Abstract
Purpose:
To determine the value of dynamic susceptibility contrast enhanced (DSC) MRI (magnetic resonance imaging) perfusion in the characterization of newly developed/enlarging lesions within irradiated regions after treatment of brain tumors.
Methods:
This prospective cross-sectional study covered 23 patients, 12 females and 11 males. All cases initially presented with histologically proven malignant brain tumors and underwent surgical intervention followed by radiotherapy (+/- chemotherapy). On follow up imaging, they presented with newly developed/progressively enhancing mass lesions at the sites of the primary tumors. All patients then underwent conventional MRI, DSC MRI perfusion and MR spectroscopy.
Results:
In our study, we found DSC MR perfusion to be a useful non-invasive method for differentiating recurrent brain tumors from radiation necrosis. This approach allows hemodynamic measurements to be obtained within the brain as the relative cerebral blood volume (rCBV) to complement the anatomic information obtained with conventional contrast enhanced MR imaging. The sensitivity and specificity of DSC MR perfusion for differentiation were found to be 77.8% and 80.0%, respectively.
Conclusion:
DSC MR perfusion is a promising technique in differentiating recurrent brain tumors from radiation necrosis as it has acceptable spatial resolution and can be routinely performed in the same settings after conventional MRI.
Keywords: Dynamic susceptibility contrast enhanced MR perfusion, recurrent brain tumors, radiation necrosis
Introduction
Neoplastic recurrence in patients with malignant brain tumors who underwent surgical resection followed by chemotherapy and/or radiation therapy, is common. Recurrence detection is crucial in patient care. (Gasparetto et al., 2009).
Due to the wide use of different types of radiotherapy, post-therapeutic sequelae have been noticed with increasing frequency. Differentiation of recurrent neoplasm from treatment related necrosis is often challenging with conventional magnetic resonance imaging because both entities share common radiological features. (Gasparetto et al., 2009)
Radiation necrosis most often occurs at and surrounding the tumor bed where the maximum radiation dose is. The MRI features of radiation necrosis are a soap bubble or Swiss cheese-like interior of the enhancing lesion which occur secondary to blood brain barrier disruption and great vulnerability to ischemic effects (Fatterpekar et al., 2012).
Physiological imaging techniques such as T2*-weighted DSC MR perfusion have markedly advanced the clinical use of MRI as it enables the measurements of heamodynamics within the brain such as the cerebral blood volume(CBV) (Barajas et al., 2009).
Brain neoplasms are characterized by high relative cerebral blood volume which is partially due to increased angiogenesis. Regions of treated radiation necrosis have low CBV, which results from endothelial cell damage, thrombosis and fibrinoid vascular necrosis. (Gasparetto et al., 2009)
So the aim of this study is to differentiate recurrent/residual brain neoplasm from radiation necrosis with a non-invasive imaging technique based on demonstrating areas of increased tumor vascularity.
Materials and Methods
Patients
Twenty three patients (11 males and 12 females with age range from 17-70 years) referred from both neurosurgical department and radiotherapy and Clinical Oncology department, Kasr AlAiny hospitals, Cairo University Table 1 were prospectively entered into the study over a 20 months period (starting from September 2014 till March 2016) on the basis of the following criteria: (a) The patient had malignant brain tumor diagnosed before standard treatment. (b) The patient had undergone standard treatment including surgical intervention and radiation therapy with or without chemotherapy. (c) New and/or increasing enhancing mass lesions at follow up MR imaging.
Table 1.
Showing Demographic Feature in the Studied Patients
| Patients (n= 23) | |
|---|---|
| Age (yrs.) | |
| Minimum-maximum | 17-70 |
| Mean ± SD | 41.61 ± 14.71 |
| Gender | |
| Female | 12 (52.2%) |
| Male | 11 (47.8%) |
Initial diagnoses in all patients were proved by examination of histologic specimens in 20 patients. Table 2
Table 2.
