Abstract
Background:
Triple-negative breast cancer (TNBC) is a sub-group of breast cancers with a particularly poor prognosis. The results of studies investigating the role of platinum-based chemotherapy (PBC) in metastatic TNBC (mTNBC) have been conflicting. In this meta-analysis, our aim was to assess the effectiveness of PBCs for mTNBCs.
Methods:
The PubMed, Cochrane Controlled Trials Register Databases, and EBSCOhost databases were accessed. The English language was used as the search language and only human studies were included. The Newcastle–Ottawa Quality Assessment Scale and the Jadad scoring system were used to evaluate the quality of the included randomized controlled studies.
Results:
Seven studies and 1,571 patients were included in this meta-analysis. The pooled hazard ratio (HR) for overall survival (OS), evaluated on the basis of six studies, showed the use of PBC regimes to be related to OS in mTNBCs (HR 0.620; 95% CI 0.513-0.749; p:<0.001). Four studies containing HR and abstract statistics used for HR calculation were included in the meta-analysis for progression-free survival (PFS). The pooled HR again indicated a significant relation (HR, 0.628; 95% CI, 0.501-0.786; p:<0.001).
Conclusions:
In this meta-analysis, we confirmed that PBC regimes provide OS and PFS advantages compared to non-PBC regimes. The use of PBC regimes could be a good choice in mTNBC patients for better quality of life and survival.
Keywords: Triple-negative breast cancer, metastasis, chemotherapy, platinum, prognosis
Introduction
Breast cancer is the second most frequent cause of death in women after lung cancer. Triple negative breast cancer (TNBC) is a sub-group of breast cancer with negative oestrogen, progester-one receptor and HER-2 expression, evaluated by using immunohistochemical (IHC) methods (Brenton et al., 2005; Avcı et al., 2014). TNBC constitutes 12% to 20% of all breast cancers and has a poorer prognosis than other sub-types, due to high recurrence ratio, short disease-free survival, and early visceral metastasis (Kilburn et al., 2008). Reported median survival rates in metastatic TNBC (mTNBC) patients are between 6 and 13.3 months (Kassam et al., 2009).
Although the effectiveness of platinum-based chemotherapies (PBC) in mTNBC patients have been shown in a vast number of studies, some of these were non-randomized and retrospective (Zhang et al., 2015). Neoadjuvant setting and metastatic patients have usually been evaluated to-gether in the meta-analyses carried out in this regard (Guan et al., 2015; Liu et al., 2013). In this meta-analysis we aimed to evaluate the effectiveness of PBCs only in mTNBC patients.
Materials and Methods
Research strategy
The PubMed, Cochrane Controlled Trials Register Databases, and EBSCOhost data-bases were searched using the terms “breast neoplasm”, “breast cancer”, “breast carcinoma”, “breast tumor”, “metastatic triple negative”, and “mTNBC” combined with “platinum”, “cisplatin”, and “carboplatin”. The last search was performed in September 2015. English was used as the search language and only human studies were included in this analysis. Additional studies were also found by searching the references of the selected studies.
Inclusions and exclusions criteria
The following criteria were used in choosing the studies to include in the meta-analysis: 1) case-control studies, retrospective and randomised prospective studies were in-cluded, (2) studies examining the effectiveness of PBC in mTNBC were included, (3) case reports and reviews were excluded, (4) studies performed with non-PBC treatment combina-tions were excluded, (5) studies without a sufficient quantity of platinum-based treatments in terms of survival in the abstract statistics were excluded, (6) duplicate studies were ex-cluded. Only articles written in English were included in this analysis.
Selection of studies
The suitability of the studies for inclusion in the meta-analysis was evaluated by two independent reviewers (MY, VK). The abstracts of all studies found in the search were read. Prospective articles for the meta-analysis were obtained in full-text. Articles included in the meta-analysis were obtained in full-text and abstract statistics were extracted from the full-text.
Study population
Patients with mTNBC, with a sufficient follow-up period and treated with PBC re-gimes were included in this study. Patients that were included in different studies by the same researcher were detected, and studies with higher quality were included in the meta-analysis.
