Abstract
Purpose:
To comparatively evaluate the results of a 2-dose human papillomavirus (HPV) vaccination programme with the AS04-adjuvanted HPV16/18 vaccine (AS04-HPV-16/18v) or HPV-6/11/16/18 vaccine (4vHPVv), in addition to cervical cancer (CC) screening, in Malaysia.
Methods:
A lifetime Markov model replicating the natural history of HPV in 13-year-old girls was adapted to Malaysia to assess the impact of vaccination on pre-cancerous lesions, genital warts and CC cases, CC deaths, quality-adjusted life years (QALYs), and costs from the perspective of the Malaysian Ministry of Health. Vaccine effectiveness was based on efficacy and HPV type distribution. Both vaccines were assumed to have equal efficacy against vaccine-type HPV but differed for protection against non-vaccine types. Vaccine price parity was used and health and cost outcomes were discounted at 3%/annum. Sensitivity analyses tested the robustness of the results.
Results:
The model predicted that AS04-HPV-16/18v would result in 361 fewer CC cases and 115 fewer CC deaths than 4vHPVv, whereas 4vHPVv averted 4,241 cases of genital warts over the cohort’s lifetime. Discounted total costs showed savings of 18.50 million Malaysian Ringgits and 246 QALYs in favour of AS04-HPV-16/18v. In one-way sensitivity analyses, the discount rate was the most influential variable for costs and QALYs, but AS04-HPV-16/18v remained dominant throughout. A two-way sensitivity analysis to assess the longevity of cross-protection for both vaccines confirmed the base-case.
Conclusions:
In Malaysia, the use of AS04-HPV-16/18v, in addition to screening, was modelled to be dominant over 4vHPVv, with greater estimated CC benefits and lower costs.
Keywords: Cost-effectiveness -Human papillomavirus, Malaysia, two-dose schedule
Introduction
Cervical cancer (CC) is the second most common cancer among Malaysian women, and was the fifth most common cause of cancer death in 2012 (Ferlay et al., 2013). Human papillomavirus (HPV) is the necessary cause of CC (Walboomers et al., 1999), most commonly associated with HPV-16/18. CC has a substantial economic burden in Malaysia, with an estimated direct medical cost of 39.2 million Malaysian Ringgits (MYR) and a further MYR 12.4 million in indirect costs due to productivity lost in 2008 (Aljunid et al., 2010).
Papanicolaou (Pap) tests for CC screening have been available in Malaysia since the 1960s, and have been free-of-charge since 1995. However, a study by Abdullah et al. (2011) found that <40% of Malaysian women reported ever having had a Pap test. In 2010, the Malaysian government implemented free HPV vaccination to all 13-year-old girls (Bruni et al., 2015; One Malaysia Community, 2009). Two vaccines are currently available: AS04-adjuvanted HPV-16/18 vaccine (Cervarix, GSK; AS04-HPV-16/18v) (Appendix A.1 (1.1MB, pdf) ) which protects against HPV-16 and -18, and HPV-6/11/16/18 vaccine (Gardasil, Merck and Co., Inc.; 4vHPVv) which in addition protects against HPV-6 and -11 associated with genital warts (GW) (Ghittoni et al., 2015).
Randomised trials comparing AS04-HPV-16/18v and 4vHPVv have reported a superior immune response with AS04-HPV-16/18v (two/three doses) than with 4vHPVv (three doses) (Einstein et al., 2014; Leung et al., 2015).
Originally, three vaccine doses were recommended. However, the World Health Organization recommends a two-dose HPV vaccine schedule in children up to 13-years (4vHPVv) or 14-years (AS04-HPV-16/18v) (World Health Organization (WHO), 2014). Though both vaccines have shown protection against non-vaccine HPV types, current data indicates this may be stronger and broader with AS04-HPV-16/18v than 4vHPVv (Brown et al., 2009; Paavonen et al., 2009; Tjalma et al., 2009). To date, immunogenicity against non-vaccine types has been sustained for up to 9.4 years (Moscicki et al., 2015).
Three HPV cost-effectiveness analyses have been published comparing both vaccines (Aljunid et al., 2010; Ezat and Aljunid, 2010) and two- and three-dose schedules (Aljunid et al., 2013).
The aim of this study is to evaluate the epidemiological and economic consequences of universal mass vaccination (UMV) with two doses of AS04-HPV-16/18v or 4vHPVv in addition to the current CC screening programme in Malaysia, taking into account protection against non-vaccine types.
Materials and Methods
Analyses were conducted from the perspective of the Malaysian Ministry of Health, including only direct medical costs.
A published Markov cohort model was adapted to assess the lifetime effects of AS04-HPV-16/18v or 4vHPVv on cases, deaths, costs, life years, and quality-adjusted life years (QALYs) due to CC and its precursors, and GW (Demarteau and Standaert, 2010).
This model type was considered appropriate to meet the study objective as it adequately reproduces the long natural history of HPV. The model consists of a series of health states through which subjects move, reflecting the progression of low-risk HPV infection to GW and cervical intraepithelial neoplasia (CIN) grade 1, and of oncogenic HPV infection to CIN1, CIN2/3 and invasive CC (Demarteau and Standaert, 2010). The model has 95 one-year cycles.
