Abstract
Backgrounds:
Colorectal (CRC) is one of the main cause of cancer worldwide. The search for noninvasive markers for diagnosis and monitoring as the use of analytical technologies such as mass spectrometry (MS), which allowed the search for lipid metabolites as candidates for probable biomarkers are needed.
Objective and Methods:
The objective was to establish the lipid profile of patients with locally advanced, unresectable or metastatic CRC. Peripheral blood was collected from patients with CRC and controls with normal colonoscopy. After lipid extraction, the samples were processed and analyzed in the MALDI TOF / TOF equipment. From the data matrix, the statistical analyzes were performed by the principal component analysis methods and the least squares discriminant analysis. The importance of the variable in the projection was used to identify the ions that had the greatest discriminatory effect between the groups.
Results:
Eight lipids were identified as potential biomarkers and a multiple logistic regression model was proposed to calculate the performance of the test where we observed values of AUC 0.87, sensitivity 88.33% and specificity 83.78% and for a validation test with 1,000 permutations a p <0.001. The classes of lipids found were sphingolipids, glycerophospholipids and policetidis. The strength of the association between the peak intensities of these lipids and the presence of CRC make these metabolites candidates for possible biomarkers. The sphingolipid (m / z = 742.98869) could be a biomarker in monitoring patients with CRC. In the survival analysis, three lipids showed a prognostic value for colorectal cancer, sphingolipid (m / z = 857.11525) and policetidis (m / z = 876.20796) and glycerophospholipid (m / z = 1031.54773).
Keywords: Lipidomic, colorectal cancer, biomarker
Introduction
Colorectal cancer (CCR) is a great health problem worldwide, with more than 1.2 million new cases per year. It is the third most common cancer and the first in the gastrointestinal tract (Allemani et al., 2015).
Cancer cells secrete various factors including matrix metalloproteinases, which degrade the basal lamina, releasing tumor cells. The cells are then guided by specific molecules to invade blood from endothelial cells, that is a barrier to reach the bloodstream. The vascular system serves as a path for the migrant cells to reach a target organ where they pass again through the barriers of endothelial cells. Upon entering these sites, they are lodged in the basal lamina and begin to proliferate leading to the formation of a tumor. Cell membranes are closely involved in these steps and are mainly composed of lipids and proteins. The lipids are responsible for at least 50% of the total membrane mass (Chiang and Massagué, 2008; Hunter et al., 2008).
Many studies look for methods that can improve the accuracy in diagnosis, prognosis and therapeutic strategies, searching for precise molecular markers tailored for each patient. Noninvasive methods are also required for the diagnosis and follow-up of CRC patients (Alexander et al., 2016; Mirnezami et al., 2012).
The advances in genomics, with the understanding that not only the knowledge of the DNA sequence but also the direct analysis of the products encoded by these genes and their metabolites, has led to the development of omics. They allow the characterization of global changes associated with disease conditions and, consequently, the identification of new biomarkers and metabolic pathways (Laterza et al., 2006; Weckwerth et al., 2004).
Recent advances in analytical technologies such as mass spectrometry (MS), which emerged as an analytical detection tool, supporting the profile determination of biologically active substances such as lipids, enabled the lipidomic research. As a branch of metabolomics, lipidomics aims the complete analysis of lipid species and their biological roles that has attracted increasing attention in recent years as a promising area for the detection of new biomarkers in CRC (Donato et al., 2015; Li et al., 2014).
The aim of this study was to establish the lipid profile of patients with locally advanced, unresectable or metastatic colorectal cancer and to identify prognostic lipid biomarkers for colorectal cancer using MALDI / TOF-MS mass spectrometry techniques.
Materials and Methods
A prospective cohort study in CRC patients with advanced tumors (T4, unresectable or metastatic) of both genders, aged between 20 and 80 years on treatment by the Oncology Group of the complex Hospital São Paulo and Universidade Federal de São Paulo was done. The study was approved by a local committee and the patients and controls signed an informed consent prior to their inclusion.
The individuals who participated in the study were divided into 2 groups: the control group and the colorectal cancer group.