Determination of the quality of studies
The quality of the studies were assessed by two independent reviewers (SP, HB) us-ing the Newcastle–Ottawa Quality Assessment Scale in the evaluation of non-randomised studies, and using the Jadad scoring system in the evaluation of randomised controlled stud-ies. Selection of the patient population, the comparability of the study and follow-up, and the results of the study were evaluated using the Newcastle–Ottawa Quality Assessment Scale. Zero to nine stars were given to the studies on the three topics. Nine stars indicated the highest quality in the quality evaluation. The Jadad scoring system is a five-point scoring system. Studies were evaluated according to the criteria meeting each point (Jadad et al., 1996). Incompatibilities between investigators were eliminated following a joint evaluation of incompatible studies and an agreement was achieved on all items.
Data extraction
Studies and data included in the meta-analysis were extracted by two independent reviewers (MY, VK). Incompatibilities between investigators were eliminated by a joint evaluation following data extraction and an agreement was reached for all items. Infor-mation on (1) basic information about the study, first author name, year of publication, country of study, (2) study design, (3) treatments, (4) survival were obtained from studies included in the meta-analysis.
Statistical analysis
The primary objective of the statistical analyses used in our study was to determine the effect of platinum-based treatment regimens on survival of TNBC patients. The hazard ratio (HR) was calculated with a 95% confidence interval (CI) for each study. Cases with HR>1 and a 95% CI that did not exceed 1 were accepted as significant. In cases without a reported HR, the HR was calculated using the abstract statistics obtained from data extrac-tion.
Homogeneity was evaluated using the χ2-based test of homogeneity test and incon-sistency index (I2). Heterogeneity was accepted when p <0.10 or I2 >50% for χ2. The results were provided using a fixed model. p <0.05 was accepted as statistically significant for ab-stract HR. Publication bias was examined by using Egger’s regression intercept, Begg-Mazumdar rank correlation analysis, and a visual inspection of the funnel plot (Egger et al., 1997; Begg and Mazumdar, 1994). Statistical analyses were carried out using Comprehen-sive Meta-analysis V 3.0.
Results
Study eligibility
A flowchart of the studies included in the meta-analysis is provided in Figure 1. The computer-based literature search using the PubMed/Medline, Cochrane Controlled Trials Register Databases, and EBSCOhost resulted in a total of 738 articles. Following the article search, 707 articles were eliminated as they were abstracts, letters, editorials, expert opin-ions, reviews, case reports, laboratory studies and articles written in a language other than English. The full-texts of the remaining articles were evaluated. Of the 31 articles evaluated in full-text, 24 were eliminated due to the inclusion of patients other than metastatic patients and lack of data necessary for estimating the HR and 95% CI.
Figure 1.
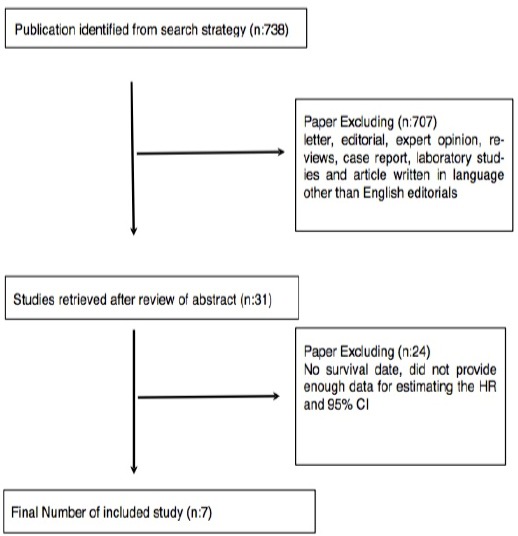
Flow Chart of the Meta-Analysis
Determination of the quality of studies included in the meta-analysis
Four of the seven studies included in this meta-analysis were retrospective, and the remaining three were prospective. The total number of patients was 1571. The median score of the prospective studies included in the meta-analysis was determined as 4, and the median score of retrospective studies was 5.
Table 1.