Pathology in the Studied Patients
| Age of the patient | Pathological diagnosis |
|---|---|
| 38 | Astrocytoma ( Grade II ) |
| 40 | Glioblastoma Multiforme (Grade IV) |
| 17 | Glioblastoma Multiforme (Grade IV) |
| 23 | Immature teratoma(Grade III) |
| 50 | Anaplastic Astrocytoma(Grade III) |
| 60 | Anaplastic Astrocytoma(Grade III) |
| 25 | Astrocytoma (Grade III) |
| 17 | Pilocytic Astrocytoma (Grade I) |
| 50 | Glioblastoma Multiforme (Grade IV) |
| 45 | Glioblastoma Multiforme(Grade IV) |
| 70 | Glioblastoma Multiforme(Grade IV) |
| 50 | Glioblastoma Multiforme(Grade IV) |
| 55 | Glioblastoma Multiforme(Grade IV) |
| 39 | Glioblastoma Multiforme(Grade IV) |
| 30 | Glioblastoma Multiforme(Grade IV) |
| 40 | Fibrillary Astrocytoma (Grade II) |
| 30 | Glioblastoma Multiforme(Grade IV) |
| 60 | Glioblastoma Multiforme(Grade IV) |
| 40 | Glioblastoma Multiforme(Grade IV) |
| 47 | Oligodendroglioma (Grade III) |
In the remaining three patients, lesions were diagnosed as high grade gliomas on MRS basis as they were surgically inaccessible.
Six patients were excluded from this study due to poor MR perfusion image quality associated with hemorrhage, or patient motion or susceptibility artifacts caused by surgical clips.
Materials and Methods
A written informed consent was obtained from all patients and approval for this study was obtained from Review Board of the Cairo University Hospitals. All patients were subjected to written clinical history, revision of the previous operative data, pathological diagnosis and therapeutic data, previous imaging available upon presentation of the patient, conventional MR studies, perfusion-sensitive contrast-enhanced MR images, MR Spectroscopy examinations. The Conventional MRI study, Perfusion and MRS studies were all done at the same setting to allow exact comparison of results.
Patient Preparation
All contraindications to MRI examination including cochlear implants, cardiac pacemakers or metallic foreign bodies were excluded.
No pre-medications or sedatives were required. A 21 gauge intravenous needle was inserted and retained in the vein of right antecubital fossa.
Conventional MRI Imaging protocol
MRI studies were obtained in all cases with a 1.5 T superconducting system, (Achieva; Philips Medical systems, BEST Netherland) using the standard head coil at Kasr Al Ainy hospitals, Cairo University.
The MR imaging protocol consisted of
Pre- contrast axial and coronal T1 weighted imaging (TR/TE:500/20)
Axial and Sagittal T2 weighted imaging (TR/TE:3000/100)
Axial fluid attenuation inversion recovery (FLAIR)imaging (TR/TI/TE : 6000/2000/120).
Post contrast axial, sagittal and coronal T1 weighted images
Interpretation and Analysis
The conventional images demonstrated the morphology of the tumor, area of abnormal signal abnormality and pathological enhancement, its location and its anatomical relationships.
Comparison was made between the previous and current imaging concerning the size, extent and mass effect of the newly developed/progressively increasing mass lesion by measuring the largest cross sectional dimensions of the enhancing component and the greatest dimension perpendicular to it.
Perfusion Weighted imaging
DSC was performed by using gradient-echo echo-planar images with TR=1000–1500 ms, TE=40–50 ms, matrix: 128x128, flip angle: 60°, section thickness =5 mm, intersection gap 1-3mm, sections =12–18. Multisection image data were acquired every second for a total of 60 seconds with the contrast injection beginning at 10 seconds. Gadodiamide (Omniscan 0.5mmol/ml GE healthcare Ireland Cork, Ireland) was power-injected though a peripheral intravenous catheter at doses standardized by patient body weight (0.2 ml/kg body weight, to a maximum of 20 ml) at 2–5 ml/s and immediately followed by a 20-mL saline flush at the same rate.
Data processing
The DSC data were transferred along with the anatomic data to a dedicated workstation (Extended MR work space 2.6 32, 2009, CG, Philips medical system Nederland)
From the time course images the change in tissues T2* relaxation rate (∆R2*) was calculated, this parameter is proportional in to the tissue concentration of the contrast agent. T2*-weighted signal intensity–time curves were derived on a voxel-by voxel basis. Correction for leakage was performed by using γ-variate curve fitting to approximate the curve without recirculation and leakage.
Cerebral blood volume maps were calculated, coregistered with the contrast T1-weighted images, and displayed as color overlays.
Multiple circular ROIs (region of interest) were placed in the areas of maximum abnormality as visually determined from rCBV. Among the patients, the ROI size ranged from 110 mm2 to 250 mm2.
For each lesion ROI measurements were also acquired from a ROI of identical size positioned on the contra lateral homologous normal-appearing white matter for a lesion in the white matter and on the contra lateral normal gray matter for a lesion in the gray matter.
The normalized rCBV ratio was calculated by dividing the CBV of the lesion by the CBV of the normal appearing area.