All patients in the control group had a normal colonoscopy. Blood was collected after 12 hours of fasting. In the control group before the colonoscopy and in the cancer group before the surgical procedure. In 18 patients with CRC, group blood was collected 30-60 days after colorectal cancer resection.
After centrifugation for 15min at 3,000 rpm, for plasma separation, the lipids were extracted according to the protocol described by Bligh-Dyer. The samples were resuspended in 8μL of methanol, placed in duplicate of 1μL per spot on MTP 384 plate (Bruker Daltonics, Bremen, Germany) and topped with 1μL of 2,5-dihydroxybenzoic acid (DHB) and 4-hydroxycinnamic acid (CHCA), both at the concentration of 7mg/ml, 0.01% trifluoroacetic acid (TFA), dissolved in 70% methanol and 30% water. The spectra acquisition was performed in the AUTOFLEX SPEEDY MALDI TOF / TOF equipment (Bruker Daltonics, Bremen, Germany) in a positive mode in the range of 700-1200 Da, with frequency 500 u.a., 1,000 shots / second and 90% power.
Statistical Analysis
Statistical processing was done in MetaboAnalyst 3.0 software (http://www.metaboanalyst.ca).
For multivariate analysis, the principal component analysis (PCA) and the least squares discriminant analysis (PLS-DA) were used. The values of R2 and Q2, found through cross-validation, were used to determine the quality of the models formed by PLS-DA. The parameter R2 was a representation of how much variation within the set was explained by the components of the model and the parameter Q2 indicates the power of projection of the model. The PLS-DA models were constructed and the importance of the variable in the projection was used to identify the 10 ions that had the greatest discriminatory effect between the groups in the component with the highest projection power.
ROC curves were built to evaluate the performance of potential biomarkers, using univariate and multivariate analysis. For each lipid survival curves of Kaplan Meir was done according to the cutoff value found by the ROC curve.
Results
Thirty-seven subjects had been included in the control group and 36 patients in the cancer group. The CRC group had a great number of males (66.67%). The mean age was similar between the groups. Most of the tumors were located on the left side (left colon, sigmoid or rectum) and were well or moderately differentiated (44.44%). Most of the patients had distant metastases (66%) (Table 1).
Table 1.
Descriptive Analysis of the Cancer and Control Groups
| Group Control (n = 37) Group Cancer (n = 36) | |||||
|---|---|---|---|---|---|
| Gender | |||||
| Male | 15 | 33.33% | 24 | 66.67% | p=0.01 |
| Female | 22 | 66.67% | 12 | 33.33% | |
| Age | |||||
| (mean +/- SD) | 58.24 | (14,49) | 61.69 | (12,27) | p=0.13 |
| Max | 84 | 85 | |||
| Min | 28 | 36 | |||
| BMI | |||||
| Underweight | 2 | 5.56% | |||
| Normal weight | 21 | 58.33% | |||
| Overweight | 13 | 36.11% | |||
| Location of the tumor | |||||
| Colon right | 4 | 11.11% | |||
| Colon left | 32 | 88.89% | |||
| Degree of differentiation | |||||
| Well-differentiated | 10 | 27.78% | |||
| Moderately differentiated | 16 | 44.44% | |||
| Poorly differentiated | 10 | 27.78% | |||
| Metastasis | |||||
| Regional lymph nodes | 6 | 16.67% | |||
| Distant | 24 | 66.67% | |||
| Without metastasis | 6 | 16.70% | |||
| Pretreatment | |||||
| Without pretreatment | 17 | 47.22% | |||
| QT e/ou RT | 19 | 52.78% | |||
From the data matrix generated by the MS of the 73 samples of lipids, 371 Ions was obtained. PLS-DA was used to evaluate whether there was a difference between the groups. Supervised methods such as PLS-DA try to find components that separate the observations considering the previously known classes (Figure 1)
Figure 1.
