4 |
| Mass | 1 | 1.2 |
| Normal | 18 | 21.7 |
The mean total lung score was found to be 9.1±4.9 for the entire patient population. The value was 3.6±2.4 for the upper lobes, 2.7±1.8 for the right-middle lobe and the lingula and, 3.0±1.9 for the lower lobes. The difference among the lobes was statistically significant (p<0.05). The patients displayed upper lobe predominance.
While the total lung score were not been statistically different in TBB positive and negative patients, the total lob scores were statistically significant in TBB positive when compared to negative ones. (8.6 ± 6.6, 8.8 ± 4.1, p=0.88) and1.8 ± 0.9, 1.7 ± 0.7, p=0.007, respectively) (Table 5).
Table 5.
Total Pulmonary and Lobe Scores Variables in TBB Positive and Negative Groups
| Positive Biopsy (n: 25) | Negative Biopsy (n: 38) | P-value | |
|---|---|---|---|
| Total Pulmonary Score | 8.8 ± 4.1 | 8.6 ± 6.6 | 0.882 |
| Total Lobe Score | 1.8 ± 0.9 | 1.7 ± 0.7 | 0.007 |
Discussion
Bronchoscopic biopsies have been performed for decades for the diagnosis of sarcoidosis. In the previous studies, the diagnostic efficiency of TBB has found to vary between 40 - 90% (Leonard et al., 1997; ATS Board of Directors,1999; Chapman and Mehta, 2003; Costabel et al., 2005; Costabel et al., 2008; Kıter et al., 2011). The results of TBB are highly dependent on the disease stages. The diagnostic yield is 56-60% in stage 1, and 75-76% in stage 2 patients (Morales et al., 1994; Descombes et al., 1994; Tournoy et al., 2010; Navani et al., 2011; Mehta and Jain, 2013).
In another study, TBB had the highest diagnostic yield of 68.7% followed by EBUS-TBNA (57.1%). The best possible diagnostic yield was observed with a combination of TBB and EBB in patients with endobronchial abnormalities (92.8%) (Goyal et al., 2014). In the study of Kıter G, et al. with Sarcoidosis Working Group of Turkish Thoracic Society, TBB was the most frequently used method with 48.8% success rate (Kıter et al., 2011).
In the previous meta-analysis of Li-Xing et al., (2016) indicated that EBUS-TBNA provided a much higher diagnostic yield then TBB. The results of this meta analysis also suggest that EBUS-TBNA+TBB+EBB could be used for the diagnosis of sarcoidosis, if available. At medical center without EBUS-TBNA, TBNA+TBB+EBB could be used instead.
In our study, TBB resulted in 39.7% diagnostic yield. That was 20% in stage 1, and 68% in stage 2 patients. Those results were less than the ones previously reported particularly for stage 1 patients. There may be several reasons for low success rate; 1) We did not perform TBB under fluoroscopy. Safety concerns might have restricted the performance. 2) Although 4–6 samples and up to 10 samples are found to be required for stage 2 and 1 patients (Costabel, 2008), we mostly perform 4 samplings in our routine practice. The number of samples was not recorded in the medical records for those particular patients. 3) the procedure was limited only to one lobe for most of patients.
Another factor that affects TBB positivity is race. Black people display a higher TBB diagnosis rate than white people (74% vs. 50%) (Torrington et al., 1997). We expected our TBB success rates to be lower because our study group consisted of white people. However, there at least several studies published on the diagnostic performance of bronchoscopy in Turkish sarcoidosis patients and our TBB success rate was even lower compared to previous Turkish studies (Kıter et al., 2011).
Like previous studies, no correlation was found between diagnostic efficiency of TBB and symptoms, ACE level, ESR, and PFTs (Boer et al., 2009).
In our study we found like previous studies TBB+EBB+TBNA combination was the much higher diagnostic yield (72%).
Sarcoidosis is characterized with typical HRCT patterns. These can be micro and macro-nodules, irregular interlobular septal thickening, ground-glass appearance, consolidation, fibrosis and, honeycomb pattern. In our study, significant correlation was found between increased diagnostic yield of TBB and HRCT patterns. This was associated to the fact that almost half of our patients displayed micro-nodule pattern in all groups and in TBB positive group. In the study of Boer et al., HRCT patterns was scored and their relationship with TBB was analyzed, they found TBB positivity was correlated with reticular and ground-glass pattern in HRCT. It was argued that despite being more common, nodules led unsuccessful results in diagnostic yield of TBB, depending on displaying a patchy distribution (Boer et al., 2009). We might have had a selection bias during the data management since we have excluded several patients due to lack of complete data and we had included micronodule dominated patients. However, we think patients with micronodules are good candidates for parenchymal biopsies in general.
Hence, we did find significant relation between intensity scoring and TBB positivity.
There might be several reasons to explain that, the most important one just similar to HRCT pattern, the intensity scores were similar for all patients group. And secondly most probably in our institute, the success of TBB was mostly dependent on performance quality itself and not the HRCT distribution. The third most important reasons was that the data was retrospective and depended on the archive records.