Malaysian data were used where available, or were supplemented by data from another (Asian) country. CC clinical experts reviewed and validated data inputs and assumptions used in the model (Appendix A.2 (1.1MB, pdf) ).
Cohort size was 259,000 13-year-old girls (Department of Statistics Malaysia, 2015a). Age-specific female mortality rates from 2013 were used as background mortality in the model (Appendix Table B.1 (1.1MB, pdf) ).
HPV incidence in Malaysian women was calculated from data reported by the HPV information centre (Bruni et al., 2015) (Appendix Table B.2 (1.1MB, pdf) ).
As Malaysian data on GW incidence could not be identified, data from Japan were used (Kumamoto et al., 2004) (Appendix Table B.3 (1.1MB, pdf) ), and approved by the experts consulted. Appendix Table C.1 (1.1MB, pdf) lists the yearly transition probabilities used to determine how subjects move between health states in the absence of HPV vaccination.
In 2013, a HPV immunisation coverage of 94% was reported (MoH Malaysia, 2014). Two-dose schedules for both vaccines have been approved in Malaysia. The base-case assumed 100% vaccination coverage with two doses (National Pharmaceutical Control Bureau (NPCB) and MoH Malaysia, 2016).
AS04-HPV-16/18v and 4vHPVv efficacy were sourced from randomised controlled trials (Appendix C.2 (1.1MB, pdf) ). As vaccination was considered before sexual activity (age 13), data from women HPV-naïve at study entry were used. Protection against non-vaccine HPV types for both vaccines was considered.
Vaccine effectiveness depends on vaccine efficacy against each HPV type. Efficacy for both vaccines against all endpoints related to vaccine-type HPV (HPV types 6/11/16/18) were assumed equal at 98% (Paavonen et al., 2009; The FUTURE II Study Group, 2007) (Appendix Table C.3 (1.1MB, pdf) ). Protective efficacy against grouped non-vaccine types were differentiated per vaccine based on the reported clinical trial values (Appendix C.4 (1.1MB, pdf) ). HPV type distributions in CIN2/3 and CC were specific for Malaysia, data for CIN1 were from South-Eastern Asia, as insufficient data for Malaysia were available (Bruni et al., 2015). HPV type distribution for GW was based on expert opinion.
Overall vaccine effectiveness was estimated based on vaccine-type and non-vaccine-type efficacies and HPV type distribution for each lesion type and calculated for each model cycle to allow for less than lifetime vaccine protection. The base-case analysis assumed life-long efficacy related to vaccine-type and non-vaccine-type HPV.
In Malaysia, 20–65-year-old women are recommended to undergo Pap testing every 3 years (MoH Malaysia and Academy of Medicine, 2003). However, the experts felt that the reported screening coverage of 59.7% within 3 years (Abdullah et al., 2011) probably represented women who had ever had a Pap smear. Similarly, Gakidou et al., (2008) found that, in 2002, 46.8% of women reported never having had a pelvic examination. Overall, they estimated an effective 3-year screening coverage of 30.5%. However, this did not reflect the number of women who underwent CC screening every 3 years. Therefore, it was assumed that 29.9% would have a Pap test at 30-years and 29.9% at 45-years. The detection rates of CIN1 and CIN2/3 were 0.580 and 0.610, respectively (Appendix Table C.1 (1.1MB, pdf) ). It was estimated that 5.5% of Pap tests would be positive (Bergeron et al., 2005; Fender et al., 2003).
Only direct medical costs were included due to a lack of data on other costs. The same price per dose was used for both vaccines.
Costs of regular screening and treatment were derived from: (1) resource use from a previous publication by Aljunid et al. (2010) and (2) unit costs retrieved from the 2010 accounting database of two Malaysian public hospitals (Appendix C.1b (1.1MB, pdf) ). Costs retrieved represented 2012 costs and were updated to 2015 values using the relative price index for health components from 2012 to August 2015, resulting in a 10% increase (Department of Statistics Malaysia, 2015b, c).
Regular screening costs for subjects with negative or false-positive Pap tests were MYR 30 or 1,190, respectively (Aljunid et al., 2016). Annual treatment costs for GW, CIN1, CIN2/3 and CC were MYR 1,834, 2,102, 2,461, and 62,537, respectively. It was assumed that no CIN1-detected patients would undergo treatment (MoH Malaysia and Academy of Medicine, 2003) but that there would be additional follow-up costs. It was assumed that all CIN2/3-detected patients would undergo treatment (Expert opinion; Appendix Table C.1 (1.1MB, pdf) ).
Yearly disutilities for precancerous/cancerous states published in HPV cost-effectiveness analyses were used (Appendix C.5 (1.1MB, pdf) ). The model runs in yearly cycles using disutility values for a single year, equalling the proportion of a single QALY lost if the disease occurs within that year. All conditions except CC, CC death, all-cause mortality and CC survival are modelled to occur over a single year. The disutility for CC is applied over the number of years the health state is modelled to last. In the current analysis, the average retention in the CC health state is 2.25 years. For CC survival, the disutility is applied over the remaining lifetime of the subject. Disutilities were subtracted from a baseline utility of 1 across all ages for every year in the model. No decrements were assumed by age for non-diseased subjects.
