Abstract
Objective:
To compare a complex physical therapy (CPT) protocol alone or combined with complex physical therapy muscle more strength training (CPT+ST) in patients with lymphedema after breast cancer treatment regarding strength and limb muscle volume.
Methods:
In this controlled clinical trial, consecutive patients treated from breast cancer from April 2014 to December 2015 were allocated in two groups, the CPT group 1 and the CPT+ST group 2, that performed CPT associated with muscle strengthening. Patients in the CPT group 1 received the routine protocol of care, consisting of manual lymphatic drainage (MLD), multilayer bandage compression therapy, skin care and regular exercises. Patients CPT+ST performed, 2 sets of 10 repetitions exercises at 40% of maximal voluntary contraction the first week, increasing to 3 sets with 10 repetitions during the second and third weeks, 3 sets with 15 repetitions, for 8 weeks, 50 minutes per session, twice per week. Strength and muscle volume were analyzed.
Result:
In the study period, 42 patients were enrolled, 22 in the CPT group 1 and 20 in the CPT+ST group 2. Only 36 completed treatment. Both groups showed similar increases in the range of movement in: shoulder flexion, extension, adduction, abduction and external rotation. Internal rotation showed less improvement in CPT+ST group 2 (p = 0,034). Strength improvement was similar between groups. The CPT+ST group 2 seemed to have a greater volume in the upper limb when compared to CPT group 1, but it was not possible to prove any significant difference (p = 0.555).
Conclusion:
There was no difference of muscular limb volume between the two interventions. This means that strengthening exercises can be performed by patients with lymphedema safely, without the risk of increasing upper limb volume with edema.
Keywords: Breast neoplasms, lymphedema, rehabilitation, strength training
Introduction
According to the World Health Organization (2017) figures released this week indicate that each year 8.8 million people die from cancer, mostly in low- and middle-income countries. One problem is that many cancer cases are diagnosed too late. Even in countries with optimal health systems and services, many cancer cases are diagnosed at an advanced stage, when they are harder to treat successfully.
The involvement of axillary lymph nodes remains the most important prognostic factor for breast cancer. In the last two decades, the concept of a sentinel lymph node biopsy (SLNB) has led to an increase in the preemptive surgical removal of axillary lymph nodes, as a way to address metastasis dissemination. Despite the higher incidence of complications resulting from the removal of lymph nodes, lymphedema is still one of the most common complications (Sclafani and Baron, 2008). However, lymphedema affects approximately 25% of women submitted to axillary interventions, and most of them develop arm swelling during the first two years after treatment (DiSipio and Newman, 2013). Axillary edema may occur any time after the surgery (Bevilacqua et al., 2012).
The gold-standard treatment for lymphedema is a complex physical therapy (CPT) protocol, including manual lymphatic drainage (MLD), compression therapy, exercises without resistance and skin care (Patel et al., 2015). CPT is divided into an intensive phase, with compression with multilayer bandage, and a maintenance phase, which replaces bandage for compression stockings (Forner-Cordero et al., 2010). Exercise and movements may also influence the lymphatic system and drainage (Foldi and Foldi, 2012).
Previous studies have suggested that patients with lymphedema should not perform high-intensity exercise because it was believed that the degree of lymphedema could be aggravated due to excessive physical exertion. However, more recent studies have shown that muscular strength exercises, when properly guided in their intensity and duration, can help reduce lymphedema (Hayes et al., 2008).
The objective of this study was to evaluate, in a controlled trial, the safety and effectiveness of resistance training added to CPT in women with lymphedema after breast cancer treatment compared to CPT alone.
Materials and Methods
Trial design, setting and ethics
This is a controlled clinical trial, using a convenience sample of consecutive patients, and conducted in the mastology clinic of a public university hospital in Brazil. The institutional Ethics Committee in Research approved the study protocol (CAAE: 25747413.8.0000.5505, approval number 565479). This study was registered in the Clinical Trials.gov platform (NCT02574780).
Participants and allocation
Participants and allocation The participants in this study were all patients consecutively admitted to the mastology clinic of Hospital São Paulo, where they received free treatment by the public service of Brazil, for lymphedema were selected between April 2014 and December 2015. They were divided into two groups according to the order of admission: the first patient was allocated to the treatment group, next to the control group and so on. Lymphedema was defined as a difference in arm circumference of at least two centimeters at two different sites, and the patient with minor edema was excluded from the study at the first evaluation (Ribeiro et al., 2017).