Details of the Studies Inclued in the Meta-Analysis
| Study | Country | Study Design | Total Number of Patients | Median Age | Median Follow-up Time (Month) | Treatment Arms | HR (OS) | P value (OS) | HR (DFS) | P value (DFS) | Study Qulatiy Score | Conclusion | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carey L,2012 | USA | Prospective Phase 2 | 102 71 |
52(28-83) | 26 | Cetuximab Cetuximab+Carboplatin |
0.381 | 0.354 | 4 | NS | 13 | ||
| Fan Y,2012 | China | Prospective Phase 2 | 53 27 |
48(32-67) | 24 | Dosetaksel+Cisplatin Dosetaksel+Capesitabine | 0.41 | 0.027 | 0.029 | <0,001 | 3 | S | 11 |
| Khalaf D,2013 | Canada | Retrospective | 58 | 48.9 | NR | Various platin based regime | 0.97 | NR | 5 | NS | 17 | ||
| Garza CV, 2013 | Canada | Retrospective | 153 58 |
53.2 | NR | Various platin based regime | 0.57 | 0.002 | 5 | S | 14 | ||
| Hong R, 2014 | China | Retrospective | 79 34 |
46 | 67 | Various platin based regime | 0.425 | 0.001 | 5 | S | 15 | ||
| Hu CX, 2015 | China | Prospective Phase 3 | 236 118 |
47(42-57) | 16.3 | Gemcitabine +Cisplatin Gemcitabine + Pacitaxel |
0.902 | 0.611 | 0.692 | 0.009 | 4 | NS | 16 |
| Zhang J, 2015 | China | Retrospective | 364 218 |
49(25-76) | 15.9 | Various platin based regime | 0.63 | 0.002 | 0.59 | 0.046 | 6 | S | 4 |
Overall survival
Pooled HR for overall survival (OS) was evaluated in six studies. Pooled HR for OS indicated that the use of PBC regimes was related to OS in mTNBC (HR 0.620; 95% CI 0.513-0.749; p:<0.001; Figure 2). Studies included in the meta-analysis were not heteroge-neous (p:0.227 I2:27.638) and thus the pooled HR was calculated using the fixed model for OS.
Figure 2.
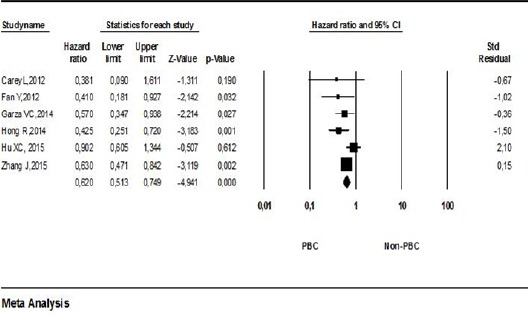
Meta-Analysis of Overall Survival among Patients PBC or Non-PBC
Progression-free survival
Four studies containing HR ratios and abstract statistics used for HR calculation were included in the meta-analysis for progression-free survival (PFS). The pooled HR for PFS indicated that the use of PBC regimes was related to PFS in mTNBC (HR, 0.628; 95% CI, 0.501-0.786; p:<0.001; Figure 3). Studies included in the meta-analysis for DFS were not heterogeneous (p:0.107 9 I2:50.803) and thus the pooled HR was calculated using the fixed model for DFS.
Figure 3.
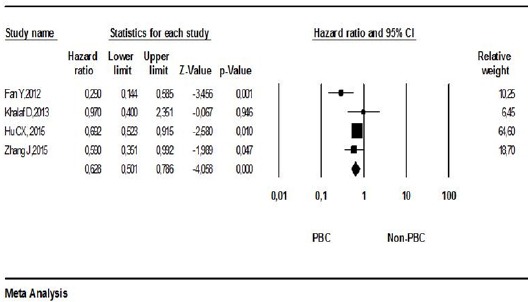
Meta-Analysis of Progression-Free Survival among Patients PBC or Non-PBC
Publication bias
No significant publication bias was detected for OS (Begg’s test, p=0.18; Egger test, p-0.264). The funnel plot did not indicate a publication bias for OS (Figure 4). It was calcu-lated that 35 contrary studies were required to invalidate the OS results of the meta-analysis. No significant publication bias was detected for PFS (Begg’s test, p:0.49; Egger test, p:0.649). The funnel plot did not indicate a publication bias for PFS (Figure 5). It was calculated that 14 contrary studies were required to invalidate the PFS results of the meta-analysis.
Figure 4.
