Abstract
Background:
Breast cancer is a leading cause of death in women worldwide. Genetic polymorphisms have been reported to be important etiological factors. Murine double minute 2 (MDM2) T309G interacts with p53 and mutations in p53 are present in approximately 50% of all cancers. However, it has been reported that effect of the polymorphism on breast cancer risk may vary in different populations. Here, we therefore investigated whether there is an association between MDM2 T309G (rs2279744) polymorphism and breast cancer in a Turkish population.
Materials and Methods:
We analysed 110 patients with breast cancer and 138 matched? controls. For genotyping, polymerase chain reaction and restriction length fragment polymorphism methods were used.
Results:
A significant difference was observed between case and control groups with regard to the distribution of the MDM2 T309G polymorphism (p<0.05). There was a significantly higher frequency of the TT genotype in the control group (p=0.028; OR, 2.42; 95% CI, 1.09-5.37). However, we did not find any relationships among tumor grade and metastasis status and this polymorphism.
Conclusion:
This study indicates that the MDM2 T309G polymorphism GG genotype and the TG+GG combination may be risk factors for breast cancer in our Turkish population.
Keywords: Breast cancer, Murine double minute 2, MDM2 T309G, polymorphism
Introduction
Breast cancer is the most common frequently diagnosed malignancy among women and leading cause of cancer death in women worldwide (Siegel et al., 2017). And also, the incidence rate of breast cancer has been significantly increasing in Turkish population (Dogan and Toprak, 2014). It is well known that some potential risk factors may contribute to the onset of breast cancer. These risk factors include environmental, genetic alterations. Additionally genetic polymorphisms have also been reported to be one of the most important etiological factors which can affect the incidence of breast cancer (Wolff and Weston, 1997; Hulka and Moorman, 2001; Zhao et al., 2012). But the exact molecular mechanisms of breast cancer are still under intensive investigation.
The association between polymorphisms of genes with various functions and susceptibility to breast cancer has been reported by many studies (Zhao et al., 2012). MDM2 in these pathways contain functional single nucleotide polymorphisms (SNPs) which are related with tumour growth, DNA repair, apoptosis and angiogenesis (Hulka and Moorman, 2001; Boersma et al., 2006; Akisik et al., 2011). MDM2 gene is known as an oncogene, codes for a negative regulator of p53 that directly binds to and inhibits the p53 protein in absence of stress and exerts negative effect on DNA double strand break repair (Boersma et al., 2006; Akisik et al., 2011). SNP at nucleotide 309 characterized as a change of T to G. T-to-G SNP in the promoter region of MDM2 leads to increased MDM2 expression by binding of the transcriptional activator to promoter of MDM2. MDM2 T309G interacts with p53 and mutations in p53 are present in approximately 50% of all cancers (Bond et al., 2004; Copson et al., 2006; Wilkening et al., 2007; Zhao et al., 2012). Several studies have showed that there has been associated between MDM2 T309G and breast, colon cancer (Bond et al., 2006b), lymphoma and soft tissue sarcomas (Bond et al., 2006a).
In a previous study, we investigate the effect of MDM2 gene T309G polymorphism on development of lung cancer and gastric cancer in a Turkish population (Tas et al., 2017; Yilmaz et al., 2017). In the present study, we aimed to investigate the association between genetic polymorphisms of the MDM2 gene T309G polymorphism and breast cancer in Turkish population.
Materials and Methods
Study population and samples collection
Total 248 individuals consisted of 110 patients with breast cancer and 138 controls were investigated in this study. Patient group was admitted and performed to the definitive diagnosis by Department of Medical Oncology, Cumhuriyet University in Central Anatolia (Sivas) in the year of 2012 and 2013. In addition, histological classifications and clinic-pathological staging of cancer were performed according to criteria of the UICC Tumour-Node-Metastasis Classification of Malignant Tumours (TNM), seventh edition, 2010 (for lung cancer ICD-O C18-C20). Control group was selected from healthy, voluntary individuals that the individuals were matched with patients according to distribution of age. The study was confirmed by local ethics committee in Sivas (Ethics Committee of Cumhuriyet University). The decision number is 2014-04/37. Both patients and controls were informed about this study and written informed consent form. After a form consisted of questions about demographic features of individuals was filled for both groups, five ml of whole blood samples from 248 individuals were collected in EDTA containing tube.
