Abstract
Objective:
The aim of this study was to determine the degree of association of BRCA1 promoter methylation with breast cancer in Asia.
Methods:
The study sample for the present meta-analysis was provided by published research articles on associations of BRCA1 promoter methylation with breast cancer in Asia accessed through databases on PubMed, ProQuest and EBSCO published between 1997 and November 2017. Pooled odds ratios (OR) were calculated with fixed and random-effect models. Data were processed using Review Manager 5.3 (RevMan 5.3).
Results:
Of a total of 475 articles, 11 studies were included in our systematic review with meta-analysis of relevant data. The results showed a highly significant association between BRCA1 promoter methylation with breast cancer in Asia (OR = 8.78 [95% CI 4.15-18.56, p < 0.00001]).
Conclusion:
This analysis confirmed association between BRCA1 promoter methylation and breast cancer in Asia. BRCA1 promoter assessment might be a predictive or diagnostic aid for breast cancer prediction.
Keywords: BRCA1, methylation, breast cancer-Asia
Introduction
Breast cancer ranks first among all cancer diseases in women encountered worldwide (Torre et al., 2015). An estimated 23% or 1,383,500 new cases a year and 14% or 458,400 cases will end in death (Jemal et al., 2011).
Breast cancer in biomolecular level is a disease caused by gene mutations triggered by multifactor: dietary factors, environmental factors, and heredity. There are several risk factors such as age (over 50 years), reproductive factors (nullipara, early menarche, late menopause), family history, history of benign tumors. There are also some risk factors that can not be fully understood, such as malnutrition, alcohol consumption, no exercise, radiation and hormonal therapy (Harahap et al., 2017; Nindrea et al., 2017). In addition, there is also one factor that is suspected to be the cause of breast cancer is the influence of loss of expression of tumor suppressor gene.
One of the tumor suppressor genes that play an important role in carcinogenesis of breast cancer is BRCA1 (Breast Cancer gene 1). The BRCA 1 protein has a role to maintain genome stability through cellular function, such as gene transcription, response to DNA damage, cell cycle regulation, and ubiquitination. Inactivation of the BRCA1 gene interferes with gene stability, particularly in breast and ovarian cancers (Bar-Sade et al., 1998). The inactivation of this BRCA1 gene may occur in hereditary, the mutation of the gene sequence and can also be sporadic. The composition of the BRCA1 gene is substantially fixed, but there is a methylation on CpG Island at the location of the BRCA1 promoter. Focal hypermethylation at the tip regions of 5’CpG island causes the gene to not be transcribed. Changes in DNA methylation profiles can lead to sporadic breast cancer without alteration of the base order in the BRCA1 gene (Iwamoto et al., 2010).
Several previous studies have shown that BRCA1 promoter methylation is associated with an increased risk of breast cancer (Dobrovic and Simfendorfer, 1997; Iwamoto et al., 2011). However, these results also have differences that suggest BRCA1 promoter methylation has no effect on the occurrence of breast cancer in women (Cho et al., 2010). However, the research results are not always consistent. Therefore, this study aims to determine the relationship of BRCA1 promoter methylation with breast cancer in Asia with some research through the Meta-analysis study so that the conclusion drawn have stronger strength.
Materials and Methods
Study design and research sample
This research is a quantitative research with Meta-analysis study design. Meta-analysis is used to find out the association of BRCA1 promoter methylation with breast cancer in Asia. The research sample is a published research article on the internet through the database on PubMed, ProQuest and EBSCO published between 1997 and November 2017. The inclusion criteria of this study sample research with case-control and cohort study, research is in the region of Asia. Exclusion criterion is research which not available in full-text form.
Operational definitions
Variables in this study include independent variable is BRCA1 promoter methylation. While the dependent variable is breast cancer.
Research procedure
This study is conducted by collecting data through the identification of published research articles on association of BRCA1 promoter methylation with breast cancer in Asia on the internet on PubMed, ProQuest and EBSCO databases Figure 1.
Figure 1.