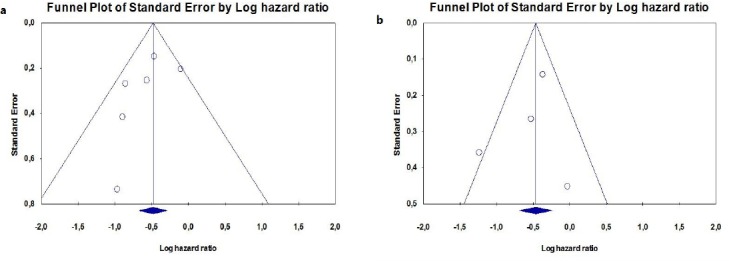
(a,b): Publication Bias Determination Using Funnel Plot for (a) Overall Survival and (b) Progression-Free Survival
Discussion
Breast cancer is a heterogeneous disease consisting of many biological sub-types. TNBC is the only clinical sub-type without a specified targeted treatment method and has poor results due to the aggressive course with early visceral metastasis (Dent et al., 2009). Some small retrospective and small patient-numbered neoadjuvant treatment series have in-dicated that TNBC is more sensitive to agents that cause DNA damage, such as cisplatin, although there are also studies indicating otherwise (Fan et al., 2013).
Bhattacharyya et al., (2009) studied the effect of adding cisplatin to cyclophosphamide and methotrexate in 126 patients diagnosed with mTNBC. In this study, they found a response ratio of 62% for the arm with platinum and 30% for the arm without platinum. They indicated that the results of the arm with platinum were better in pa-tients with visceral disease and more than two metastatic regions.
Carey et al., (2012) studied the role of EGFR inhibition and the effectiveness of combination treatment with platinum in mTNBC patients in a Phase 2 multi-centric study. In this study, a benefit of the cetuximab-carboplatinum combination was shown in PFS against the cetuximab arm; however, no difference was shown for OS.
Fan et al., (2013) compared a cisplatin and docetaxel (TP) combination in the first arm against a docetaxel and capecitabin (TX) combination in the second arm in a Phase II study in 53 mTNBC patients. The primary endpoint of this study was the objec-tive response rate (ORR) and the secondary endpoint was PFS and OS. The ORR ratio was higher in the TP arm (63% vs. 15.4% p=0.001) in the median 24-month follow-up. Moreo-ver, the PFS was doubled (10.9 months vs. 4.8 months p<0.001). An improvement was also observed in OS (32.8 months vs. 21.5 months p=0.027). The authors argued that TP usage was superior compared to the TX regime, in terms of ORR, PFS, and OS in mTNBC pa-tients.
Villarreal-Garza et al., (2015) retrospectively evaluated mTNBC patients treated at four large cancer centers in Canada between 2003 and 2010. They showed that patients in the group receiving PBC had longer OS with respect to non-PBC (14.5 months vs. 10 months, p=0.041). Hong et al., (2014) conducted a similar study in China. Patients treated for mTNBC between 2003 and 2014 were retrospectively evaluated in this study. The study had a follow-up period of 67 months and median PFS was found to be 10 months in PBC patients (95% CI: 6.6 months-13.4 months) and 5 months (95% CI: 3.7 months-6.3 months) in non-PBC patients. Median OS was found to be 32 months in PBC patients and 21 months in non-PBC patients (p=0.002). Another retrospective study in this regard was carried out by Zhang et al., (2015). In this study, it was shown that taking multiple steps of PBC improved OS and resulted in a 27% decrease in mortality risk based on the Cox hazards model.
Hu et al., (2015) compared a paclitaxel and cisplatin combination against a paclitaxel and gemcita-bine combination in a randomized Phase III, hybrid-designed study, conducted at 12 centers in China. In this study, it was shown that the cisplatin and paclitaxel arm was non-inferior and more superior.
Despite restrictions due to the number of patients included and the fact that it was an observation study, in a retrospective study by Khalaf et al., (2014) a PBC regime in TNBC patients did not have any apparent or significant advantage in comparison to standard chemotherapy. In another retrospective study, Staudacher et al., (2011) showed a response rate of 33.3% with PBC. In this study, a high response ratio tendency for providing a survival (in terms of OS or PFS) advantage was found with PBC in mTNBC. The increase in toxicity due to platinum inclusion was found to be at an accepta-ble level.