In conclusion, TBB is one of the preferred diagnostic methods for pulmonary sarcoidosis, with a wide range of diagnostic accuracy. TBB diagnostic efficiency was found to be 39.7% for the last 10 year period in our institute. A significant relationship was found between TBB success rates and HRCT patterns and total lobe scores. The success rate was lower than expected. This may depend on several technical limitations. Since the new era brought more sophisticated methods with higher success rates, institutions should figure out their own limitations and find new strategies to overcome these limitations. EBUS guided TBNA and new technologies have been arising each year and sarcoidosis patients should have a chance to benefit from them.
References
- 1.Baughman RP. Pulmonary sarcoidosis. Clin Chest Med. 2004;25:521–30. doi: 10.1016/j.ccm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Boer S, de Milne DG, Zeng I, et al. Does CT scanning predict the likelihood of a positive transbronchial biopsy in sarcoidosis? Thorax. 2009;64:436–39. doi: 10.1136/thx.2008.105031. [DOI] [PubMed] [Google Scholar]
- 3.Chapman JT, Mehta AC. Bronchoscopy in sarcoidosis:diagnostic and therapeutic interventions. Curr Opin Pulm Med. 2003;9:402–7. doi: 10.1097/00063198-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Costabel U, Guzman J, Drent M. Diagnostic approach to sarcoidosis. Eur Respir Mon. 2005;32:259–64. [Google Scholar]
- 5.Costabel U, Ohshimo S, Guzman J. Diagnosis of sarcoidosis. Curr Opin Pulm Med. 2008;14:455–61. doi: 10.1097/MCP.0b013e3283056a61. [DOI] [PubMed] [Google Scholar]
- 6.Descombes E, Gardiol D, Leuenberger P. Transbronchial lung biopsy:an analysis of 530 cases with reference to the number of samples. Monaldi Arch Chest Dis. 1997;52:324–9. [PubMed] [Google Scholar]
- 7.Fraser GF. Diagnosis of disease of the chest. fourth edition. Vol. 3. Philadelphia: W.B.Saunders Company; 1999. pp. 1533–83. [Google Scholar]
- 8.Goyal A, Gupta D, Agarwal R, et al. Value of different bronchoscopic sampling techniques in diagnosis of sarcoidosis:a prospective study of 151 patients. J Bronchol Intervent Pulmonol. 2014;21:220–26. doi: 10.1097/LBR.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 9.Hansell DM, Bankier AA, Mac Mahon H, et al. Glossary of Terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 10.Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee. Statement on sarcoidosis. Am J Respir Crit Car Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 11.Kıter G, Müsellim B, Cetinkaya E, et al. Clinical presentations and diagnostic work-up in sarcoidosis:a series of Turkish cases (clinics and diagnosis of sarcoidosis). Sarcoidosis Working group of Turkish Thoracic Society. Tuberk Toraks. 2011;59:248–58. [PubMed] [Google Scholar]
- 12.Leonard C, Tormey VJ, O'Keane C, Burke CM. Bronchoscopic diagnosis of sarcoidosis. Eur Respir J. 1997;10:2722–4. doi: 10.1183/09031936.97.10122722. [DOI] [PubMed] [Google Scholar]
- 13.Li Xing H, Ru Xuan C, Hui H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration versus standard bronchoscopic modalities for diagnosis of sarcoidosis:A meta-analysis. Chin Med J. 2016;129:1607–20. doi: 10.4103/0366-6999.184458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lync JP. Computed tomographic scanning in sarcoidosis. Semin Respir Crit Care Med. 2003;24:393–418. doi: 10.1055/s-2003-42375. [DOI] [PubMed] [Google Scholar]
- 15.Mehta AC, Jain P. Interventional bronchoscopy: A clinical guide, respiratory medicine. 2013;10 DOI 10.1007/978-1-62703-395-4_2, ©Springer Science+Business Media New York. [Google Scholar]
- 16.Morales CF, Patefield AJ, Strollo PJ, Jr, Schenk DA. Flexible transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 1994;106:709–11. doi: 10.1378/chest.106.3.709. [DOI] [PubMed] [Google Scholar]
- 17.Navani N, Booth HL, Kocjan G, et al. Combination of endobronchialultrasound-guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology. 2011;16:467–72. doi: 10.1111/j.1440-1843.2011.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ors F, Gumus S, Aydogan M, et al. HRCT findings of pulmonary sarcoidosis;relation to pulmonary function tests. Multidiscip Respir Med. 2013;8:1–8. doi: 10.1186/2049-6958-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torrington KG, Shorr AF, Parker JW. Endobronchial disease and racial differences in pulmonary sarcoidosis. Chest. 1997;111:619–22. doi: 10.1378/chest.111.3.619. [DOI] [PubMed] [Google Scholar]
- 20.Tournoy KG, Bolly A, Aerts JG, et al. The value of endoscopic ultrasound after bronchoscopy to diagnose thoracic sarcoidosis. Eur Respir J. 2010;35:1329–35. doi: 10.1183/09031936.00111509. [DOI] [PubMed] [Google Scholar]
- 21.Von Bartheld MB, Dekkers OM, Szlubowski A. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis. JAMA. 2013;309:2457–64. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]