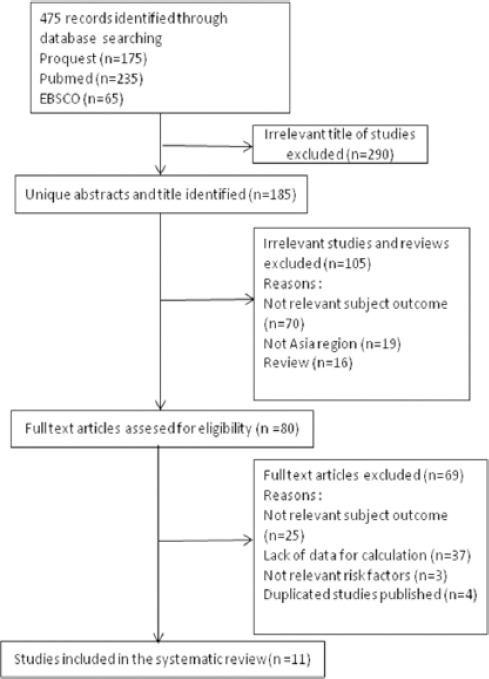
Flow Diagram Research Procedure
Data collection technique
Search is limited only to English language articles. This article type is limited to journal articles. Research subjects are limited only to research with subjects of a human. The time of publication is limited from 1997 to November 2017. Articles with potentially relevant titles are reviewed abstract, while irrelevant articles are excluded. Furthermore, the article is reviewed abstract. Articles that have potentially relevant abstracts will then be reviewed in full-text. While irrelevant articles are excluded. Furthermore, the article is excluded based on the location of the study that is not specific or outside the Asia region, research variables (the independent variable is BRCA1 promoter methylation and the dependent variable is breast cancer) and the design of the study (case-control or cohort study).
Data analysis
The analysis held to get the value of pooled odds ratio which is the combined odds ratio value from the research. Data analysis by Mantel-Haenszel method using fixed effect model and DerSimonian-Laird random-effect model. Data is analyzed by using Review Manager 5.3 (RevMan 5.3).
Results
Identification of 475 articles, done by review through the title of the articles, then reviewed abstract, then reviewed in full-text form. Irrelevant articles are excluded. Selection of studies conducted to obtain 11 studies related to the association of BRCA1 promoter methylation with breast cancer in Asia Table 1.
Table 1.
Systematic Review of Association of BRCA1 Promoter Methylation with Breast Cancer in Asia
| First Author, Year | Region | Methods | Materials | Patients Characteristics | Number of Patients | |
|---|---|---|---|---|---|---|
| Cases | Control | |||||
| Chen et al., 2003 | China | MSP | Tissue | Invasive ductal breast cancer | 93 | 20 |
| Bhavani et al., 2009 | India | MSP | Tissue | Sporadic breast cancer | 104 | 48 |
| Jing et al., 2010 | China | MSP | Blood | Sporadic breast cancer | 50 | 50 |
| Sharma et al., 2010 | India | MSP | Tissue | Operable primary breast cancer | 100 | 30 |
| Al-moghrabi et al., 2011 | Saudi Arabic | MSP | Blood | Sporadic breast cancer | 7 | 73 |
| Bal et al., 2012 | India | MSP | Tissue | Sporadic breast cancer | 74 | 21 |
| Hsu et al., 2013 | Taiwan | PCR | Tissue | Early-stage breast cancer | 21 | 21 |
| Hasan et al., 2013 | India | MSP | Tissue | Sporadic breast cancer | 29 | 26 |
| Otani et al., 2014 | Japan | PCR | Tissue | Primary breast cancer | 15 | 15 |
| Saelee et al., 2014 | Thailand | MSP | Tissue | Invasive ductal breast cancer | 38 | 7 |
| Cai et al., 2016 | China | Pyrosequencing | Tissue | Sporadic breast cancer | 154 | 154 |
Based on the results of systematic review there are 11 studies analyzed by meta-analysis Table 2. Meta-analysis of the association of BRCA1 promoter methylation with breast cancer in Asia Figure 2.
Table 2.
Meta-analysis Association of BRCA1 Promoter Methylation with Breast Cancer in Asia
| First Author, Year | Weight (%) | Pooled Odds Ratio (95% CI) | p-value | Heterogeneity Test | ||
|---|---|---|---|---|---|---|
| χ2 | p value | I2 (%) | ||||
| Study data (BRCA1 promoter methylation) | 8.78 (4.15-18.56) | <0.0001 | 18.10 | 0.05 | 45 | |
| Chen et al., 2003 | 5.3 | |||||
| Bhavani et al., 2009 | 11.7 | |||||
| Jing et al., 2010 | 5.3 | |||||
| Sharma et al., 2010 | 5.3 | |||||
| Al-moghrabi et al., 2011 | 9.7 | |||||
| Bal et al., 2012 | 5.2 | |||||
| Hsu et al., 2013 | 12.2 | |||||
| Hasan et al., 2013 | 13.0 | |||||
| Otani et al., 2014 | 10.8 | |||||
| Saelee et al., 2014 | 7.4 | |||||
| Cai et al., 2016 | 14.1 | |||||
Figure 2.