In a meta-analysis evaluating both palliative chemotherapy at metastatic stage and neoadjuvant chemotherapy, Guan et al., (2015) found a statistically significant difference between PBC and non-PBC and argued that better response ratios and pathologically complete re-sponse ratios were obtained with PBC in TNBC. Another meta-analysis performed by Liu et al., (2013) evaluated also both palliative chemotherapy and neoadjuvant chemotherapy. Higher clinically complete response ratios and pathologi-cally complete response ratios in TNBC were found with neoadjuvant treatment in this me-ta-analysis. No difference was observed in PFS ratios between the groups in ad-vanced/metastatic patients. Unlike this meta-analysis, studies including only patients at the metastatic stage were included in our meta-analysis.
In this meta-analysis, we have shown that PBC regimes provide an OS and PFS ad-vantage with respect to non-PBC regimes. The use of PBC regimes could be a good choice in mTNBC patients for better quality of life and survival.
Funding Statement
None declared.
Acknowledgements
None declared.
References
- 1.Avcı N, Ulaş A, Ordu Ç, et al. Metaplastic breast cancer and EGFR expression. JCAM. 2015;6:616–9. [Google Scholar]
- 2.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for pub-lication bias. Biometrics. 1994;50:1088–01. [PubMed] [Google Scholar]
- 3.Bhattacharyya GS, Basu S, Agarwal V, et al. Single institute phase II study of week-ly cisplatinum and metronomic dosing of endoxan and methotrexate in second line metastat-ic breast cancer triple-negative. Eur J Cancer. 2009;7:18. (Late breaking abstract) [Google Scholar]
- 4.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecu-lar forecasting of breast cancer:ready for clinical application? J Clin Oncol. 2005;23:7350–60. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 5.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001:randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–3. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent R, Hanna WM, Trudeau M, et al. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–8. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 7.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–4. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y, Xu BH, Yuan P, et al. Docetaxel-cisplatin might be superior to docetaxel-capecitabine in the first-line treatment of metastatic triple-negative breast cancer. Ann Oncol. 2013;24:1219–5. doi: 10.1093/annonc/mds603. [DOI] [PubMed] [Google Scholar]
- 9.Guan X, Ma F, Fan Y, et al. Platinum-based chemotherapy in triple-negative breast cancer:a systematic review and meta-analysis of randomized-controlled trials. Anticancer Drugs. 2015;26:894–01. doi: 10.1097/CAD.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 10.Hong R, Ma F, Xu B, et al. Efficacy of platinum-based chemotherapy in triple-negative breast cancer patients with metastases confined to the lungs:a single-institute experience. Anticancer Drugs. 2014;25:1089–4. doi: 10.1097/CAD.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 11.Hu XC, Zhang J, Xu BH, et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006):a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–6. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of random-ized clinical trials:Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer:implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–3. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 14.Khalaf D, Hilton JF, Clemons M, et al. Investigating the discernible and distinct ef-fects of platinum-based chemotherapy regimens for metastatic triple-negative breast cancer on time to progression. Oncol Lett. 2014;7:866–70. doi: 10.3892/ol.2014.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilburn LS. ‘Triple negative’ breast cancer:a new area for phase III breast cancer clinical trials. Clin Oncol. 2008;20:35–9. doi: 10.1016/j.clon.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Mo QG, Wei CY, et al. Platinum-based chemotherapy in triple-negative breast cancer:A meta-analysis. Oncol Lett. 2013;5:983–1. doi: 10.3892/ol.2012.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staudacher L, Cottu PH, Diéras V, et al. Platinum-based chemotherapy in metastatic triple-negative breast cancer:the Institut Curie experience. Ann Oncol. 2011;22:848–6. doi: 10.1093/annonc/mdq461. [DOI] [PubMed] [Google Scholar]
- 18.Villarreal-Garza C, Khalaf D, Bouganim N, et al. Erratum to:platinum-based chemotherapy in triple-negative advanced breast cancer. Breast Cancer Res Treat. 2015;150:697. doi: 10.1007/s10549-015-3345-z. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Fan M, Xie J, et al. Chemotherapy of metastatic triple negative breast can-cer:Experience of using platinum-based chemotherapy. Oncotarget. 2015;6:43135–3. doi: 10.18632/oncotarget.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]