Model outcomes included: CIN1, CIN2/3, CC, deaths due to CC, GW, costs, life years, and QALYs. Incremental cost-effectiveness ratios (ICERs) were computed. Results were presented as undiscounted values (for all outcomes) and discounted values (for cost, QALYs, life years, and ICERs).
As per Malaysian guidelines, health and cost outcomes were discounted at 3% per annum (ISPOR, 2015).
One-way sensitivity analyses were performed to evaluate robustness of the results for changes in selected model variables. Ninety-five% confidence intervals (CIs) were used for vaccine efficacy related to protection against non-vaccine types (Appendix Table D.1 (1.1MB, pdf) ). Other inputs were varied ±20% of the base-case values, except for the discount rate, which varied from 1.5-5%. A specific analysis was added to assess the impact of using an unadjusted distribution for HPV-16/18.
A probabilistic sensitivity analysis (PSA) was performed to assess parameter uncertainty in the model. Ten thousand replicates were generated (Appendix Table D.2 (1.1MB, pdf) ).
A separate scenario assessed the impact of a lower (39.7%) 5-year survival rate for CC (Razak et al., 2013) instead of 57.2% used in the base-case.
A two-way sensitivity analysis was performed to assess the impact of the longevity of cross-protection for both vaccines. The duration of protection varied from 5-100 years with 5-year increments for both vaccines. Cost and QALY were calculated for each of the values and tabulated (Appendix E (1.1MB, pdf) ).
Results
Model validation
The model adequately reproduced age-dependent CC incidence, CC mortality in Malaysia and GW incidence in Japan in 2002 (Ferlay et al., 2013; Kumamoto et al., 2004) (Appendix Figure C.6 (1.1MB, pdf) , C.7 (1.1MB, pdf) and C.8 (1.1MB, pdf) ).
Health and economic impacts
In the base-case, AS04-HPV-16/18v prevented no cases of GW whereas 4vHPVv prevented 4,241 GW cases, but AS04-HPV-16/18v resulted in 361 fewer CC cases and 115 fewer deaths over the cohort’s lifetime versus 4vHPVv (Table 1). AS04-HPV-16/18v resulted in CC and CIN cost savings that outweighed 4vHPVv associated with GW cost savings. AS04-HPV-16/18v dominated 4vHPVv with MYR 18.50 million cost savings and 246 QALYs gained.
Table 1.
Cases Remaining, Total Costs, Life Years and QALYs Gained for Unvaccinated, AS04-HPV-16/18v and 4vHPVv, Incremental Vvalues AS04-HPV-16/18v vs. 4vHPVv
| Base-case: 5-year cervical cancer survival 57.2% | Alternative scenario: 5-year cervical cancer survival 39.7% | |||||||
|---|---|---|---|---|---|---|---|---|
| No vaccination | 4vHPVv | AS04-HPV-16/18v | Difference AS04-HPV-16/18v vs. 4vHPVv | No vaccination | 4vHPVv | AS04-HPV-16/18v | Difference AS04-HPV-16/18v vs. 4vHPVv | |
| Cases | ||||||||
| CIN1 screening-detected | 1,008 | 636 | 555 | -81 | 1,007 | 636 | 555 | -81 |
| Genital warts | 5,648 | 1,407 | 5,648 | 4,241 | 5,647 | 1,407 | 5,648 | 4,241 |
| CIN2/3 screening-detected | 684 | 287 | 163 | -124 | 684 | 287 | 163 | -124 |
| Cervical cancer | 2,552 | 885 | 524 | -361 | 2,552 | 885 | 524 | -361 |
| Cervical cancer deaths | 811 | 280 | 165 | -115 | 1,184 | 412 | 245 | -167 |
| Undiscounted costs (Malaysian Riggits; MYR) | ||||||||
| Vaccine | 0 | 69,412,000 | 69,412,000 | 0 | 0 | 69,412,000 | 69,412,000 | 0 |
| Screening | 167,163,323 | 169,761,617 | 169,470,060 | -291,557 | 167,163,400 | 169,761,621 | 169,470,082 | -291,539 |
| Genital warts | 15,904,119 | 3,963,704 | 15,904,498 | 11,940,794 | 15,903,788 | 3,963,687 | 15,904,401 | 11,940,714 |
| CIN1 treatment | 2,117,541 | 1,335,837 | 1,166,268 | -169,569 | 2,117,463 | 1,335,775 | 1,166,246 | -169,529 |
| CIN2/3 treatment | 1,684,049 | 706,916 | 401,733 | -305,183 | 1,684,049 | 706,916 | 401,733 | -305,183 |
| Cervical cancer | 749,055,510 | 260,059,686 | 153,985,579 | -106,074,107 | 963,093,783 | 333,112,601 | 196,415,195 | -136,697,406 |
| Total cost differences | – | – | – | -94,899,622 | – | – | – | -125,522,943 |
| Undiscounted outcomes | ||||||||
| Life years | 12,699,197 | 12,702,717 | 12,703,476 | 759 | 12,696,180 | 12,701,540 | 12,702,695 | 1,155 |
| Quality-adjusted life years (QALYs) | 12,694,333 | 12,701,225 | 12,702,455 | 1,230 | 12,690,780 | 12,699,937 | 12,701,616 | 1,679 |
| Incremental cost-effectiveness ratio (ICERs) | AS04-HPV-16/18v is dominant | AS04-HPV-16/18v is dominant | ||||||
| Discounted results | ||||||||
| Total costs (Malaysian Riggits; MYR) | 272,272,530 | 215,835,194 | 197,330,893 | -18,504,301 | 311,889,505 | 229,685,818 | 205,593,197 | -24,092,621 |
| Life years | 6,052,441 | 6,053,275 | 6,053,455 | 180 | 6,051,657 | 6,052,970 | 6,053,253 | 283 |
| Quality-adjusted life years (QALYs) | 6,051,232 | 6,052,900 | 6,053,146 | 246 | 6,050,364 | 6,052,579 | 6,052,936 | 357 |
| Incremental cost-effectiveness ratios (ICERs) | AS04-HPV-16/18V is dominant | AS04-HPV-16/18v is dominant | ||||||
CIN, cervical intraepithelial neoplasia; CIN1/2/3, cervical intraepithelial neoplasia grade 1/2/3; HPV, human papillomavirus; AS04-HPV-16/18v, AS04-adjuvanted HPV-16/18 vaccine (Cervarix); 4vHPVv, HPV-6/11/16/18 vaccine (Gardasil); ICER, incremental cost-effectiveness ratio; MYR, Malaysian Ringgits; QALYs, quality-adjusted life years
Sensitivity analyses
One-way sensitivity analysis was assessed on discounted cost and QALY separately. The discount rate was the most influential variable for costs (Figure 1) and QALYs closely followed by a change in HPV-16/18 distribution (Figure 2). A discount rate between 1.5-5% did not change the dominance of AS04-HPV-16/18v over 4vHPVv.