DNA isolation
Peripheral blood samples (2 ml) were obtained and collected into citrate-containing tubes from all subjects. The DNA was extracted from whole blood using the salting out procedure as soon as the samples reached the laboratory (Miller et al., 1988).
MDM2 Genotyping
The MDM2 T309G polymorphism was analyzed using the polymerase chain reaction (PCR)-restriction fragment length polymorphism method. The following primers were used: Forward, 5’-CGC GGG AGT TCA GGG TAA AG-3’ and reverse, 5’-CTG AGT CAA CCT GCC CAC TG-3’ to amplify the MDM2 polymorphism. Amplification was performed using the following: 25 pmol each primer, 200 mM total dNTP, 1.5 mM MgCI2, 1µl PCR buffer and 2.5 U Taq DNA polymerase and 50-100 ng DNA in a total volume of 50 µl. The PCR program was initiated with denaturation at 95°C for 5 min, followed by 30 cycles of 94°C for 60 sec, 55°C for 60 sec (annealing) and 72°C for 60 sec (extension). The PCR was completed with a final extension cycle at 72°C for 5 min. Following confirmation of PCR amplification by 1.5% agarose gel electrophoresis, the amplified product was digested overnight with MspA1I restriction enzyme at 37°C and electrophoresed on 3% agarose gel stained with ethidium bromide and visualized under UV light. Genotypes were identified for the polymorphism as TT (157 bp), TG (157, 110 and 47 bp), or GG (110 and 47 bp) (Tas et al., 2017) (Figure 1).
Figure 1.
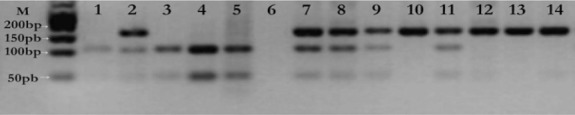
Imaging of RFLP Products of MDM2 T309G Polymorphism on 2.5% Agarose Gel. M-Marker (50 bp marker-Biolab); 6, Negatif control; 1, 3-5, GG homozygous polymorphic type (110 and 47 bp); 2, 7-9,11, TG heterozygoud genotype (157, 110, 47 bp); 10,12-14, TT homozygous wild type (157 bp).
Statistical Analysis
All statistical analyses were performed by using Statistical Package for Social Sciences Program-version 22.0 (SPSS inc., Chicago, IL, USA). Frequencies of characteristics and distributions of allele-genotype for this polymorphism between patients and controls were calculated with chi-square test (χ2). Hardy–Weinberg equilibrium (HWE) in genotype distributions of both groups was also evaluated via a χ2 test. We detected in our study that frequencies of genotype for controls fixed HWE, whereas genotype frequencies for patient group were not consisted with HWE. Therefore, we used Cochran’s and Mantel-Haenszel statistics for to calculate of odds ratio (OR). The crude OR in 95% confidence interval (CI) was calculated using Fisher’s exact test. P values of <0.05 were considered statistically significant.
Results
A total of 248 Turkish women were studied (110 patients with breast cancer and 138 healthy controls) in current study. The main characteristics of study groups have been shown in Table 1. No statistically significant difference was observed between cases and controls regarding the age (p=0.141). The family history of cancer, smoking habit and alcohol consumption frequencies were same in both groups (p>0.05). Frequencies of genotypes and OR values in both groups for MDM2 rs2279744 polymorphism were shown in table II. In statistical analysis using Chi-square (χ2) test, we observed that there was a significant difference between case and control groups for distribution of MDM2 rs2279744 polymorphism (p<0.05). There was a significantly increased frequency of the TT genotype in control group (p=0.028; OR, 2.42; 95% CI, 1,09-5,37).
Table 1.
Distribution of Selected Variables in Breast Cancer Cases and Controls
| Variables | Breast Cancer | Controls | χ2 | p |
|---|---|---|---|---|
| N (%) (n =110) | N (%) (n= 138) | |||
| Age (year±SD) | 51.67±14.02 | 54.08±11.03 | - | 0.14 |
| Age range | (30-80) | (21-90) | ||
| Smoking Habit | ||||
| No | 91 (82.7) | 123 (89.1) | 2.12 | 0.14 |
| Yes | 19 (17.3) | 15 (10.9) | ||
| Family History | ||||
| No | 82 (74.5) | 94 (68.1) | 1.22 | 0.26 |
| Yes | 28 (25.5) | 44 (31.9) | ||
| Tumour Size (Tx-T4) | ||||
| Tx | 3 (2.7) | |||
| T1 | 35 (31.8) | |||
| T2 | 60 (54.5) | |||
| T3 | 11 (10.0) | |||
| T4 | 1 (0.9) | |||
| Tumour Grade | ||||
| G1 | 25 (22.7) | |||
| G2 | 64 (58.2) | |||
| G3 | 21 (19.1) | |||
| Lymph node + | 69 (62.7) | |||
| Metastases + | 21 (19.1) | |||
SD, Standard derivation
Table 2.