Inclusion criteria were: adult females (≥ 18 years old), with upper limb (UL) lymphedema resulting from unilateral surgery for breast cancer treatment, and available to participate in the study twice a week for eight weeks, the bandages were removed before each session so that the skin care could be performed, _(from the 16 exercise sessions proposed, they should be present at least in 12).
Patients were excluded if they had bilateral breast cancer; renal dysfunction; acute inflammation; deep vein thrombosis; heart disease; skin infections; metastatic lymphedema; or had participated in any treatment involving the reduction of lymphedema in the previous three months. Stage 3 lymphedema (the most severe form of lymphedema, with a full failure of the lymphatic system) was an exclusion criterion for this study too.
Intervention
CPT group 1
Patients in the complex physical therapy (CPT) received the routine protocol of care, consisting of manual lymphatic drainage (MLD), multilayer bandage compression therapy, skin care and regular exercises twice a week during eight weeks, as follows. MLD is a massage technique carried out with slow, rhythmic, and gentle maneuvers involving the skin surface and following the lymphatic anatomical pathways of the body (Damstra et al., 2009). Skin care consisted of hydration of the upper limb with lymphedema before the application of padding bandages (Bergmann et al., 2014). Exercises consisted muscular contractions of various intensities while moving the shoulder girdle, shoulder, elbow, wrist, and hand.
Multilayer bandage compression therapy was made with the application of elastic bandages in the longitudinal direction of the limb after lymphatic drainage procedure. The bandages were bought by the patients, all of the same model and manufacturer (Nevada, Neve, São Paulo), made of cotton and spandex, with high strength and elasticity. The bandages width could vary from 6 cm to 20 cm. Bandages were made twice a week. Patients were instructed to maintain the bandages until the next session, but they were allowed to wash the arm. The bandage should be maintained in place until the next session.
CPT+ ST group 2
The complex physical therapy+ strength training (CPT+ST) group 2 held CPT associated with a muscular strengthening protocol (MSP), also twice a week during eight weeks. For this protocol, physiotherapy equipment like Thera Band (load), stick, and a little ball were used. Exercises were applied as described in detail below and were applied to the upper limbs only.
The method chosen to determine the maximal repetition (MR) workload was the muscular strength percent, as described in Table 1. This method used the maximal repetition test (1-MR) or maximal load test (Badger et al., 2004). As the population of the study would probably consist of patients who were beginners in MSP, to avoid the risk of muscle injury, a one-repetition maximum with free weights was performed, which was then converted to the 1-MR at 40% of the maximal.
Table 1.
Baseline Characteristics of Participants n = 42
| Characteristics | CPT Group 1 (n= 22) | % | ST Group 2 (n =20) | % | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 25 a 29.9 | 13 | 59.1 | 6 | 30.0 |
| ≥30 | 9 | 40.9 | 14 | 70.0 | |
| Lymphadenectomy | Yes | 22 | 100.0 | 18 | 90.0 |
| No | 0 | 0.0 | 2 | 10.0 | |
| Breast reconstruction | Yes | 10 | 45.5 | 7 | 35.0 |
| No | 12 | 54.5 | 13 | 65.0 | |
| Radiotherapy | Yes | 21 | 95.5 | 18 | 90.0 |
| No | 1 | 4.5 | 2 | 10.0 | |
| Quimiotherapy | Yes | 20 | 90.9 | 16 | 80.0 |
| No | 2 | 9.1 | 4 | 20.0 | |
| Mastectomy | Yes | 18 | 81.8 | 16 | 80.0 |
| No | 4 | 18.2 | 4 | 20.0 | |
| Quadrantectomy | Yes | 4 | 18.2 | 4 | 20.0 |
| No | 18 | 81.8 | 16 | 80.0 | |
| Lymphedema stage | I | 16 | 72.7 | 4 | 20.0 |
| II | 6 | 27.3 | 16 | 80.0 |
Exercise protocol for the CPT+ST group 2
The exercises began with heating and circumduction movements of the shoulders, performed five times, both forwards and backwards. Then, specific exercises were performed throughout the program:
- Week 1: two sets of 10 repetitions and a determination of the 1-MR
- Week 2: three sets of 10 repetitions
- Week 3 to 8: three sets of 15 repetitions
The specific exercises were:
Shoulder abduction (AB) with TheraBands to exercise the scapular stabilizers (isometric exercise)
Protraction and retraction of the shoulder blades with a stick to work the serratus anterior muscles
Shoulder flexion up to 90° with a halter
Shoulder AB to 90° with a halter
External rotation (ER) with the TheraBand to strengthen the rotator cuff
Internal rotation (IR) with the TheraBand to strengthen the rotator cuff
Elbow flexion with the halter to exercise the biceps (during the first week, the movement was performed without gravity)
Fist flexion with a halter (flexor carpi radialis muscle).