Figure 1.
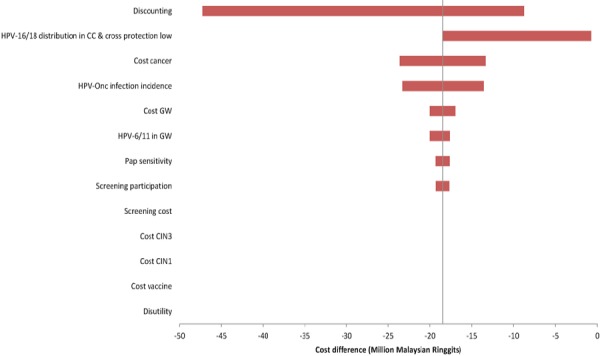
Univariate Sensitivity Analyses on Cost for AS04-HPV-16/18v vs. 4vHPVv. CC, cervical cancer; CIN1, cervical intraepithelial neoplasia grade 1; CIN3, cervical intraepithelial neoplasia grade 3; GW, genital warts; HPV, human papillomavirus; HPVonc, oncogenic human papillomavirus; Pap, Papanicolaou.
Figure 2.
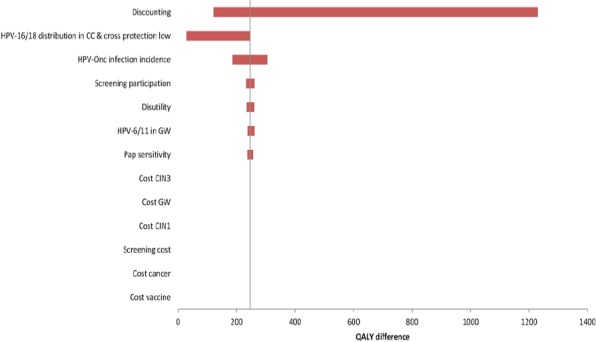
Univariate Sensitivity Analyses on QALY for AS04-HPV-16/18v vs. 4vHPVv. CC, cervical cancer; CIN1, cervical intraepithelial neoplasia grade 1; CIN3, cervical intraepithelial neoplasia grade 3; GW, genital warts; HPV, human papillomavirus; HPVonc, oncogenic human papillomavirus; Pap, Papanicolaou; QALY quality-adjusted life year.
The PSA results (Appendix Figure D.3 (1.1MB, pdf) ) showed AS04-HPV-16/18v was dominant in 96.3% of cases and be dominated in 2.4% of cases.
The 5-year survival rate for CC of 39.7% instead of 57.2% showed more CC deaths (167) were avoided with AS04-HPV-16/18v (Table 1). The difference in total discounted cost savings was predicted to increase to MYR 24.09 million, as was the number of QALYs gained (357).
Duration of cross-protection was varied from 5-100 years. AS04-HPV-16/18v was dominant or very cost-effective (i.e., <1xGDP/capita) in all scenarios tested. CC mortality by Razak et al. (2013) did not change this conclusion. Unadjusted HPV-16/18 distribution in CC resulted in AS04-HPV-16/18v being dominant or cost-effective in 57% of the cases (Appendix E (1.1MB, pdf) ).
Discussion
UMV with AS04-HPV-16/18v in 13-year-old Malaysian girls plus CC screening dominated 4vHPVv, with 361 fewer CC cases and 115 fewer deaths, 246 discounted additional QALYs, and MYR 18.5 million saved over the lifetime of the cohort. This was due to the higher reported cross-protection for non-vaccine oncogenic HPV types with AS04-HPV-16/18v (Appendix Table C.3 (1.1MB, pdf) ).