Stratification Analyses between MDM2 SNP309 (rs2279744) Genotypes and Breast Cancer Risk
| Genotypes | Controls | Breast Cancer | p | Crude OR |
|---|---|---|---|---|
| n=138. N (%) | n=110. N (%) | |||
| TT | 46 (33.3) | 19 (17.3) | - | - |
| TG | 70 (50.7) | 69 (62.7) | 0.006* | 2.38 (1.27-4.47) |
| GG | 22 (16.0) | 22 (20.0) | 0.028* | 2.42 (1.09-5.37) |
| TG+GG | 92 (66.7) | 91 (82.7) | 0.004* | 2.39 (1.30-4.39) |
| TT+TG | 116 (84.0) | 88 (80.0) | 0.406 | 1.31 (0.68-2.53) |
p< 0.05 was considered as significant
We also investigated the effect of MDM2 rs2279744 polymorphism on tumor grade and metastasis status. Regarding these factors there were no significant effects of MDM2 rs2279744 polymorphism as shown in table III.
Table 3.
Comparison between Genotypes and Clinical Parameters
| Tumour Grade | Genotypes N (%) | χ2 value | p value | ||
|---|---|---|---|---|---|
| TT | TG | GG | |||
| G1 | 5 (20.0) | 17 (68.0) | 3 (12.0) | 8.818 | 0,066 |
| G2 | 7 (10.9) | 44 (68.8) | 13 (20.3) | ||
| G3 | 7 (17.3) | 8 (62.7) | 6 (20.0) | ||
| Metastases | |||||
| No | 16 (18.0) | 54 (60.7) | 19 (21.3) | 0.871 | 0,647 |
| Yes | 3 (14.3) | 15 (71.4) | 3 (14.3) | ||
| Tumour Size | |||||
| Tx | 0 (0) | 2 (66.7) | 1 (33.3) | 10.99 | 0,137 |
| T1 | 7 (20.0) | 24 (68.6) | 4 (11.4) | ||
| T2 | 11 (18.3) | 37 (61.7) | 12 (20.0) | ||
| T3 | 0 (0) | 6 (54.5) | 5 (45.5) | ||
| T4 | 1 (100.0) | 0 (0) | 0(0) | ||
| Lymph Node | |||||
| Nx | 1 (25.0) | 2 (50.0) | 1 (25.0) | 6.95 | 0,504 |
| N1 | 6 (16.2) | 26 (70.3) | 5 (13.5) | ||
| N2 | 9 (26.5) | 18 (52.9) | 7 (20.6) | ||
| N3 | 2 (10.0) | 14 (70.0) | 4 (20.0) | ||
Discussion
Early onset breast cancer is a multifactorial disease, and the genesis and progression of most breast cancers are influenced by both environmental and genetic factors (Beral et al., 2004). Potentially modifiable factors associated with increased breast cancer risk include weight gain after the age of 18 and/or being overweight or obese for postmenopausal breast cancer, use of combined estrogen and progestin, physical inactivity, and alcohol consumption. In addition, recent research indicates that long-term, heavy smoking may also increase breast cancer risk, particularly among women who start smoking before their first pregnancy (American Cancer Society). In this study, we investigated that between MDM2 SNP309 was associated with breast cancer risk in smoker. But there were no statistically significant of breast cancer risk (p=0,14, χ2=2,12). Any case-control study not reported that the interaction between MDM2 SNP309 was associated with evaluated breast cancer risk in smoker in the world. A family history of breast cancer seems to be the greatest risk factor for early onset breast cancer, and the risk is a function of the number of affected relatives, the degree of relationship, and the time of onset (Beral et al., 2004). Our study, there was no statistically significant difference was observed between cases and controls regarding the family history of cancer (p=0,26, χ2=1,22). Similar to our study, in a study that has made Koh et al. (2011) there was no statistically significant difference between cases and controls regarding the family history of cancer (OR: 1,49, %95 CI: 0,55–4,03) (Koh et al., 2011). But, Compbell et al., (2006) found that statistically significant difference between breast cancer cases and controls regarding the family history of cancer (OR: 0,99, %95 CI: 0,62–1,59) (Campbell et al., 2006).