MRS Data Analysis
MR Spectroscopy data were evaluated using a Philips work station. Signal intensities of the following metabolites were calculated: Total choline (tCho), Total creatine (tCr), N-acetylaspartate (NAA), Myo-inositol (mI) and Lactate and fatty acids.
The following signal intensity ratios were analyzed for the different lesions (residual/recurrence or radiation injury) after the end of treatment:
Cho/Cr
NAA/Cr
Cho/NAA
The final diagnosis was based on the MRS findings as well as on the consensus of multidisciplinary neuro-oncology team based on clinical course and MRI data analysis.
Patients were diagnosed to be neoplastic based on
MRS criteria (Cho/Cr or Cho/NAA >1.79)
During follow up when the enhancing lesion progressively increased in size on serial MR examinations and the patient`s clinical condition deteriorated progressive during follow up period.
Patients were considered to be non neoplastic enhancing tissue based on
MRS criteria
The enhanced lesions disappeared or decreased in size on subsequent MR examination, or were present but unchanged on serial follow-up MR examinations, accompanied by neurologic improvement during the follow-up.
Statistics
Results are expressed as mean ± SD or number (%). Association between different variables was performed using Chi square test. Agreement between variables was performed using Kappa test. Standard diagnostic indices including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic efficacy were calculated as described by Galen (1980). SPSS computer program (version 19 windows) was used for data analysis. P ≤ 0.05 was considered significant (Galen, 1980).
Results
A total of 23 patients (11 males and 12 females) were included in this study. Their age ranged from 17-70 years old.
Among the 23 patients included in our study, 20 patients (87 %) were pathologically proven to be high grade tumor (III and IV) and 3 patients (13 %) to be low grade tumor (grade I and II).
The time between the operation and the time of presentation ranged from 5-53 months with mean 21.15 and since the end of radiotherapy and/or chemotherapy ranged from 3- 48 months with mean 15.48 months.
The reason for performing additional radiological assessment was to differentiate between radiation necrosis and recurrence/progression of the tumor.
In all 23 patients, Perfusion-sensitive contrast enhanced studies as well as conventional MR study and MR spectroscopy was done.
Newly enhancing or progressively increasing lesions with a normalized rCBV ratio above 1.8 was observed in 15 cases representing tumoral tissue. According to MRS criteria, 14 out of 15 cases showed high Cho/Cr ratio consistent with tumor recurrence Figure 1. The remaining case did not show tumoral tissue criteria on MRS (Cho/CR ratio=1.6).
Figure 1a and b.
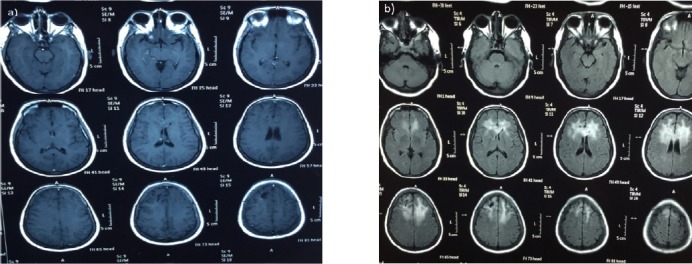
40 Years Old Female Patient Underwent Surgical Removal of Right Frontal Brain Tumor, Which was Proved to be Fibrillary Astrocytoma (Grade II), followed by radiotherapy.
Figure 1c, d and e.
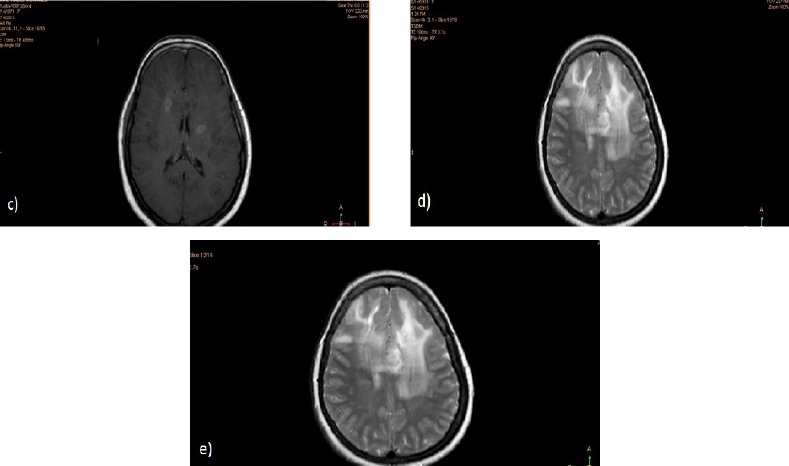
Post Contrast Axial T1 (c and d) and FLAIR (e) Weighted Images Showing Evidence of Newly Developed Well-Defined Lobulated Lesion at the Left High Parietal Region, Periventricular in Location, Involving the Corpus Callosum, Appearing of High T2 Signal Intensity with Heterogeneous Post Contrast Enhancement. It is seen surrounded by high T2 and FLAIR signal suggestive of vasogenic edema. Similar but smaller lesion is also noted at the right high parietal region
Figure 1f.