4vHPVv prevented 4,241 GW cases vs. AS04-HPV-16/18v. GW are not associated with mortality, so in the current analysis, the benefit of 4vHPVv on GW was outweighed by the benefits of AS04-HPV-16/18v on CC and precancerous lesions.
The model used a two-dose schedule for HPV vaccination. Aljunid et al., (2013) predicted that a two-dose HPV schedule was cost-saving compared with a three-dose schedule. Studies in the United Kingdom and Canada predicted that a two-dose schedule was cost-effective versus screening alone, if protection lasts for ≥10 years, but adding a third dose if protection exceeds ≥20 years (Jit et al., 2015) or >30 years (Laprise et al., 2014) was not cost-effective.
Two analyses comparing AS04-HPV-16/18v and 4vHPVv reported conflicting results (Aljunid et al., 2010; Ezat and Aljunid, 2010). Ezat and Aljunid (2010) concluded that 4vHPVv had a lower ICER than AS04-HPV-16/18v, due to its efficacy against GW. However, they did not consider the increased protection against non-vaccine types with AS04-HPV-16/18v or present incremental results for both vaccines. Aljunid et al. (2010) evaluated higher protection against non-vaccine types for AS04-HPV-16/18v, and predicted that AS04-HPV-16/18v provided the greatest potential economic benefit (savings of MYR 45.4 million vs. MYR 42.9 million with 4vHPVv).
Analyses comparing AS04-HPV-16/18v and 4vHPVv in other countries also reported conflicting results. Aponte-Gonzalez et al., (2013); Bresse et al., (2013); Brisson et al., (2013); Dee and Howell (2010); Lee et al., (2011); Westra et al. (2013), found more favourable cost-effectiveness with 4vHPVv. Westra et al., (2013) reported a marginal economic difference with 4vHPVv, but better cancer protection with AS04-HPV-16/18v. Jit et al., (2011) (UK) concluded 4vHPVv might reduce healthcare costs and QALYs lost versus AS04-HPV-16/18v, but that AS04-HPV-16/18v might prevent more CC deaths. Capri et al., (2011); Demarteau et al. (2012); Lee et al., (2015) found AS04-HPV-16/18v would provide a better health and economic value than 4vHPVv. In these models, the inputs varied according to country and model used, and Dee and Howell (2010) did not account for the higher non-vaccine HPV type protection with AS04-HPV-16/18v. Notably, models with high discount rates for outcomes and/or high GW rates resulted in better results for 4vHPVv (Bresse et al., 2013; Dee and Howell, 2010; Lee et al., 2015). Westra et al., (2013) investigated the value of protecting against CC alone and against CC and GW, questioning the willingness-to-pay threshold for GW protection.
Both vaccines have different strengths: 4vHPVv protects against GW, but AS04-HPV-16/18v seems to provide greater protection against CC, resulting in cost savings for AS04-HPV-16/18v. Results were largely driven by AS04-HPV-16/18v cross-protection and distribution of HPV types. The base-case assumption was life-long duration for cross-protection. Variation in cross-protection duration did not change the conclusions, but using unadjusted HPV-16/18 distribution had an important effect on the results (Appendix Tables E.7 (1.1MB, pdf) , E.8 (1.1MB, pdf) , E.9 (1.1MB, pdf) ).
HPV type varies by country, and AS04-HPV-16/18v protection against non-vaccine HPV types will provide more incremental benefits in countries with a higher prevalence of non-HPV-16/18-related disease. Differences in GW incidence, screening and treatment costs vary between countries impacting the cost-effectiveness of both vaccines.
This study has limitations. Markov cohort models don’t account for dynamic effects (e.g., herd protection) or population changes that could have improved the results. This analysis may, therefore, represent a conservative estimate of the benefits of HPV vaccination in Malaysia. Although Malaysian data were used where possible, it was supplemented with regional data when unavailable. Additionally, the nine-valent HPV vaccine was not considered for analysis, which may become available to the Malaysian public in the near future. This should be addressed in a future analysis.
There are no head-to-head vaccine efficacy studies; therefore vaccine effectiveness was estimated based on HPV type distribution and efficacies from individual trials. The model also combined all oncogenic and low-risk HPV types and did not account for the differential progression/regression of each HPV type, which may have favoured protection associated with non-HPV-16/18 types in our analysis, as cancers associated with HPV-16/18 types are reported to burden younger populations more than other HPV types (de Sanjose et al., 2013).
It is unknown whether HPV vaccination will have any long-term effects on type replacement (Drolet et al., 2015). It is also unclear how long protection will last for and whether booster vaccinations will be required. However, long-term follow-up studies for AS04-HPV-16/18v have shown maintained seropositivity for >9 years (De Vincenzo et al., 2014). Furthermore, Aregay et al. (2013) modelled that AS04-HPV-16/18v antibodies against vaccine-type HPV are expected to last ≥50 years. Kemp et al., (2012) reported persistence of cross-neutralising antibody titres 3 years after AS04-HPV-16/18v vaccination. Similarly, immunogenicity against non-vaccine types was sustained for >9 years (Moscicki et al., 2015), which strengthens our assumption that protection against non-vaccine types may be long-lasting. A meta-analysis by Malagón et al. (2012) concluded that differences in cross-protection with AS04-HPV-16/18v and 4vHPVv may not be statistically significant nor long-lasting. Taylor et al., (2016) recently summarised that AS04-HPV-16/18v has consistently demonstrated long-lasting (7-9 years), cross-protective efficacy in vaccine recipients, which was also demonstrated in the UK vaccination programme. This strengthens our assumptions on long-lived protection against non-vaccine types with AS04-HPV-16/18v.