Fist extension with a halter (extensor carpi radialis brevis muscle).
Elbow extension with the TheraBand to strengthen the triceps brachii muscle.
Press or move the ball to activate intrinsic muscles of the hand.
Following the MSP, relaxation was performed with circumduction of the shoulder through forward and backward movements, five times each, for warming up.
Outcomes evaluation
Patients in both groups were evaluated for strength, volume and range of motion of the upper limb before the first session of exercising and again in the 16th session. The evaluations were performed as follows.
Strength evaluation: Evaluations were performed with a manual dynamometer (Hand Held Dynamometer Model 01163 brand Lafayette Instrument Company) that recorded the peak force (kg) for a 5-second muscle isometric contraction. The beginning and end of the contraction were signaled with a buzzer. Various movements were performed including flexion, extension, adduction, abduction, and internal and external arm rotation. Two measures were performed per patient and the average of these measures was used for statistical analysis.
Volumetry: To evaluate lymphedema, the circumference of the affected upper limb was measured in all sessions at seven different evaluation points, with a measuring tape. The measured points included: point A = 14 cm above the olecranon; point B = 7 cm above the olecranon; point C = the circumference of the olecranon; point D = 7 cm below the olecranon; point E = 14 cm below the olecranon; and point F = 21 cm below the olecranon. Another measurement site was point G = hand measurement, which was a measure of the entire circumference of the dorsum and palm, at the line of the metacarpals with the base of the finger (Makluf et al., 2006) To analyze the variable volumetry, the following formula was used: V= h. (C2 + C.c + c2)/(π.12), where V is the estimated volume, h is the distance between the circumferences (C), C and c are circumferences (Lanza et al., 2015).
Shoulder range of motion (ROM): The shoulder ROM measurements were made with a manual large plastic goniometer (model sh5205, Carci, São Paulo) for the following movements: flexion, extension, adduction, abduction, internal rotation, and external rotation. The movements were evaluated bilaterally from the active-free mode with the patient in the sitting position.
Statistical analysis
Data were first described as means, medians, minimum and maximum values, standard deviations, and absolute and relative frequencies (percentage). Statistical analysis was made using Shapiro-Wilk tests to verify the normality of the variables, and Student’s t and Mann-Whitney tests for independent groups, for the goniometry and muscle strength variables (Neter et al., 1996) in order to compare the limb circumference measures between the groups over time.
Data were entered in 2010 Excel spreadsheets for proper storage. Statistical analyses were performed with the Statistica software, version 12.
All conclusions with inferential analyses used an alpha significance level of ≤ 5% to reject the null hypothesis.
Results
All the 64 breast cancer patients who had had unilateral breast surgery in the institution in the study period and were suffering from upper limb lymphedema were recruited for this study. As described in Figure 1, 22 were excluded because they had bilateral breast cancer, lymphedema stage 3, renal dysfunction, acute inflammation, metastatic lymphedema or had been treated for lymphedema in the previous three months.
Figure 1.