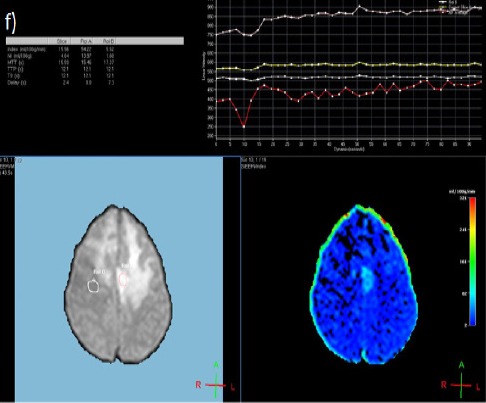
DSC MR Perfusion. The normalized rCBV was calculated and it showed value of 8.7. The color map showed evidence of vascularity
Figure 1g.
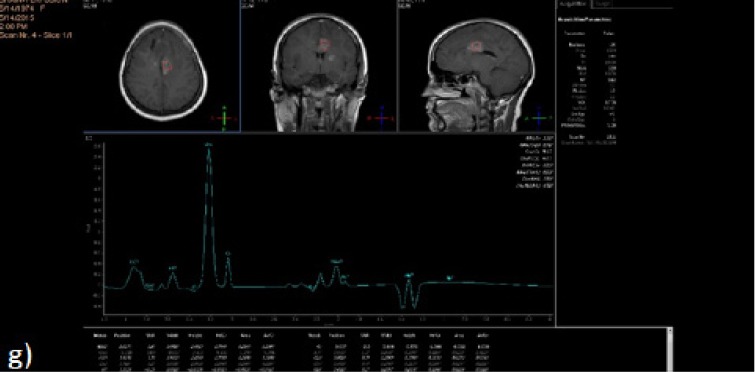
MRS Single Voxel was Applied at the Enhancing Component of the Left High Parietal Lesion Using Short, Long and Intermediate TEs. It showed elevated Cho/Cr of 9.1 denoting Tumoral component. Result: This case was diagnosed as recurrent brain tumor
Lesions with normalized rCBV ratio less than 1.8 were observed in 8 cases Figure 2. Based on MRS criteria, 4 out of 8 cases were also diagnosed as non-tumoral tissue, consistent with radiation necrosis Table 3.
Figure 2a.
Post Contrast Axial T1 Weighted Images for Post Operative Follow up of a 17 Years Old Female Patient with Right Temporal SOL (Pathologically Proven to Glioblastoma Multiforme) Received Radiotherapy and Chemotherapy. No evidence of residual enhancing component at the operative bed.
Figure 2b and c.
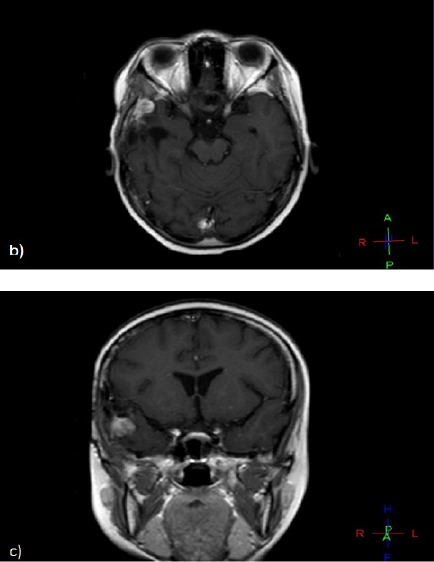
On Her Regular Follow up, Post Contrast Axial and Coronal T1 Weighted Images Showing Newly Developed Right Temporal Enhancing SOL at the Operative Bed.
Figure 2d.