We assumed an optimistic, yet reasonable, vaccine coverage of 100%, based on a 94% reported coverage in Malaysia in 2013 (MoH Malaysia, 2014). Changing vaccination coverage in the model would only affect the number of avoided cases but not ICER (Suárez et al., 2008). Current data on Malaysian screening coverage was unavailable, therefore, was largely based on expert opinion. Varying the screening rate in sensitivity analyses did not have a profound effect on results as the changes in outcomes for a given change in screening participation would be similar for both vaccines. We assumed price parity for both vaccines, so that any differences in outcomes were not attributable to vaccine cost, but this is likely not a reality.
Szarewski et al., (2013) recently reported that AS04-HPV-16/18v confers moderate protection against HPV-6/11, further supported by English data demonstrating a marked reduction in GW incidence in a AS04-HPV-16/18v-vaccinated population. They concluded that the patterns of decline by age/gender suggest a moderately protective effect of AS04-HPV-16/18v against GW (Canvin et al., 2016). The current model did not take any efficacy of AS04-HPV-16/18v against GW into account. Had these data been incorporated, results would have been further in favour of AS04-HPV-16/18v.
In conclusion, AS04-HPV-16/18v was predicted to reduce CC cases and deaths in Malaysia, compared with 4vHPVv, due to higher reported rates of protection against non-vaccine HPV types. AS04-HPV-16/18v also resulted in more discounted QALYs gained and lower medical costs. Therefore, UMV with AS04-HPV-16/18v is predicted to dominate in Malaysia when added to the current screening programme, allowing for the reinvestment of saved costs into the needs of the Malaysian population.
Abbreviations
CC, cervical cancer; CI, confidence interval; CIN1/2/3, cervical intraepithelial neoplasia grade 1/2/3; GW, genital warts; HPV, human papillomavirus; AS04-HPV-16/18v, AS04-adjuvanted HPV-16/18 vaccine (Cervarix); 4vHPVv, HPV-6/11/16/18 vaccine (Gardasil); ICER, Incremental cost-effectiveness ratio; MYR, Malaysian Ringgits; Pap, Papanicolaou test; QALY, quality-adjusted life year; UMV, universal mass vaccination; UK, United Kingdom.
Trademarks
Cervarix is a trademark owned by or licenced to the GSK group of companies. Gardasil is a trademark of Merck and Co. Inc.
Authors’ Disclosures
GVK, WYS, and JC are employees of the GSK group of companies. IHL was an employee of the GSK group of companies at the time of the study. SMA, RS, and CMY report no conflicts of interest.
Acknowledgements
GlaxoSmithKline Biologicals S.A. funded this study/research and all costs associated with the development of this manuscript.
Highlights
Effects of HPV vaccination over the lifetime of 259,000 Malaysian girls were modelled.
361 less cervical cancer cases are predicted with AS04-HPV-16/18v vs. 4vHPVv.
115 less cervical cancer deaths are predicted with AS04-HPV-16/18v vs. 4vHPVv.
4,241 fewer cases of genital warts are predicted with 4vHPVv vs. AS04-HPV-16/18v.
AS04-HPV-16/18v is predicted to be dominant (246 more QALYs; MYR 18.50 million savings).
References
- 1.Abdullah F, Aziz NA, Su TT. Factors related to poor practice of Pap smear screening among secondary school teachers in Malaysia. Asian Pac J Cancer Prev. 2011;12:1347–52. [PubMed] [Google Scholar]
- 2.Aljunid S, Zafar A, Saperi S, Amrizal M. Burden of disease associated with cervical cancer in Malaysia and the potential costs and consequences of HPV vaccination. Asian Pac J Cancer Prev. 2010;11:1551–9. [PubMed] [Google Scholar]
- 3.Aljunid S, Namaitijiang M, Zafar A, et al. Cost-effectiveness analysis of two versus three doses HPV vaccination in Malaysia. Abstract FC 2.8 presented at the 22nd Malaysian International Congress of Obstetrics and Gynaecology (MICOG), 30 May - 2 June 2013, Kuala Lumpur, Malaysia. Malaysian J Obstet Gynaecol. 2013;8:48. [Google Scholar]
- 4.Aljunid S, Maimaiti N, Nur AM, Noor MR, Puteh SE. Cost-effectiveness of HPV vaccination regime:comparing twice versus thrice vaccinations dose regime among adolescent girls in Malaysia. BMC Public Health. 2016;16:71. doi: 10.1186/s12889-016-2754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aponte-Gonzalez J, Fajardo-Bernal L, Diaz J, et al. Cost-effectiveness analysis of the bivalent and quadrivalent human papillomavirus vaccines from a societal perspective in Colombia. PLoS One. 2013;8:e80639. doi: 10.1371/journal.pone.0080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aregay M, Shkedy Z, Molenberghs G, David MP, Tibaldi F. Model-based estimates of long-term persistence of induced HPV antibodies:A flexible subject-specific approach. J Biopharm Stat. 2013;23:1228–48. doi: 10.1080/10543406.2013.834917. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron C, Cartier I, Guldner L, et al. Lésions précancéreuses et cancers du col de l'utérus diagnostiqués par le frottis cervical, Ile-de-France, enquête Crisap 2002. Bull Epidemiol Hebd. 2005;2:5–6. [Google Scholar]
- 8.Bresse X, Adam M, Largeron N, Roze S, Marty R. A comparative analysis of the epidemiological impact and disease cost-savings of HPV vaccines in France. Hum Vaccin Immunother. 2013;9:823–33. doi: 10.4161/hv.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brisson M, Laprise JF, Drolet M, et al. Comparative cost-effectiveness of the quadrivalent and bivalent human papillomavirus vaccines:A transmission-dynamic modeling study. Vaccine. 2013;31:3863–71. doi: 10.1016/j.vaccine.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 10.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV;types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 11.Bruni L, Barrionuevo-Rosas L, Albero G, et al. Information centre on HPV and cancer (HPV information Centre):Human papillomavirus and related diseases in Malaysia - summary report 19 April 2017. 2015. [(accessed 05 October 2015)]. http://www.hpvcentre.net/statistics/reports/XWX.pdf .