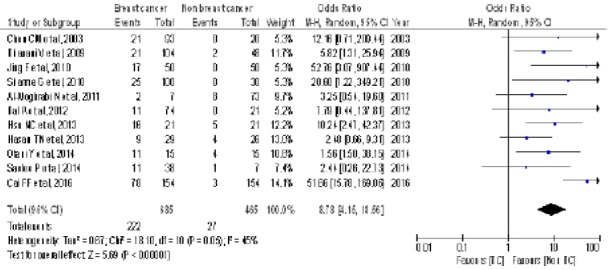
Forest Plots Association of BRCA1 Promoter Methylation with Breast Cancer in Asia
Figure 2 meta-analysis of BRCA1 promoter methylation with breast cancer in Asia (OR = 8.78 [95% CI 4.15-18.56, p <0.00001]). Funnel plot of association of BRCA1 promoter methylation with breast cancer in Asia Figure 3.
Figure 3.
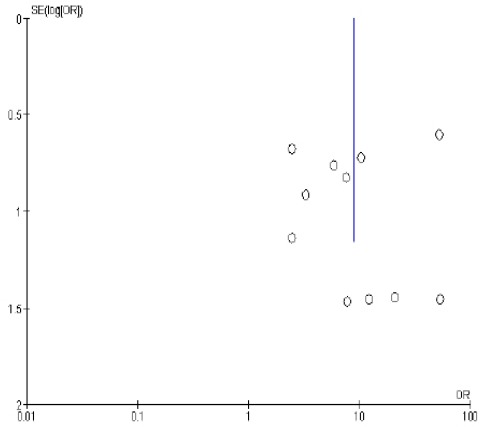
Funnel Plots Association of BRCA1 Promoter Methylation with Breast Cancer in Asia
Figure 3 shows BRCA1 promoter methylation has a variation of homogeneous research for the occurrence of breast cancer, this is because the plot is symmetrical based on the vertical line means that if the analysis is done on the population, time and place and different conditions then the results will be consistent.
Discussion
The result of a meta-analysis of BRCA1 promoter methylation with breast cancer in Asia (OR = 8.78 [95% CI 4.15-18.56, p <0.00001]). BRCA1 promoter methylation has a variation of homogeneous research or no heterogeneity between studies for the occurrence of breast cancer.
Several studies have suggested the role of BRCA1 methylation in the aggressiveness of breast cancer, and BRCA1 tumor methylation is found primarily in tumors of grade III rather than grade I and II (Wei at al., 2005; Birgisdottir et al., 2006; BenGacem et al., 2012). In previous studies, loss of gene expression in breast cancer, often associated with BRCA1 promoter hypermethylation. For example, several studies have shown that BRCA1 promoter methylation is associated with a decrease in mRNA BRCA1 levels in clinical breast cancer specimens, and also with decreased levels of breast cancer cell protein and sporadic breast cancer (Baldwin et al., 2000; Mirza et al., 2007; Bal et al., 2012).
Methylation is not the only mechanism to reduce BRCA1 protein expression (Sharma et al., 2010). Therefore, some mechanisms affect the inactivation of BRCA1 function in breast cancer (Rice et al., 2000). Several studies have shown that mutations, loss of heterozygosity, and deletion may also suppress BRCA1 expression in invasive sporadic breast tumors (Birgisdottir et al., 2006)
BRCA1 promoter methylation is common in sporadic breast cancer. BRCA1 promoter methylation measurements can actually be a new diagnostic tool (Jemal et al., 2011; Li et al., 2013; Pace and Keating, 2014). Previous research has shown that CpG island hypermethylation promoters to tumor suppressor genes may occur earlier in tumor development, which has implications for early detection of cancer, especially in people with breast cancer risk with a family history of previous breast cancer (Stefansson et al., 2011; Wong et al., 2011). Patients who had BRCA1 promoter methylation had significantly worse disease-free survival than patients with non-methylated BRCA1 promoters (Xu et al., 2011). Attempts to perform BRCA1 promoter methylation detection will enable adjustment of antitumor therapy and apply the appropriate treatment and care protocols to patients.