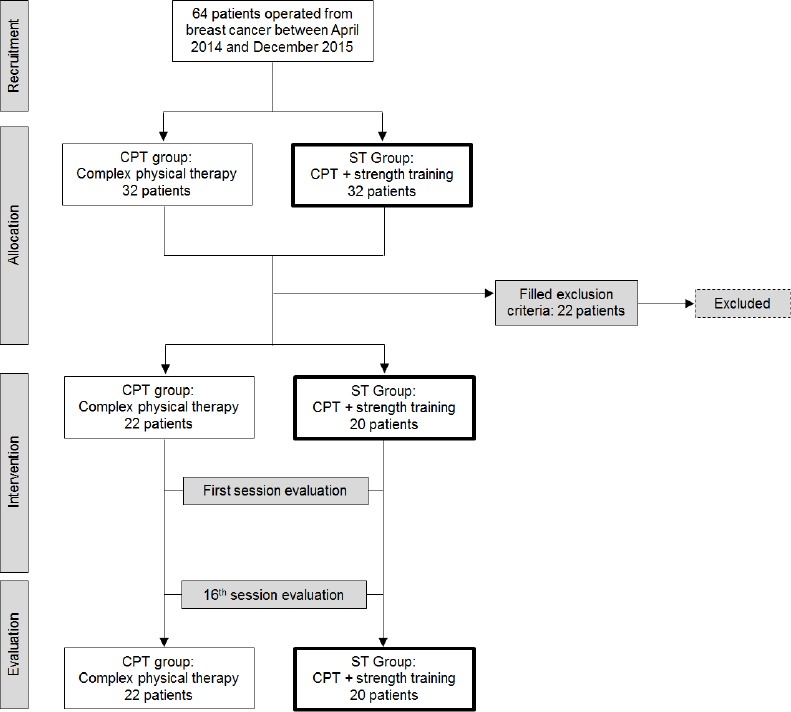
The mean age of the 42 patients analyzed was 59.5 years. Almost half of them had failed to complete high school education (45.2%) and only 23.9% had superior education. More than half had been born in other cities (54.8%). The majority were housewives (4.8%) or retired women (47.6%). Most (61.9%) did not have any comorbidity, but 2.4% had fibromyalgia, 4.8% had sleep disorders and 30.9% had other diseases such as osteoporosis or arthritis. Among the 42 participants, 34 had undergone mastectomy CPT group 1 (81,8%), ST group 2 (80,0%) and 8 had undergone quadrantectomy CPT group 1 (18,2%), ST group 2 (20,0 %). Their BMI, the treatments they underwent and lymphedema stage are described in Table 1. There were patients with lymphedema stage I (16 of them in the CPT group 1 and 4 ST group 2) and patients were stage II (06 in the CPT group 1 + 16 ST group 2).
Both groups showed similar gains and increased the ROM in the following variables: shoulder flexion, extension, adduction, abduction, and external rotation, as shown in Table 2. The ST group had less improvement in the internal rotation compared to the CPT group 1 (p = 0.034). Both groups had equal improvements in shoulder strength Table 3. Limb volume was also similar between groups, the two groups decreased volume after the intervention (Table 4).
Table 2.
Shoulder Range of Motion after Exercising
| Shoulder range of motion | CPT group | ST group | p-value | ||
|---|---|---|---|---|---|
| Mean (degrees) | SD (degrees) | Mean (degrees) | SD (degrees) | ||
| Flexion | 9.22 | 17.5 | 7.75 | 15.7 | 0.962 |
| Extension | 11.61 | 16.6 | 12.3 | 21.4 | 0.074* |
| Abduction | 23.2 | 32.3 | 15.6 | 23.7 | 0.635 |
| Aduction | 12.7 | 28.2 | 5.3 | 23.6 | 0.318 |
| Internal rotation | 17.6 | 32.6 | 15.6 | 23.7 | 0.034 |
| External rotation | 1.16 | 11.4 | 6.19 | 15.9 | 0.149 |
Student’s t test (all others: Mann-Whitney test)
Table 3.
Shoulder Strength after Exercising
| Shoulder strength | CPT group | ST group | p-value | ||
|---|---|---|---|---|---|
| Mean (kg/s) | SD (kg/s) | Mean (kg/s) | SD (kg/s) | ||
| Flexion | 13.1 | 22.4 | 12.3 | 33.3 | 0.438 |
| Extension | 19.4 | 36.6 | 12.6 | 27.2 | 0.669 |
| Abduction | 19 | 27.6 | 21.8 | 40.4 | 0.837 |
| Aduction | 10.1 | 21.2 | 13.6 | 17.6 | 0.563* |
| Internal rotation | 13.6 | 28.6 | 8.4 | 13.3 | 0.987 |
| External rotation | 4.19 | 16.6 | 9.8 | 18.2 | 0.447 |
Student’s t test (all others: Mann-Whitney test)
Table 4.