- 12.Canvin M, Sinka K, Hughes G, Mesher D. Decline in genital warts diagnoses among young women and young men since the introduction of the bivalent HPV (16/18) vaccination programme in England:an ecological analysis. Sex Transm Infect. 2016;93:125–28. doi: 10.1136/sextrans-2016-052626. [DOI] [PubMed] [Google Scholar]
- 13.Capri S, Gasparini R, Panatto D, Demarteau N. Cost-consequences evaluation between bivalent and quadrivalent HPV vaccines in Italy:the potential impact of different cross protection profiles. Gynecol Oncol. 2011;121:514–21. doi: 10.1016/j.ygyno.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 14.de Sanjose S, Wheeler CM, Quint WGV, et al. Age-specific occurrence of HPV16- and HPV18-related cervical cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1313–8. doi: 10.1158/1055-9965.EPI-13-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vincenzo R, Conte C, Ricci C, Scambia G, Capelli G. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999–1010. doi: 10.2147/IJWH.S50365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dee A, Howell F. A cost-utility analysis of adding a bivalent or quadrivalent HPV vaccine to the Irish cervical screening programme. Eur J Public Health. 2010;20:213–9. doi: 10.1093/eurpub/ckp141. [DOI] [PubMed] [Google Scholar]
- 17.Demarteau N, Standaert B. Modelling the economic value of cross- and sustained-protection in vaccines against cervical cancer. J Med Econ. 2010;13:324–38. doi: 10.3111/13696998.2010.490481. [DOI] [PubMed] [Google Scholar]
- 18.Demarteau N, Tang CH, Chen HC, Chen CJ, Van Kriekinge G. Cost-effectiveness analysis of the bivalent compared with the quadrivalent human papillomavirus vaccines in Taiwan. Value Health. 2012;15:622–31. doi: 10.1016/j.jval.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Department of statistics Malaysia. Population by age and sex, Malaysia 2014. 2015a. [(accessed 30 September 2015)]. http://pqi.stats.gov.my/searchBI.php?tahun=2014andkodData=2andkodJadual=1andkodCiri=3andkodNegeri=00 .
- 20.Department of statistics Malaysia. Consumer price index Malaysia August 2015. 2015b. [(accessed 30 September 2015)]. https://www.statistics.gov.my/index.php?r=column/cthemeByCatandcat=106andbul_id=SGxRcHdFOU9BMUNtUTlicmtEcFdSZz09andmenu_id=bThzTHQxN1ZqMVF6a2I4RkZoNDFkQT09 .
- 21.Department of statistics Malaysia. Time series data:Malaysia economic statistics - Time series 2013. 2015c. [(accessed 30 September 2015)]. https://www.statistics.gov.my/index.php?r=column/ctimeseriesandmenu_id=NHJlaGc2Rlg4ZXlGTjh1SU1kaWY5UT09 .
- 22.Drolet M, Bénard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes:a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–80. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Einstein MH, Levin MJ, Chatterjee A, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years:Follow-up through Month 48 in a Phase III randomized study. Hum Vaccin Immunother. 2014;10:3455–65. doi: 10.4161/hv.36117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezat SW, Aljunid S. Comparative cost-effectiveness of HPV vaccines in the prevention of cervical cancer in Malaysia. Asian Pac J Cancer Prev. 2010;11:943–51. [PubMed] [Google Scholar]
- 25.Fender M, Schott J, Baldauf JJ, et al. EVE, a regional campaign for the screening of cervical cancer. Organization, 7-years results and perspectives. Presse Med. 2003;32:1545–51. [PubMed] [Google Scholar]
- 26.Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v1.0, Cancer incidence and mortality worldwide:IARC CancerBase No 11 [Internet] Vol. 2013. Lyon, France: International Agency for Research on Cancer (IARC); 2013. [(accessed 31 January 2014)]. http://globocan.iarc.fr . [Google Scholar]
- 27.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries:low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghittoni R, Accardi R, Chiocca S, Tommasino M. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience. 2015;9:526. doi: 10.3332/ecancer.2015.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ISPOR. Pharmacoeconomic guidelines ground the world. 2015. [(accessed 13 February 2015)]. http://www.ispor.org/PEguidelines/index.asp .