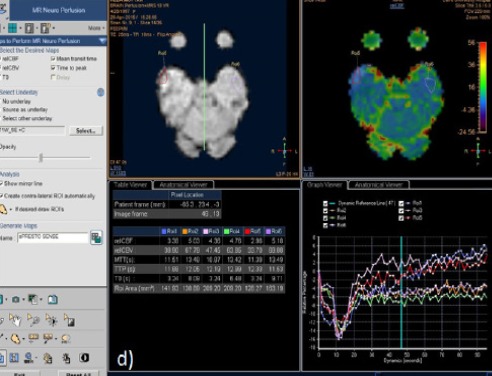
DSC MR Perfusion: The Normalized rCBV was calculated and It showed value of 0.48. The color map showed no evidence of vascularity. This suggests non-neoplastic nature of this lesion
Figure 2e.
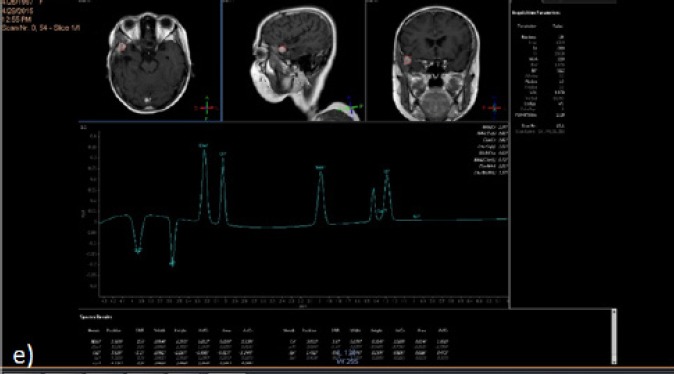
Single Voxel Technique Was Applied to the Enhancing Component of the Left Frontal SOL Using Long TE. It revealed Cho/Cr ratio of 1.6. This confirmed the non neoplastic nature of the newly developed mass lesion. Result: This case diagnosed as radiation effect
Table 3.
Association Between MRS and Perfusion in the Studied Patients
| MRS | p value | |||
|---|---|---|---|---|
| Non tumor (n= 5) | Tumor (n= 18) | |||
| Perfusion | Non tumor (n= 8) | 4 (80.0%) | 4 (22.2%) | 0.016* |
| TN | FN | |||
| Tumor (n= 15) | 1 (20.0%) | 14 (77.8%) | ||
| FP | TP | |||
Data are expressed as number (%);
p< 0.05, significant; Kappa value, 0.475; TP, true positive; TN, true negative; FP, false positive; FN, false negative
Discrepancy among the two techniques was observed in 4 patients that showed low normalized rCBV ratio (consistent with non tumoral tissue) and high Cho/Cr ratio (consistent with tumor recurrence).
The positive predictive value and negative predictive value, sensitivity, specificity and efficacy of contrast enhanced MR perfusion was as follow: sensitivity 77.8%, specificity 80%, PPV 93.3%, NPV 50% and Efficacy (78.3%).
Among the 15 cases diagnosed as tumor recurrence based on perfusion criteria, 80% of the cases were originally diagnosed as high grade tumor (grade III and IV) and 20 % as low grade tumor (grade I and II).
And based on MRS criteria, among the 18 patients diagnosed as tumor recurrence, 83.3% (15 cases) were originally diagnosed as high grade tumor (grade III and IV) and 16.7% (3 cases) as low grade tumor (grade I andII).
Discussion
The present study highlighted the role of contrast enhanced MR perfusion in managing cases of recurrent/residual brain tumors versus post radiation effects. The results of this study showed that DSC MR Perfusion is a promising technique in differentiating recurrent/residual brain tumors from radiation necrosis. This technique has been described by several authors to be useful in trials to differentiate recurrent tumor from radiation effect. (Matsusue et al., 2010 ; Prat et al., 2010; Gahramanov et al., 2013; Kim et al., 2014).
Over the past years, management for primary and metastatic brain tumors has become very aggressive as neurosurgeons, neurooncologists and radiation oncologists attempt to cure patients or at least provide them with a longer disease-free survival. Surgical resection and chemotherapy alone have proved to be insufficient in many instances. As a result, the various types of radiotherapy have all become important therapeutic adjuncts. Therefore, radiation necrosis is being seen with increased frequency (Sugahara et al., 2000)
Postradiation damages occur after a delay of months from the time of radiation and consist principally of necrosis caused by blood-brain barrier disruption and radiation induced demyelination leading to white matter injury. Radiation necrosis most commonly occurs at the site of the previous tumor (Sugahara et al., 2000).
There also has been increasing awareness of therapy-related parenchymal injury, so called post treatment radiation effect (PTRE). The mild and self-limited pseudoprogression develops within 3–6 months of radiotherapy, frequently earlier than adjuvant TMZ is finished. As a consequence, wrong interpretation of pseudoprogression as treatment failure can lead to premature stoppage of chemotherapy. In contrast, RN develops 6 months after radiotherapy and is more severe, typically requiring steroids or surgical intervention (Hu et al., 2012).