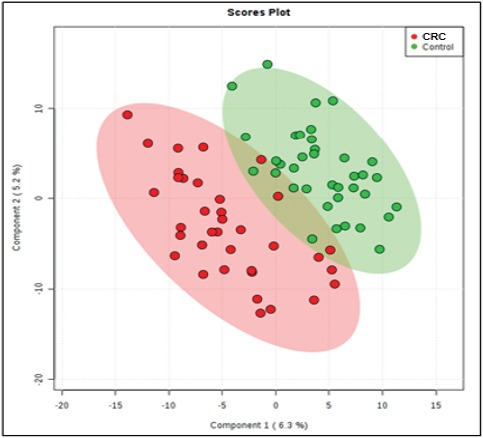
PLS-Da Score Plot Shows Groups Separation According to the Principal Components 1 (6.3%) and 2 (5.2%). The PCA analysis indicates intrinsic differences between the groups, which could be observed by the MALDI-TOF MS metabolomic profiling.
Figure 2 shows the ROC curves of the eight first VIP lipids listed in Table 3 in cancer patients. The accuracy, sensitivity, and specificity of each lipid for detecting cancer patients were shown in Table 2. In addition, the OR was performed and the association between the higher and lower ions expression and the presence of CRC were evaluated. The ions 881, 882, 907 and 707 presented higher strength of association for CRC were increased whereas the ions 704, 857, 876 and 1031 had a higher binding strength for CRC were lower, when compared between groups.
Figure 2.
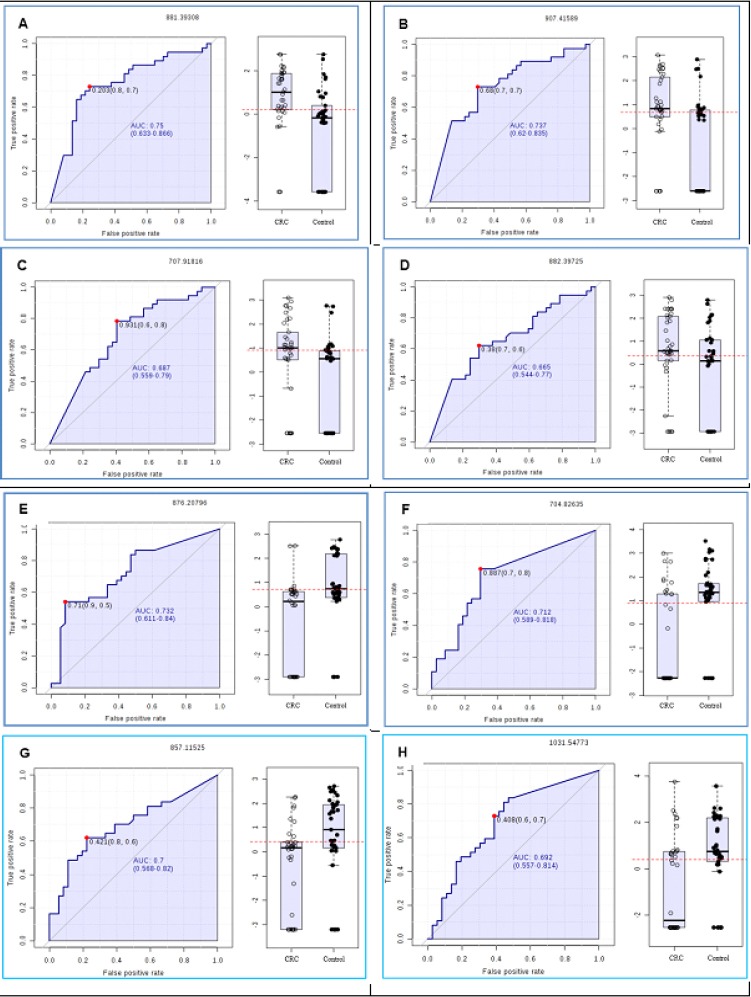
A. The ROC Curve Analysis and Box-Plot Shows AUCs, Variable that Indicates the Accuracy of the Each Biomarker for CRC Group (A,B, C and D) and Classification in Controls (E,F,G and H). Note that the ROC curve analysis was performed only considering the biomarkers selected by PLS analysis
Table 3.