- 30.Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines:economic evaluation based on transmission model. BMJ. 2011;343:d5775. doi: 10.1136/bmj.d5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jit M, Brisson M, Laprise JF, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules:cost effectiveness analysis based on transmission model. BMJ. 2015;350:g7584. doi: 10.1136/bmj.g7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemp TJ, Safaeian M, Hildesheim A, et al. Kinetic and HPV infection effects on cross-type neutralizing antibody and avidity responses induced by Cervarix((R)) Vaccine. 2012;31:165–70. doi: 10.1016/j.vaccine.2012.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumamoto Y, Tsukamoto J, Sugiyama T, et al. National surveillance of sexually transmitted diseases of Japan in 2002. Japanese Journal of Sexually Transmitted Diseases. 2004;15:17–45. [Google Scholar]
- 34.Laprise JF, Drolet M, Boily MC, et al. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination:A transmission-dynamic modelling study. Vaccine. 2014;32:5845–53. doi: 10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Kim B-G, Hur S, et al. Cost-effectiveness analysis of AS04-adjuvanted human papillomavirus 16/18 vaccine compared with human papillomavirus 6/11/16/18 vaccine in adolescent girls in Korea, with the new 2-dose schedule. Abstract n°VACC2015_0281 presented at the 9th Vaccine and ISV Congress, 18 - 20 October 2015, Seoul, Korea. 2015 [Google Scholar]
- 36.Lee VJ, Tay SK, Teoh YL, Tok MY. Cost-effectiveness of different human papillomavirus vaccines in Singapore. BMC Public Health. 2011;11:203. doi: 10.1186/1471-2458-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung TF, Liu AP-Y, Lim FS, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9-14 years:Results to month 12 from a randomized trial. Hum Vaccin Immunother. 2015;11:1689–702. doi: 10.1080/21645515.2015.1050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines:a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–9. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 39.MoH Malaysia academy of medicine. Management of cervical cancer. Clinical practice guidelines. 2003. [(accessed 01 December 2015)]. http://www.acadmed.org.my/cpg/CPG-Management%20of%20Cancer%20Cervix.pdf .
- 40.MoH Malaysia. Childhood immunisation coverage. 2014. [(accessed 20 May 2015)]. www.acadmed.org.my/view_file.cfm?fileid=191 .
- 41.Moscicki AB, Harper DM, Naud P, et al. Abstract presented at the 30th International Papillomavirus Conference and Clinical and Public Health Workshops, 28 February - 04 March 2015. Lisbon, Portugal: 2015. HPV-16/18 AS04-adjuvanted vaccine:sustained immunogenicity against HPV-31 and HPV-45 non-vaccine oncogenic types up to 9.4 years follow-up. [Google Scholar]
- 42.National pharmaceutical control bureau (NPCB), MoH Malaysia. Maklumat tambahan indikasi untuk upload pada laman web Year 2014 - Products approved for additional indication (DCA 281 - 24 November 2014) 2016. [(accessed 06 May 2016)]. http://npra.moh.gov.my/en/images/NewProduct/add-indication/Maklumat-tambahan-indikasi-DCA-274.pdf .
- 43.One Malaysia Community. HPV vaccine in Malaysia. 2009. [(accessed 30 October 2015)]. https://ecofrenone.wordpress.com/2009/10/02/hpv-vaccine-in-malaysia/
- 44.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA):final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 45.Razak NA, Mn K, Zubairi YZ, Naing NN, Zaki NM. Estimating the five-year survival of cervical cancer patients treated in hospital universiti sains malaysia. Asian Pac J Cancer Prev. 2013;14:825–8. doi: 10.7314/apjcp.2013.14.2.825. [DOI] [PubMed] [Google Scholar]
- 46.Suárez E, Smith JS, Bosch FX, et al. Cost-effectiveness of vaccination against cervical cancer:A multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccine. 2008;26:29–45. doi: 10.1016/j.vaccine.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 47.Szarewski A, Skinner SR, Garland SM, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against low-risk HPV types (PATRICIA randomized trial):an unexpected observation. J Infect Dis. 2013;208:1391–6. doi: 10.1093/infdis/jit360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor S, Ryser M, Mihalyi A, van Effelterre T. Response letter regarding the letter to the editors by Brown et-al. Hum Vaccin Immunother. 2016;12:1943–6. doi: 10.1080/21645515.2016.1151598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The Future II study group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 50.Tjalma W, Paavonen J, Naud P, et al. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against abnormal cytology and low-grade histopathological lesions in an oncogenic HPV-naïve population. Abstract n°A-171-0004-01446 presented at the 16th international meeting of the European society for gynaecological oncology (ESGO), 11 - 14 Oct, Belgrade, Serbia. Int J Gynecol Cancer. 2009;19:1008. [Google Scholar]
- 51.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Westra T, Stirbu-Wagner I, Dorsman S, et al. Inclusion of the benefits of enhanced cross-protection against cervical cancer and prevention of genital warts in the cost-effectiveness analysis of human papillomavirus vaccination in the Netherlands. BMC Infect Dis. 2013;13:75. doi: 10.1186/1471-2334-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization (WHO) Human papillomavirus vaccines:WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89:465–91. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