Limb Volume Reduction after Exercising
| Volume | CPT group | ST group | |||
|---|---|---|---|---|---|
| Mean (mm) | SD (mm) | Mean (mm) | SD (mm) | p-value | |
| First session | 2272 | 434.05 | 2626 | 507.52 | 0.555* |
| 16th session | 2153 | 438.05 | 2329 | 472.85 | |
Student’s t test
Discussion
In this study, we proposed strength therapy to women operated for breast cancer and suffering from upper limb lymphedema. Strength exercising proved safe for these women, as evidenced by the upper limb volume that was similar to that of patients performing the standard protocol. The patients who received weight training had an increase in strength without worsening of the lymphedema condition, and both groups showed a reduction in upper limb volumes from the first to the 16th sessions of exercise.
At baseline, there were more cases of lymphedema stage II in the ST + CPT group. Patients were not distributed equally with regard to lymphedema stages between groups. Lymphedema stage II, as defined by the International Society of Lymphology, is the condition where there is no reduction of edema with limb elevation, in addition there are tissue changes that increase the risk of fibrosis, causing infections and skin lesions (Internacional Society of Lymphology, 2013). Also, there were more patients undergoing mastectomy than quadrantectomy, and possibly the type of surgery may have had an impact on the baseline lymphedema stage and the number of axillary lymph nodes removed Leidenius et al., (2003) and Bergmann et al., (2007). Patients undergoing axillary lymph node dissection are four times more likely to develop lymphedema compared to those who had a SLNB (DiSipio et al., 2013). Risk factors related to treatment are not modifiable and are dictated by the type and stage of disease. If patients remain at the same BMI range and continue the habit of physical exercising following breast cancer treatment Eakin et al., (2007) and Smith et al., (2010) lymphedema may be prevented. The number of patients receiving one and another intervention was balanced in this study, though, and both resulted in equal gains in ROM, strength and limb volume reduction.
Early studies had doubts whether exercises applied to lymphedema patients would be safe. However, recent studies have indicated that exercise can be beneficial for patients with lymphedema what is Lymphedema (2013) and Morris et al., (2015) but no specific resistance training was provided, and in some of them, patients were not supervised. In this project, we observed that the exercise with or without load, associated with the standard CPT, reduced volume and increased shoulder strength pattern. In addition, it was possible to show a gain of muscle strength in the upper limbs of both groups.
The maximum load reached in this study was 2 kg by the end of treatment. The load was not higher because the vast majority of the sample was comprised of sedentary women, which may have been a limiting factor to further increasing the load. This study offered training twice a week for 8 consecutive weeks.
The lack of a more prominent lymphedema reduction might also be attributed to the type of bandage that was used in our setting. Patients included in this study were being seen in a public hospital, and they do not have financial resources to buy the same bandages that were used in other studies the Rosidal bandage (Partsch and Mortimer, 2015) that in Brazil can cost something like five times more, not the public service pays for it. For homogeneity purposes, we decided to ask our patients to buy always the same model and brand of bandages (the Nevada, from Neve). It is possible that the strength and elasticity varies comparing the two brands, but a specific study should address this. Nevertheless, the same bandages were used in both groups, with reductions in lymphedema.
Another limitation to be pointed is that, in the current study, chemotherapy was performed in CPT group 190,9 % and ST group 280,00% of the sample; however, it was not possible to recover data on the laterality of intravenous administration of chemotherapy, in order to correlate the side of breast surgery and of chemo administration with the severity of lymphedema.
In conclusion, there was no difference of muscular limb volume between the two interventions. This means that strengthening exercises can be performed by patients with lymphedema safely, without the risk of increasing upper limb volume with edema.