Ions Identified Through the Analysis of PLS-DA
| m/z | Categoria | Formula | Sub Classe |
|---|---|---|---|
| 907.41589 | Sphingolipids (SP) | C79H140N4O37 | Gangliosides (SP0601) |
| 857.11525 | Sphingolipids (SP) | C114H201N5O58 | Galβ1-4GlcNAcβ1-3Galβ1-4Glc- (Neolacto series) [SP0505] |
| 704.82635 | Sphingolipids (SP) | C64H117NO27 | GalNAcβ1-3Galα1-4Galβ1-4Glc- (Globo series) [SP0502] |
| 881.39308 | Glycerophospholipid (GP) | C76H133O38P | Diacilglicerofosfoinositolglicano (GP1501) |
| 876.20796 | Policetidios (PK) | C36H43O25 | Anthocianidina (PK1201) |
| 1031.54773 | Glycerophospholipid (GP) | C50H89O18P | Diacilglicerofosfoinositolglicano (GP1501) |
| 707.91816 | Sphingolipids (SP) | C64H125NO25P2 | Ceramida fosfoinositol (SP0303) |
| 882.39725 | Glycerophospholipid (GP) | C76H135O38P | Diacilglicerofosfoinositolglicano (GP1501) |
Table 2.
Analysis of the Protection and Risk of Colorectal Cancer According to the Intensity of Each Ion
| Ion [cut-off] | Cancer (%) | Control (%) | Odds Ratio (IC 95%) | P | SEN. | ESP. | AUC | |
|---|---|---|---|---|---|---|---|---|
| 704.82635 | < cut-off | 26 (35.62) | 9 (12.33) | 0.12 | <0.001 | 0.757 | 0.722 | 0.718 |
| [825] | ≥ cut-off | 10 (13.70) | 28 (38.36) | (0.04 - 0.35) | ||||
| 857.11525 | < cut-off | 31 (42.47) | 24 (32.88) | 0.3 | 0.033 | 0.22 | 0.778 | 0.702 |
| [1130] | ≥ cut-off | 5 (6.85) | 13 (17.81) | (0.09 - 0.95) | ||||
| 876.20796 | < cut-off | 33 (45.21) | 28 (38.36) | 0.28 | 0.06 | 0.541 | 0.917 | 0.732 |
| [918] | ≥ cut-off | 3 (4.11) | 9 (12.33) | (0.07 - 1.15) | ||||
| 1031.54773 | < cut-off | 23 (31.51) | 10 (13.70) | 0.21 | 0.001 | 0.73 | 0.611 | 0.693 |
| [486] | ≥ cut-off | 13 (17.81) | 27 (36.99) | (0.08 - 0.57) | ||||
| 882.39725 | < cut-off | 15 (20.55) | 25 (34.25) | 2.92 | 0.025 | 0.62 | 0.694 | 0.669 |
| [866] | ≥ cut-off | 21 (28.77) | 12 (16.34) | (1.12 - 7.58) | ||||
| 907.41589 | < cut-off | 15 (20.55) | 28 (38.36) | 4.36 | 0.003 | 0.73 | 0.694 | 0.731 |
| [743] | ≥ cut-off | 21 (28.77) | 9 (12.33) | (1.60 - 11.86) | ||||
| 881.39308 | < cut-off | 21 (28.77) | 30 (41.10) | 3.06 | 0.033 | 0.73 | 0.75 | 0.745 |
| [1420] | ≥ cut-off | 15 (20.55) | 7 (9.59) | (1.06 - 8.80) | ||||
| 707.91816 | < cut-off | 19 (26.03) | 30 (41.10) | 3.83 | 0.009 | 0.784 | 0.611 | 0.701 |
| [912] | ≥ cut-off | 17 (23.29) | 7 (9.59) | (1.34 10.97) | ||||
SEN., Sensitivity; ESP., Specitivity; AUC, area under curve (accuracy)
The identification of the ions was done through the online database Lipid Maps (http://www.lipidmaps.org). Hydrogen (H+), sodium (Na+) and potassium (K+) adducts were identified and accepted since they are in the composition of the solvents and the mass error determined as the maximum of 50 parts per million (ppm).