Methylation status in cancer tissue can be used as a tool to understand the gradual molecular changes in the phase of carcinogenesis. Breast cancer shows a drastic change in the status of DNA methylation which further leads to chromosomal instability and suppressor tumor silencing gene. From the epigenetic analysis of cancer tissue found an association between epigenetic and cancer histopathologic types (Kanal, 2008). Methylation of BRCA1 promoters can be used as a treatment guide, breast cancer cell cultures with BRCA1 promoter hypermethylation (UACC3199 and HCC-38 cell culture) are equally as good as cisplatin chemotherapy, such as culture cells with BRCA1 mutation (MDA-MB-436 cell culture) (Stefansson et al., 2011). In addition, Triple Negative Breast Cancer (TNBC) has a poor prognosis and response. TNBC by methylation of BRCA1 promoters has a good response to chemotherapy on the basis of Anthraxline in sporadic breast cancer (Ignatov et al., 2013). Likewise, the use of anti-methylation drugs, such as 5 Aza and hydralazine which can relinquish methyl groups binding to CpG Island can provide new hope in breast cancer patients, especially with advanced stages.
However, certain limitations of the study should be considered. First, the number of studies contained in the present meta-analysis is relatively small, particularly in Asia populations, and the results should be confirmed in large samples. Second, the associations among BRCA1 promoter methylation, the prognosis of patients and the negative status of the breast cancer-related therapeutic target receptors should be further investigated.
In conclusion, the results of this meta-analysis show that BRCA1 promoter methylation was associated with an increased risk of breast cancer. The results of this study recommend the need for large-scale studies that use uniform criteria on detection methods for methylation and sample materials before the BRCA1 promoter method can be a predictive or diagnostic biomarker useful for breast cancer patients and applied to future therapeutic strategies.
Acknowledgements
The authors would like to thank Assoc. Hasni Hasanuddin, MPH for collecting data. Mac Arif Hamdanas, MA for translating.
References
- 1.Al-Moghrabi N, Al-Qasem ABJS, Aboussekhra A. Methylation-related mutations in the BRCA1 promoter in peripheral blood cells from cancer-free women. Int J Oncol. 2011;39:129–35. doi: 10.3892/ijo.2011.1021. [DOI] [PubMed] [Google Scholar]
- 2.Bal A, Verma S, Joshi K, et al. BRCA1-methylated sporadic breast cancers are BRCA-like in showing a basal phenotype and absence of ER expression. Virchows Arch. 2012;461:305–12. doi: 10.1007/s00428-012-1286-z. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin RL, Nemeth E, Tran H, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma:a population-based study. Cancer Res. 2000;60:5329–33. [PubMed] [Google Scholar]
- 4.Bar-Sade RB, Kruglikova A, Modan B, et al. The 185delAG BRCA1 mutation originated before the dispersion of Jews in the diaspora and is not limited to Ashkenazim. Hum Mol Genet. 1998;7:801–5. doi: 10.1093/hmg/7.5.801. [DOI] [PubMed] [Google Scholar]
- 5.Ben Gacem R, Hachana M, Ziadi S, et al. Contribution of epigenetic alteration of BRCA1 and BRCA2 genes in breast carcinomas in Tunisian patients. Cancer Epidemiol. 2012;36:190–7. doi: 10.1016/j.canep.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Bhavani V, Srinivasulu M, Ahuja YR, Hasan Q. Role of BRCA1, HSD17B1 and HSD17B2 methylation in breast cancer tissue. Cancer Biomark. 2009;5:207–13. doi: 10.3233/CBM-2009-0105. [DOI] [PubMed] [Google Scholar]
- 7.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, et al. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai FF, Chen S, Wang MH, et al. Pyrosequencing quantified methylation level of BRCA1 promoter as prognostic factor for survival in breast cancer patient. Oncotarget. 2016;7:27499–510. doi: 10.18632/oncotarget.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CM, Chen HL, Hsiau THC, et al. Methylation target array for rapid analysis of CpG island hypermethylation in multiple tissue genomes. Am J Patol. 2003;163:37–45. doi: 10.1016/S0002-9440(10)63628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho YH, Yazici H, Wu HC. Aberrant promoter hypermethylation and genomic hypomethylation in tumor, adjacent normal tissues and blood from breast cancer patients. Anticancer Res. 2010;30:2489–96. [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrovic A, Simpfendorfer D. Methylation of the BRCA1 gene in sporadic breast cancer. Cancer Res. 1997;57:3347–50. [PubMed] [Google Scholar]