The activation of p53 protein over cellular stress such as DNA damage and oncogene activation leads to induction of cell cycle detention and the activation of apoptotic cell death, and may account for the role of this tumor suppressor protein in preventing the accumulation of genomic alterations and tumor development (Jin and Levine, 2001). Somatic inactivating mutations of the p53 gene are found in over 50% of all human tumors (Lain and Lane, 2003). MDM2 directly binds to p53 and acts as a crucial negative modulator for maintaining function of p53 through regulating its location, stability, and activity (Harris and Levine, 2005). A subset of tumors overexpresses MDM2, which is associated with accelerated cancer progression (Freedman and Levine, 1999) and poor prognosis (Poyurovsky and Prives, 2006). Therefore, inherently consisting functional polymorphisms of the p53 gene have also been investigated for possible association with human susceptibility to cancer. In our study, we investigated that stratification analyses between MDM2 SNP309 genotypes and breast cancer risk. We found a significant association between MDM2 SNP309 and breast cancer risk for TG and GG genotypes in Turkish population when individuals with TG and GG genotypes were compared individuals with TT genotype (TG genotype: OR:2,38, %95 CI: 1,27–4,47 and GG genotype: OR: 2,42 %95 CI: 1,09–5,37) (Table II). Similarly, Alshatwi et al., (2012) found that MDM2 SNP309 (TG/GG) carriers among arab population associated with higher breast cancer risk (TG genotype: OR: 1,43, %95 CI: 1,12–2,02 and GG genotype: OR: 2,79 %95 CI: 2,04–3,92). But, Petenkaya et al., (2006) did not find significant association between for TG and GG genotypes in Turkish population (TG genotype: OR: 1,20, %95 CI: 0,67–2,12 and GG genotype: OR:1,14 %95 CI: 0,59–2,22). Yadav et al., (2016) is similar to their study did not find a significant correlation between TG/TG genotypes and breast cancer (TG genotype: OR:0,90, %95 CI: 0,66–1,23 and GG genotype: OR:1,11 %95 CI: 0,77–1,62). In this study we also investigated the relationship between SNP309 MDM2 polymorphism and clinical parameters, such as tumor grade, metastase, tumor size, lymph nodes. But we did not find a significant relationship between their (Table III). In the same way, Yadav et al., (2016) found no significant relationship their, but excluding metastasis (p=0, 04).
This study provides basic information about the genotype frequency distributions of polymorphisms of rs2279744 in the MDM2 gene studied. The MDM2 polymorphisms in other studies conducted in Turkey has not been evaluated in terms of clinical parameters, which is very important to understand whether or how much impact associated polymorphisms of MDM2 SNP309 and breast cancer risk. The results of the present study, in conjunction with the results regarding MDM2 SNP309 gene polymorphisms in a Turkish population, provide a framework for further studies concerning the role of this gene as a susceptibility many diseases, including certain cancers
Competing interest
The all authors have no conflicts of interest to declare.
Acknowledgements
Laboratory facilities in Department of Research Centre, Cumhuriyet University, and Faculty of Medicine (CUTFAM) were used in this study.