Using the ions with a high contribution to identify the difference between the groups we build a multiple logistic regression model to calculate the logit test performance (P) = log (P / (1-P) = -3.578 + 0.111 704.82635 + 0.354 857.11525 + 0.329 (Figure 5). Through this equation we observe AUC= 0.87, sensitivity 83.33%, specificity 83.78% (Figure 3) and for a validation test with 1000 permutations a statistically significant result of p<0.001.
Figure 3.
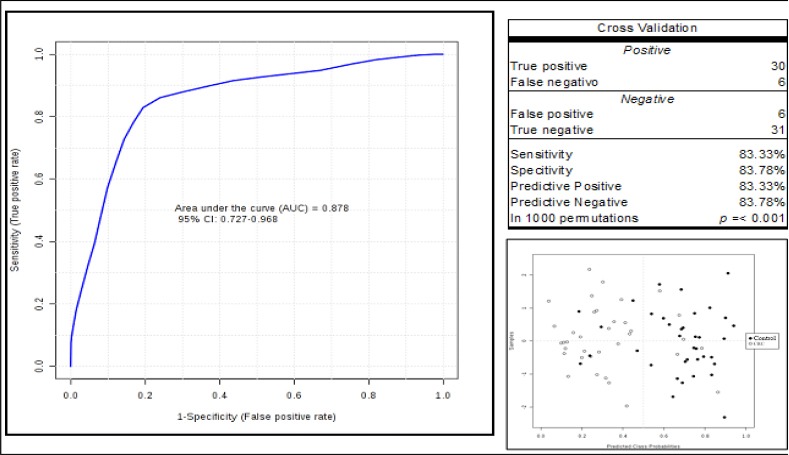
Predictive Model ROC Curve - The blue line in the graph is the ROC curve for the predictive model. The AUC values, sensitivity and specificity of the predictive model obtained through the multiple logistic regression analysis were 87.80%, 83.33% and 83.78%, respectively, and the optimum cut-off value was 0.41.
A survival curve was calculated according to the concentration (above or below the cut off) for each of the eight ions with the greatest contribution to group separation (Figure 4). We observed statistically significant differences for the lipids: SP (m = z 857.11525) p = 0.04 (Figure 4C), PK (m / z = 876.20796) p = 0.02 (Figure 4D) and GP (m / z = 1031.54773) p = 0.04 (Figure 4H).
Figure 4.
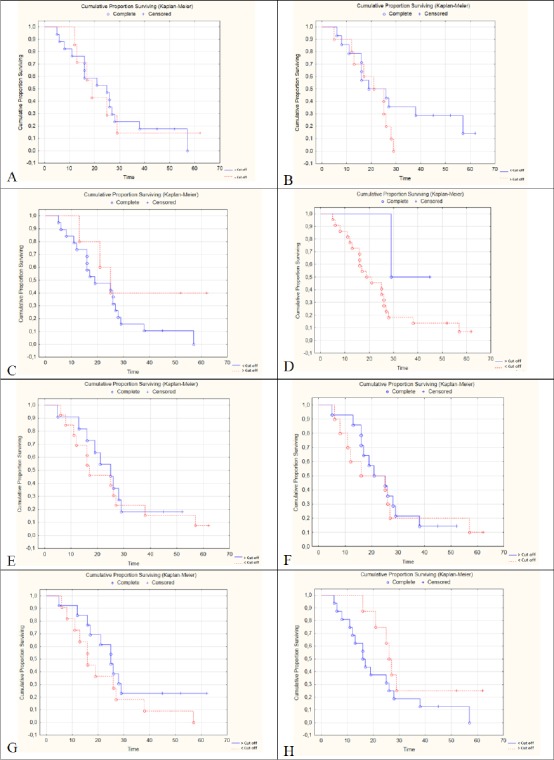
Kaplan Meier Curve Comparing Groups According to [Cut off] of the Lipids with Greater Contribution to Separation of the Groups. Lipids and (m / z): SP (704.82635), SP (707.91816), SP (857.11525), PK (876.20796), GP (881.39308), GP (882.39725), SP (907.41589) and GP (1031.54773) corresponding to Figures A, B, C, D, E, F and G respectively.