Depending on conventional imaging to distinguish tumor from PTRE leads to diagnostic inaccuracy and potential clinical dilemma. In addition, Consecutive follow up imaging requires months to reach a diagnosis, during which time tumor progression may have occurred. Consequent delay in suitable therapy can reduce treatment efficacy or increase toxicity (Hu et al., 2012).
Stereotactic or image-guided biopsies are frequently performed to achieve a histological diagnosis in these cases, but as the procedure morbidity rates range from 1% to 4.7%, and mortality rates from 0% to 2.3%, and results may be inconclusive, non invasive methods will be of great value (Prat et al., 2012).
Perfusion-sensitive contrast-enhanced MR imaging allows measuring vascularity within brain lesions and can be acquired during the same session as conventional MR imaging. The vascularity of malignant tumor differs significantly from that of irradiated brain tissue, specifically radiation necrosis. Thus, Perfusion sensitive contrast enhanced MR imaging allows differentiating areas of tumor recurrence within irradiated lesions from regions of radiation necrosis (Sugahara et al., 2000).
The aim of this study was to determine the value of DSC MRI perfusion in the characterization of newly developed/enlarged enhancing lesions within irradiated regions after the treatment of brain tumors.
This study was conducted at the radiology department in Kasr AlAiny hospitals, Cairo University including 23 patients (11 males and 12 females). Their age ranged from 17-70 years. All patients included in this study were pathologically proven to have brain tumors. They underwent surgical intervention followed by radiotherapy (+/- chemotherapy).
On their follow up conventional imaging, they could not be diagnosed as tumoral tissue or post therapeutic effect.
The preoperative tumor grade has no role in differentiating tumor recurrence from radiation necrosis as all cases of low grade tumors showed recurrence. Recurrent tumors after resection of low grade malignant gliomas showed transformation into malignant gliomas according to Sugahara (2000).
In this study, DSC MR perfusion was done in all patients. This technique relies on the T2* signal drop caused by the susceptibility effect of gadolinium based contrast agents in brain tissue. The drop in signal correlates with the concentration of the contrast agent and can be used to measure the relative cerebral blood volume (rCBV) which is the most widely used quantitative variable derived from DSC.
The normalized rCBV was calculated by dividing the CBV of the lesion by the CBV of the normal appearing area of the contralateral side. A normalized rCBV threshold value of 1.8 is suggested according to a study done by Gasparetto (2009). He proposed that calculation of the normalized CBV profile is useful for objective evaluation of recurrence versus treatment-related change, with an accuracy of 97%.
The results of assessment of rCBV were compared to those of Magnetic resonance spectroscopy. According to many previous studies, MRS has been widely used to differentiate between treatment necrosis and tumor recurrence (Rock et al., 2002 ; Sundgren et al., 2009 ; Bobek-Billewicz et al., 2010).
Weybright (2009) in a study including 29 patients found that the Cho/Cr and Cho/NAA ratios were significantly higher in tumor than in radiation injury (p < 0.0001). The author proposed that values greater than 1.8 for either Cho/Cr or Cho/NAA ratios were considered evidence of tumor.
In this study, our results showed that DSC MR perfusion was able to differentiate newly developed/enlarging enhancing mass lesion in patients with previous history of brain tumor and surgery followed by radio therapy (+/- chemotherapy) as recurrent tumor or radiation necrosis using the cut off value of normalized rCBV of 1.8 with sensitivity 77.8%, specificity 80%, positive predictive value PPV 93.3%, negative predictive value 50% and efficacy 78.3%. Statistical calculations showed P value of 0.016.
Our findings are also supported by the findings of several histologic studies that showed that tumor vasculature was markedly elevated in tissue specimens obtained from contrast-enhanced portions of GBM (Maia et al., 2005; Oh et al., 2007 ; Sadeghi et al., 2008).
A study done by Matasusue (2010) including 15 patients using DSC suggested that rCBV is a useful tool for differentiating recurrent brain tumors from radiaton necrosis with sensitivity 90% and specificity 80%.
Another study done by Hu(2009) reported a threshold value of 0.71 for the optimized differentiation of predominant tumor progression or treatment-related change with a sensitivity of 91.7% and a specificity of 100%.