References
- 1.Akisik E, Yazici H, Dalay N. ARLTS1 MDM2 and RAD51 gene variations are associated with familial breast cancer. Mol Biol Rep. 2011;38:343–8. doi: 10.1007/s11033-010-0113-3. [DOI] [PubMed] [Google Scholar]
- 2.Alshatwi AA, Hasan TN, Shafi G, et al. A single-nucleotide polymorphism in the TP53 and MDM-2 gene modifies breast cancer risk in an ethnic Arab population. Fundam Clin Pharmacol. 2012;26:438–43. doi: 10.1111/j.1472-8206.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- 3.Beral V, Bull D, Doll R, et al. Collaborative group on hormonal factors in breast cancer:Breast cancer and abortion:collaborative reanalysis of data from 53 epidemiological studies, including 83,000 women with breast cancer from 16 countries. Lancet. 2004;363:1007–16. doi: 10.1016/S0140-6736(04)15835-2. [DOI] [PubMed] [Google Scholar]
- 4.Boersma BJ, Howe TM, Goodman JE, et al. Association of breast cancer outcome with status of p53 andMDM2 SNP309. J Natl Cancer Inst. 2006;98:911–9. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 5.Bond GL, Hirshfield KM, Kirchhoff T, et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006a;66:5104–10. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- 6.Bond GL, Hu W, Bond EE, et al. A single nucleotide polymorphism in theMDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Bond GL, Menin C, Bertorelle R, et al. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet. 2006b;43:950–2. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell IG, Eccles DM, Choong DY. No association of theMDM2 SNP309 polymorphism with risk of breast or ovarian cancer. Cancer Lett. 2006;240:195–7. doi: 10.1016/j.canlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Copson ER, White HE, Blaydes JP, et al. Influence of theMDM2 single nucleotide polymorphismSNP309 on tumour development inBRCA1 mutation carriers. BMC Cancer. 2006;6:80. doi: 10.1186/1471-2407-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dogan N, Toprak D. Female breast cancer mortality rates in Turkey. Asian Pac J Cancer Prev. 2014;15:7569–73. doi: 10.7314/apjcp.2014.15.18.7569. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DA, Levine AJ. Regulation of the p53 protein by theMDM2 oncoprotein--thirty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1999;59:1–7. [PubMed] [Google Scholar]
- 12.Harris SL, Levine AJ. The p53 pathway:positive and negative feedback loops. Oncogene. 2005;24:2899–908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 13.Hulka BS, Moorman PG. Breast cancer:hormones and other risk factors. Maturitas. 2001;38:103–13. doi: 10.1016/s0378-5122(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 14.Jin SK, Levine AJ. The p53 functional circuit. J Cell Sci. 2001;114:4139–40. doi: 10.1242/jcs.114.23.4139. [DOI] [PubMed] [Google Scholar]
- 15.Koh WP, Van Den Berg D, Jin A, et al. Combined effects of MDM2 SNP309 and TP53 R72P polymorphisms, and soy isoflavones on breast cancer risk among Chinese women in Singapore. Breast Cancer Res Treat. 2011;130:1011–9. doi: 10.1007/s10549-011-1680-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lain S, Lane D. Improving cancer therapy by non-genotoxic activation of p53. Eur J Cancer. 2003;39:1053–60. doi: 10.1016/s0959-8049(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 17.Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petenkaya A, Bozkurt B, Akilli-Ozturk O, et al. Lack of association between the MDM2-SNP309 polymorphism and breast cancer risk. Anticancer Res. 2006;26:4975–7. [PubMed] [Google Scholar]
- 19.Poyurovsky MV, Prives C. Unleashing the power of p53:lessons from mice and men. Genes Dev. 2006;20:125–31. doi: 10.1101/gad.1397506. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer Statistics 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 21.Tas A, Atabey M, Caglayan G, et al. Investigation of the association between theMDM2 T309G polymorphism and gastric cancer. Biomed Rep. 2017;7:469–73. doi: 10.3892/br.2017.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkening S, Bermejo JL, Hemminki K. MDM2 SNP309 and cancer risk:a combined analysis. Carcinogenesis. 2007;28:2262–7. doi: 10.1093/carcin/bgm191. [DOI] [PubMed] [Google Scholar]
- 23.Wolff MS, Weston A. Breast cancer risk and environmental exposures. Environ Health Perspect. 1997;105:891. doi: 10.1289/ehp.97105s4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav P, Masroor M, Tanwer K, et al. Clinical significance ofTP53 (R72P) andMDM2 (T309G) polymorphisms in breast cancer patients. Clin Transl Oncol. 2016;18:728–34. doi: 10.1007/s12094-015-1425-5. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz M, Tas A, Kacan T, et al. Is there a relation between Murine double minute 2 T309G polymorphism and lung cancer risk in the Turkish population? Turk J Biochem Journal. 2017;42:123–9. [Google Scholar]
- 26.Zhao E, Cui D, Yuan L, et al. MDM2 SNP309 polymorphism and breast cancer risk:a meta-analysis. Mol Biol Rep. 2012;39:3471–7. doi: 10.1007/s11033-011-1119-1. [DOI] [PubMed] [Google Scholar]