Discussion
The association between lipid metabolism and CRC has been revealed in recent decades (Agnoli et al., 2014), Lipids not only serve as an energy source, structural components of several cell membranes but play an important role in cytokine biosynthesis, cell signaling, energy metabolism, material transport, proliferation, differentiation and development (Lands, 2012; Wüstner, 2007).
In this study, we investigate the plasma lipid profile in patients with advanced CRC. An analytical system composed of MALDI - TOF mass spectrometry in order to find biomarkers of lipid metabolites that allow the diagnosis, prognosis, and follow-up of more advanced colorectal cancer cases.
We observed that the spectra obtained from MALDI spectrometry clearly had different profiles for the studied groups. The lipid profiles of body fluids may reflect the general condition of the whole body and indicate the existence of certain diseases, such as cancer (Jelonek et al., 2013).
Data processing involved multivariate statistical analysis such as PCA main component analysis, discriminant analysis with PLS-DA partial least squares multivariate calibration, which are useful for the identification of correlations between lipid metabolites and between lipid patterns that are associated with a phenotype physiological The PLS-DA method showed better separation between groups than the PCA method. This superiority was already expected since the PCA method is an unsupervised method in which each of the main components detects the directions of greater variance in the data matrix, while the PLS-DA is a supervised method in which data is provided identifying the samples, which optimizes the separation between the groups by rotating the components of the PCA in order to obtain the maximum separation between the classes (Wishart, 2008).
ROC curves of the eight lipids of greater significance were performed to separate the groups. As a way of improving these results we propose a multiple logistic regression model composed of the 8 selected metabolites and we obtain the following predictive model: logit (P) = log (P / (1-P) = -3.578 + 0.111 (SP) 704.82635 + 0.354 (PD) 857.11525 + 0.329 (PK) 876.20796 + 0.191 (GP) 1031.54773 - 0.369 (SP) 907.41589 - 0.083 (GP) 881.39308 - 0.219 (SP) 707.91816 + 0.081 (GP) 882.39725, which showed high sensitivity and specificity in the population of AUC 0.87, sensitivity 83.33% and specificity 83.78% and for a validation test with 1,000 permutations a statistically significant result. Our results formed the basis of future prospective studies for other cohorts, therefore, its performance should be prospectively validated in other populations where blood samples should be collected through the same methods.
The ions were higher 881, 882, 907, 707 were higher and the others lower 704, 857, 876 and 1,031 was associated with CRC, showing that these lipids may play role in CRC carcinogenesis.
The lipid classes identified in this study were sphingolipids (SP), glycerophospholipid (GP) and polyketides (PK). These lipids are present in mammalian cells, whose membranes consist mainly of sterols. The SP with m/z 704.82635 and 857.11525 were hyper represented in the control group, while the SPs with m/ z 907.41589 and 707.91816 were overrepresented in the CRC group. Sphingolipids constitute a class of lipids that are essential for the cellular structural integrity and play a role in regulating lipid bilayer fluidity. Although the mechanisms by which the deregulated metabolites of glycosylated ceramide contribute to drug resistance and/or metastasis are undefined, such changes are widely observed and warrant investigation. Sphingolipids constitute a class of lipids that are essential for the cellular structural integrity and play a role in regulating lipid bilayer fluidity. Although the mechanisms by which the deregulated metabolites of glycosylated ceramide contribute to drug resistance and/or metastasis are undefined, such alterations are widely observed and warrant investigation (Byrnes et al., 2009; Saltz et al., 2008).