As shown in previous reports and our results, the threshold of normalized CBV values that can be used for differentiation of post treatment contrast-enhanced lesions in patients with glioma were variable. These rCBV values are specific to the data acquisition parameters (ie, field strength, pulse sequence, preload bolus dosing, and type and amount of contrast agent) and the available postprocessing methods (Kim et al., 2010).
The value of DSC was also investigated against other imaging techniques. A study done by Kim (2014) found that adding any perfusion MR imaging to the combination of CE T1-WI and DW imaging, compared with the combination of CE T1-weighted imaging and DW imaging, provided superior diagnostic performance of the MR imaging protocol for differentiating recurrent glioblastoma versus radiation necrosis.
In our study, we used echoplanar T2* weighted images DSC MR imaging derived rCBV. Other studies previously described the use of T1-weighted FSE dynamic contrast-enhanced MR imaging (Yun et al., 2015).
The use of T2* has several advantages over the use of T1 weighted images. First, signal acquisition is much slower with the T1-weighted sequences than with the T2*-weighted echo-planar imaging sequences. In addition, they do not allow one to estimate CBV that is directly related to microvasculature (Barajas et al., 2009).
This technique however showed some limitations. Our study included four false negative results when those cases showed no evidence of increased rCBV on their post therapeutic follow up and were proved to be recurrent tumor on MRS criteria. This can be attributed to the following reasons:
Some antiangiogenic agents that tend to normalize the blood blain barrier causing decrease in the degree of enhancement without actual decrease in the tumor bed. Pseudo response was observed in patients receiving bavcizumab. 6 month progression free survival was much improved in the form of reduced symptoms, reduced vasogenic edema and steroid dependence and quality of life, however distant and diffuse recurrence was promoted (Fatterpekar et al., 2012).
In addition, Petechial hemorrhage induced by radiation may produce susceptibility artifacts and decrease the normalized rCBV ratios when it occurs within areas of tumor recurrence. Those artifacts may become more obvious with gradient echo planar sequences (Sugahara et al., 2000).
One false positive result was observed in our study which showed increased rCBV on DSC MR Perfusion imaging and was proved to be non neoplastic on MRS basis. Authors have assumed that radiation produce venous occlusion, with subsequent development of necrosis. Physiological attempts to form collateral drainage away from the site of occlusion or congestion result in the formation of vascular telangiectasia. So, Post contrast enhanced areas could have high CBV ratios (Okeda and Shibata, 1973; Gaensler et al., 1994).
In this study, the combination of MR spectroscopy and MR perfusion have shown increase in the accuracy of differentiating necrosis from recurrent tumor in patients with primary brain tumors. Both techniques together have successfully diagnosed 18 out of 23 patients, thus decreasing the percentage of patients that might need further evaluation. Based on the results of meta-analysis of a study done by Chuang (2016), rCBV and ratios of Cho/Cr and Cho/NAA were higher in recurrent tumors than in radiation necrosis.
Both MRS and MRP have superior PPV and NVP compared to FDG-PET in discriminating tumour recurrence, grade increase and radiation necrosis. Of interest is the 100% NVP for both techniques, which implies that 100% of patients with a normal MRS and MRP have neither tumour recurrence nor grade increase (Prat et al., 2010).
There were several limitations to this study. First, our study included small number of patients. Moreover, the dichotomization of the diagnosis into tumor recurrence versus treatment related effect because most treated tumors have areas of tumor admixed with treatment-related changes (Forsyth et al., 1995).
In addition, DSC-MRI suffers from being highly sensitive to susceptibility artifacts and therefore its application in patients with hemorrhages, calcifications or surgical clips is limited.
Other limitations also included: Lack of follow up studies or histopathological correlation or correlation with molecular imaging by PET/SPECT as a gold standard to refer our results. Finally, our results were specific to the imaging parameters and image processing methods used in this study.
In our experience, MRS and MRP are the most promising techniques: they both have acceptable spatial resolution and can be routinely performed in clinical settings just after conventional MRI.
We suggest that MRS and DSC perfusion MR should both be routinely added to conventional MR imaging in the follow up of such patients as they provide more valuable physiological information, beyond the morphological criteria, of post treatment changes.
We recommend that further prospective evaluation be undertaken with a larger sample size and image-guided histopathologic correlation to confirm the efficacy of the techniques described in this article.
In conclusion, our results showed that Contrast enhanced MR Perfusion imaging can be used to differentiate between recurrent/residual brain tumours and radiation necrosis. It helps prevent unnecessary interventions in patients, reducing health care costs and improving patient survival and quality of life.