- 12.Harahap WA, Ramadhan Khambri D, et al. Outcomes of trastuzumab therapy for 6 and 12 months in Indonesian national health insurance system clients with operable HER2-positive breast cancer. Asian Pac J Cancer Prev. 2017;18:1151–7. doi: 10.22034/APJCP.2017.18.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan TN, Leena Grace B, Shafi G, Syed R. Association of BRCA1 promoter methylation with rs11655505 (c.2265C>T) variants and decreased gene expression in sporadic breast cancer. Clin Transl Oncol. 2013;15:555–62. doi: 10.1007/s12094-012-0968-y. [DOI] [PubMed] [Google Scholar]
- 14.Hsu NC, Huang YF, Yokoyama KK, et al. Methylation of BRCA1 promoter region is associated with unfavorable prognosis in women with early-stage breast cancer. PLoS One. 2013;8:e56256. doi: 10.1371/journal.pone.0056256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignatov T, Poehlmann A, Ignatov A, et al. BRCA1 promoter methylation is a marker of better response to anthracycline-based therapy in sporadic TNBC. Breast Cancer Res Treat. 2013;141:205–12. doi: 10.1007/s10549-013-2693-9. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto T, Yamamoto N, Taguchi T, Tamaki Y, Noguchi S. BRCA1 promoter methylation in peripheral blood cells is associated with increased risk of breast cancer with BRCA1 promoter methylation. Breast Cancer Res Treat. 2011;129:69–77. doi: 10.1007/s10549-010-1188-1. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Bray F, Ferlay J, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 18.Jing F, Yuping W, Yong C, et al. CpG island methylator phenotype of multigene in serum of sporadic breast carcinoma. Tumour Biol. 2010;31:321–31. doi: 10.1007/s13277-010-0040-x. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Kong X, Xu C, Zuo X, Yang Q. Prognostic significance of mammalian sterile 20-like kinase 1 in breast cancer. Tumour Biol. 2013;34:3239–43. doi: 10.1007/s13277-013-0895-8. [DOI] [PubMed] [Google Scholar]
- 20.Mirza S, Sharma G, Prasad CP, et al. Promoter hypermethylation of TMS1, BRCA1, ERalpha and PRB in serum and tumor DNA of invasive ductal breast carcinoma patients. Life Sci. 2007;81:280–7. doi: 10.1016/j.lfs.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Nindrea RD, Aryandono T, Lazuardi L. Breast cancer risk from modifiable and non-modifiable risk factors among women in Southeast Asia:A meta-analysis. Asian Pac J Cancer Prev. 2017;18:3201–6. doi: 10.22034/APJCP.2017.18.12.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otani Y, Miyake T, Kagara N, et al. BRCA1 promoter methylation of normal breast epithelial cells as a possible precursor for BRCA1-methylated breast cancer. Cancer Sci. 2014;105:1369–76. doi: 10.1111/cas.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311:1327–35. doi: 10.1001/jama.2014.1398. [DOI] [PubMed] [Google Scholar]
- 24.Rice JC, Ozcelik H, Maxeiner P, et al. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis. 2000;21:1761–5. doi: 10.1093/carcin/21.9.1761. [DOI] [PubMed] [Google Scholar]
- 25.Saelee P, Chaiwerawattana A, Ogawa K, et al. Clinicopathological significance of BRCA1 promoter hypermethylation in Thai breast cancer patients. Asian Pac J Cancer Prev. 2014;15:10585–89. doi: 10.7314/apjcp.2014.15.24.10585. [DOI] [PubMed] [Google Scholar]
- 26.Sharma G, Mirza S, Parshad R, et al. Clinical significance of promoter hypermethylation of DNA repair genes in tumor and serum DNA in invasive ductal breast carcinoma patients. Life Sci. 2010;87:83–91. doi: 10.1016/j.lfs.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Stefansson OA, Jonasson JG, Olafsdottir K, et al. CpG island hypermethylation of BRCA1 and loss of pRb as co-occurring events in basal/triple-negative breast cancer. Epigenetics. 2011;6:638–49. doi: 10.4161/epi.6.5.15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 29.Wei M, Grushko TA, Dignam J, et al. BRCA1 promoter methylation in sporadic breast cancer is associated with reduced BRCA1 copy number and chromosome 17 aneusomy. Cancer Res. 2005;65:10692–9. doi: 10.1158/0008-5472.CAN-05-1277. [DOI] [PubMed] [Google Scholar]
- 30.Wong EM, Southey MC, Fox SB, et al. Constitutional methylation of the BRCA1 promoter is specifically associated with BRCA1 mutation-associated pathology in early-onset breast cancer. Cancer Prev Res (Phila) 2011;4:23–33. doi: 10.1158/1940-6207.CAPR-10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Gammon MD, Zhang Y, et al. Gene promoter methylation is associated with increased mortality among women with breast cancer. Breast Cancer Res Treat. 2010;121:685–92. doi: 10.1007/s10549-009-0628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