Similar results were observed for GP, the m/z 1031.54773 were overrepresented in the control and the m/z 882.39725 and 881.39308 in the CRC group. Glycerophospholipids, also called phospholipids, are the main lipids of cell membranes and key components in cell metabolism and signaling. In vitro and in vivo studies suggest that cyclic phosphatidic acid (cPA), a bioactive phospholipid, inhibits the mitosis process and prevents invasion and metastasis. Cyclic nucleotide phosphodiesterase 3B (PDE3B) activity, that contributes to the cleavage of phosphodiester bonds, is also inhibited by cPA. The increase of intracellular cAMP and reduction of PDE3B activate the cAMP-dependent protein kinase A pathway (PKA), which leads to inhibition of CRC growth, and proliferation (Kurabe et al., 2013; Tsukahara et al., 2013).
Another lipid class identified with greater contribution to the separation of the groups in our study were the PK that constitute a great class of secondary metabolites (Schümann and Hertweck, 2006). The antitumor mechanisms described for anthocyanins (a subclass of PK) are attributed to their antioxidant capabilities. A study in vitro described by Zhang et al., (2005) showed inhibition of the growth of cancer cells in the stomach, colon, lung, and breast in more than 60%.
In a recent review, Zhang et al., (2017) showed that different studies have observed consistent changes in metabolite concentrations of CRC patients regardless of the heterogeneity of the groups studied.
Uehara et al., (2016) compared the lipid content of gastric cancer tissue to the adjacent non-neoplastic mucosa using the MALDI-IMS technique. The authors focused on the signal in the m/z 798.5 representing 16: 0/18: 1 PC, which was higher in the cancer tissue. In contrast, the signal intensity in m/z 496.3, 16: 0 LPC lysophosphatidylcholine, was lower in cancer lesions.
To determine if LPC class lipids can be used as markers in CRC, Zhao et al., (2007) analyzed plasma LPCs from 133 CRC cancer patients and 125 healthy controls using LC-MS. Plasma levels of different LPC forms, including 18:1 and 18:2, were significantly reduced in patients with CRC, suggesting that these lipids could represent potential biomarkers. The multivariate analysis in the validation set found specificity of 93% and a sensitivity of 82% in the development of cancer patients in contrast to healthy controls. They proposed a predictive model capable of adequately classifying 89% of the T1 tumor, suggesting that may be a marker of early CRC detection.
Dobrzyńska et al., (2005) looking for changes in the concentration of PL in the pT3 stage of CRC membranes, using qualitative HPLC and the quantitative composition of PLs on the membrane observed that the transformation of cancer was associated with an increase in the total concentration of PL.
The relationship between CRC and lipids will be essential to understand the complex system of tumorigenesis, to understand the metabolic alterations, to identify the main lipid structures, functions, and interactions with other lipids, proteins and metabolites. However, it is still a difficult and long-term task to understand how lipid profiles can be used as biomarkers for diagnosis and prognosis of CRC. The standard of preparation and extraction, analysis protocols and the database of lipid metabolomes is not well established yet, which will inevitably lead to different results. The progression of cancer involves differential regulation of multiple lipids which amplifies the complexity to investigate the lipidome in CRC. Despite these challenges, the integration of lipid metabolic strategies in cancer research may generate new opportunities to obtain information on the diagnosis, prognosis, and prediction of individualized therapies (Lee et al., 2012; Yang et al., 2009).
We consider this study an exploratory research that must be continued in a high number of patients, to better understand the differences in different stages of the disease and also in different part of the colon.
In conclusion, we observed a different lipid profile among patients with locally advanced, unresectable or metastatic colorectal cancer compared with healthy volunteers. These eight ions presented in this study could have a greater contribution to become possible biomarkers of diagnosis or therapeutically targets for the future.