References
- 1.Barajas RF, Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253:486–96. doi: 10.1148/radiol.2532090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki Ł. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol. 2010;48:81–92. [PubMed] [Google Scholar]
- 3.Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy:a meta-analysis. PLoS One. 2016;11:e0141438. doi: 10.1371/journal.pone.0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E. Treatment-related change versus tumor recurrence in high-grade gliomas:a diagnostic conundrum-use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol. 2012;198:19–26. doi: 10.2214/AJR.11.7417. [DOI] [PubMed] [Google Scholar]
- 5.Forsyth PA, Kelly PJ, Cascino TL, et al. Radiation necrosis or glioma recurrence:is computer-assisted stereotactic biopsy useful? J Neurosurg. 1995;82:436–44. doi: 10.3171/jns.1995.82.3.0436. [DOI] [PubMed] [Google Scholar]
- 6.Gaensler EH, Dillon WP, Edwards MS, et al. Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology. 1994;193:629–36. doi: 10.1148/radiology.193.3.7972799. [DOI] [PubMed] [Google Scholar]
- 7.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo-and radiation therapy:diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266:842–52. doi: 10.1148/radiol.12111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galen RS. Predictive value and efficiency of laboratory testing (1980) Pediatr Clin N Am. 27:861–9. doi: 10.1016/s0031-3955(16)33930-x. [DOI] [PubMed] [Google Scholar]
- 9.Gasparetto EL, Pawlak MA, Patel SH, et al. Posttreatment recurrence of malignant brain neoplasm:accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology. 2009;250:887–96. doi: 10.1148/radiol.2502071444. [DOI] [PubMed] [Google Scholar]
- 10.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect:direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. Am J Neuroradiol. 2009;30:552–8. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu LS, Eschbacher JM, Heiserman JE, et al. Reevaluating the imaging definition of tumor progression:perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012;14:919–30. doi: 10.1093/neuonc/nos112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Kim JH, Kim SH, Cho KG, Kim SY. Posttreatment high-grade glioma:usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence. Radiology. 2010;256:906–15. doi: 10.1148/radiol.10091461. [DOI] [PubMed] [Google Scholar]
- 13.Kim HS, Goh MJ, Kim N, et al. Which combination of MR imaging modalities is best for predicting recurrent glioblastoma?Study of diagnostic accuracy and reproducibility. Radiology. 2014;273:831–43. doi: 10.1148/radiol.14132868. [DOI] [PubMed] [Google Scholar]
- 14.Maia AC, Malheiros SM, Da Rocha AJ, et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. Am J Neuroradiol. 2005;26:777–83. [PMC free article] [PubMed] [Google Scholar]
- 15.Matsusue E, Fink JR, Rockhill JK, Ogawa T, Maravilla KR. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology. 2010;52:297–306. doi: 10.1007/s00234-009-0613-9. [DOI] [PubMed] [Google Scholar]
- 16.Oh BC, Pagnini PG, Wang MY, et al. Stereotactic radiosurgery:Adjacent tissue injury and response afterhigh-dose single fraction radiation:Part I-histology, imaging, and molecularevents. Neurosurgery. 2007;60:31–45. doi: 10.1227/01.NEU.0000249191.23162.D2. [DOI] [PubMed] [Google Scholar]
- 17.Okeda R, Shibata T. Radiation encephalopathy:An autopsy case and some comments on the pathogenesis of delayed radionecrosis of central nervous system. Pathol Int. 1973;23:867–83. doi: 10.1111/j.1440-1827.1973.tb02781.x. [DOI] [PubMed] [Google Scholar]
- 18.Prat R, Galeano I, Lucas A, et al. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18 F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J Clin Neurosci. 2010;17:50–3. doi: 10.1016/j.jocn.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Rock JP, Hearshen D, Scarpace L, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51:912–20. doi: 10.1097/00006123-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghi N, D'Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas:relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. Am J Neuroradiol. 2008;29:476–82. doi: 10.3174/ajnr.A0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugahara T, Korogi Y, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor:the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. Am J Neuroradiol. 2000;21:901–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Sundgren PC. MR spectroscopy in radiation injury. Am J Neuroradiol. 2009;30:1469–76. doi: 10.3174/ajnr.A1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weybright P, Sundgren PC, Maly P, et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. Am J Roentgenol. 2005;185:1471–6. doi: 10.2214/AJR.04.0933. [DOI] [PubMed] [Google Scholar]
- 24.Yun TJ, Park CK, Kim TM, et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy:differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology. 2015;274:830–40. doi: 10.1148/radiol.14132632. [DOI] [PubMed] [Google Scholar]