References
- 1.Badger C, Preston C, Seers K, Mortimer P. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev. 2004;18:cd 003141. doi: 10.1002/14651858.CD003141.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann A, da Costa Leite Ferreira MG, de Aguiar SS, et al. Physiotherapy in upper limb lymphedema after breast cancer treatment:a randomized study. Lymphology. 2014;47:82–91. [PubMed] [Google Scholar]
- 3.Bergmann A, Mattos IE, Koifman RJ, et al. Axillary web syndrome after lymph node dissection:results for 1004 breast cancer patients. Lymphology. 2007;40:198–203. [Google Scholar]
- 4.Bevilacqua JLB, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol. 2012;19:2580–9. doi: 10.1245/s10434-012-2290-x. [DOI] [PubMed] [Google Scholar]
- 5.Damstra RJ, Voesten HG, Van SWD, et al. Lymphatic venous anastomosis (LVA) for treatment of secondary arm lymphedema. A prospective study of 11 LVA procedures in 10 patients with breast cancer related lymphedema and a critical review of the literature. Breast Cancer Res Treat. 2009;113:199–206. doi: 10.1007/s10549-008-9932-5. [DOI] [PubMed] [Google Scholar]
- 6.DiSipio T, Rye S, Newman B, et al. Incidence of unilateral arm lymphoedema after breast cancer:a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 7.Eakin EG, Youlden DR, Baade PD, et al. Health behaviors of cancer survivors:data from an Australian population-based survey. Cancer Causes Control. 2007;18:881–94. doi: 10.1007/s10552-007-9033-5. [DOI] [PubMed] [Google Scholar]
- 8.Foldi M, Foldi E. Foldi's Textbook of lymphology:for physicians and Lymphedema therapists. 3ed. Munich: Urban and Fischer; 2012. pp. 22038–39. [Google Scholar]
- 9.Forner-Cordero I, Munoz-Langa J, Forner-Cordero A, et al. Predictive factors of response to decongestive therapy in patients with breast-cancer-related lymphedema. Ann Surg Oncol. 2010;17:744–51. doi: 10.1245/s10434-009-0778-9. [DOI] [PubMed] [Google Scholar]
- 10.Hayes SC, Janda M, Cornish B, et al. Lymphedema after breast cancer:incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26:3536–42. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 11.Internacional Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the international society of lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 12.Lanza M, Bergmann A, Ferreira MG, et al. Quality of life and volume reduction in women with secondary lymphoedema related to breast cancer. Int J Breast Cancer. 2015;2015:586827. doi: 10.1155/2015/586827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leidenius M, Leppanen E, Krogerus L, et al. Motion restriction and axillary web syndrome after sentinel node biopsy and axillary clearance in breast cancer. Am J Surg. 2003;185:127–30. doi: 10.1016/s0002-9610(02)01214-x. [DOI] [PubMed] [Google Scholar]
- 14.Makluf ASD, Dias RC, Barra A, de A. Avaliação da qualidade de vida em mulheres com câncer da mama. Revista Brasileira de Cancerologia. 2006;52:49–58. [Google Scholar]
- 15.Morris C, Wonders KY. Concise review on the safety of exercise on symptoms of lymphedema. World J Clin Oncol. 2015;6:43–4. doi: 10.5306/wjco.v6.i4.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neter JM, Kutner H, Nachtsheim CJ. Wasserman w applied linear statistical models. Chicago, IL: Irwin; 1996. p. 10. [Google Scholar]
- 17.Partsch H, Mortimer P. Compression for leg wounds. Br J Dermatol. 2015;173:359–69. doi: 10.1111/bjd.13851. [DOI] [PubMed] [Google Scholar]
- 18.Patel KM, Manrique O, Sosin M, et al. Lymphatic mapping and lymphedema surgery in the breast cancer patient. Gland Surg. 2015;4:244–56. doi: 10.3978/j.issn.2227-684X.2015.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro Pereira ACP, Koifman RJ, Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment:10 years of follow-up. Breast J. 2017;36:67–73. doi: 10.1016/j.breast.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Sclafani LM, Baron RH. Sentinel lymph node biopsy and axillary dissection:added morbidity of the arm, shoulder and chest wall after mastectomy and reconstruction. Cancer. 2008;14:216–22. doi: 10.1097/PPO.0b013e31817fbe5e. [DOI] [PubMed] [Google Scholar]
- 21.Smith SG, Chagpar AB. Adherence to physical activity guidelines in breast cancer survivors. Am Surg. 2010;76:962–5. [PubMed] [Google Scholar]
- 22.What Is Lymphedema?What Is Lymphedema?National Lymphedema Network, 1 Jan (2013) [Internet] [[captured on 30 January, 2016]]. Available on: http://www.lymphnet.org/
- 23.World Health Organization. Early cancer diagnosis saves lives, cuts treatment cost, 2017. Geneva, Switzerland: World Health Organization; 2017. Available on:[Internet]. http://www.who.int/mediacentre/news/releases/2017/early-cancer-costs/en/ [Google Scholar]