Conflicts of Interest
The authors declare no competing interests related to this manuscript
References
- 1.Agnoli C, Grioni S, Sieri S, et al. Colorectal cancer risk and dyslipidemia:A case–cohort study nested in an Italian multicentre cohort. Cancer Epidemiol. 2014;38:144–51. doi: 10.1016/j.canep.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Gildea L, Balog J, et al. A novel methodology for in vivo endoscopic phenotyping of colorectal cancer based on real-time analysis of the mucosal lipidome:a prospective observational study of the iKnife. Surg Endosc. 2017;31:1361–70. doi: 10.1007/s00464-016-5121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009:analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrnes RW, Cotter D, Maer A, et al. An editor for pathway drawing and data visualization in the Biopathways Workbench. BMC Syst Biol. 2009;3:99. doi: 10.1186/1752-0509-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrzyńska I, Szachowicz-Petelska B, Sulkowski S, et al. Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol Cell Biochem. 2005;276:113–9. doi: 10.1007/s11010-005-3557-3. [DOI] [PubMed] [Google Scholar]
- 7.Donato P, Cacciola F, Beccaria M, Dugo P, Mondello L. Lipidomics. In 'Comprehensive Analytical Chemistry', Ed PicóY. Advanced mass spectrometry for food safety and quality. Oxford: Elsevier Press; 2015. pp. 395–439. [Google Scholar]
- 8.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10:1–10. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelonek K, Ros M, Pietrowska M, et al. Cancer biomarkers and mass spectrometry-based analyses of phospholipids in body fluids. Clin Lipidol. 2013;8:137–50. [Google Scholar]
- 10.Kurabe N, Hayasaka T, Ogawa M, et al. Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer;possible involvement of LPCAT4. Cancer Sci. 2013;104:1295–302. doi: 10.1111/cas.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lands B. Consequences of essential fatty acids. Nutrients. 2012;4:1338–57. doi: 10.3390/nu4091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laterza OF, Modur VR, Crimmins DL, et al. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–21. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- 13.Lee GK, Lee HS, Park YS, et al. Lipid MALDI profile classifies non-small cell lung cancers according to the histologic type. Lung Cancer. 2012;76:197–203. doi: 10.1016/j.lungcan.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Yang L, Bai Y, et al. Analytical methods in lipidomics and their applications. Anal Chem. 2014;86:161–75. doi: 10.1021/ac403554h. [DOI] [PubMed] [Google Scholar]
- 15.Mirnezami R, Nicholson J, Darzi A. Preparing for precision medicine. N Engl J Med. 2012;366:489–91. doi: 10.1056/NEJMp1114866. [DOI] [PubMed] [Google Scholar]
- 16.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer:a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 17.Schümann J, Hertweck C. Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes. J Biothecnol. 2006;124:690–703. doi: 10.1016/j.jbiotec.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 18.Tsukahara T, Matsuda Y, Haniu H. Cyclic phosphatidic acid stimulates cAMP production and inhibits growth in human colon cancer cells. PLoS One. 2013;8:e81139. doi: 10.1371/journal.pone.0081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uehara T, Kikuchi H, Miyazaki S. Overexpression of lysophosphatidylcholine acyltransferase 1 and concomitant lipid alterations in gastric cancer. Ann Surg Oncol. 2016;23:206–13. doi: 10.1245/s10434-015-4459-6. [DOI] [PubMed] [Google Scholar]
- 20.Weckwerth W, Wenzel K, Fiehn O. Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics. 2004;4:78–83. doi: 10.1002/pmic.200200500. [DOI] [PubMed] [Google Scholar]
- 21.Wishart DS. Metabolomics:applications to food science and nutrition research. Trends Food Sci Technol. 2008;19:482–93. [Google Scholar]
- 22.Wüstner D. Fluorescent sterols as tools in membrane biophysics and cell biology. Chem Phis Lipids. 2007;146:1–25. doi: 10.1016/j.chemphyslip.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Bennett R, Strum J. Screening phosphatidylcholine biomarkers in mouse liver extracts from a hypercholesterolemia study using ESI-MS and chemometrics. Anal Bioanal Chem. 2009;393:643–54. doi: 10.1007/s00216-008-2504-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Zhang Y, Zhao W. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer:a systematic review. Oncotarget. 2017;8:35460. doi: 10.18632/oncotarget.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–72. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Xiao Y, Elson P, et al. Plasma lysophosphatidylcholine levels:potential biomarkers for colorectal cancer. J Clin Oncol. 2007;25:2696–701. doi: 10.1200/JCO.2006.08.5571. [DOI] [PubMed] [Google Scholar]


