Abstract
Background:
Gastric cancer is one of the most common malignancies worldwide. Epirubicin (EPI) is used extensively in the treatment of multiple cancers despite its tendency to induce multidrug resistance though overexpression of the ABCB1 efflux pump. However, this overexpression can be disrupted using short interfering RNAs (siRNAs).
Objective and Methods:
The aim of this study was to explore approaches to reverse EPI resistance and thus increase the success of chemotherapy treatment in an EPI-resistant gastric cancer cell subline (AGS/EPI).
Methods:
The study focused on effects of ABCB1 knockdown by siRNA technology using TaqMan gene expression assays with quantitative real-time reverse-transcription PCR (qRT-PCR). MTT assays were performed to evaluate viability and prolifer in subline. ABCB1 protein localization and EPI intracellular fluorescence intensity in AGS/EPI cells were detected by confocal microscopy.
Results:
The siRNA efficiently downregulated ABCB1 mRNA in AGS/EPI cells. Thus MDR reversal was clearly demonstrated in the AGS/EPI cells, offering the possibility of future in vitro chemoresistance assays for the GC field.
Conclusions:
ABCB1 knockdown decreased EPI efflux and increased EPI sensitivity in AGS/EPI cells. This result provides a novel strategy for targeted gene therapy to reverse EPI resistance in gastric cancer.
Keywords: RNA interference, gastric cancer, ABCB1, drug resistance, epirubicin
Introduction
Gastric cancer (GC) remains the second leading cause of cancer-related death and the fourth most common malignancy, with 989,600 new cases and 738,000 deaths estimated to have occurred worldwide in 2008 (Jemal et al., 2011). Consequently, the development of effective therapies with minimal toxicities remains a field of intense investigation. The regimen involving epirubicin, cisplatin, and fluorouracil (ECF) described by Findlay et al., (1994) in the 1980s improves overall patient survival with metastatic or advanced GC. Epirubicin (EPI) is used extensively in the clinic despite the tendency for this anthracycline to induce multidrug resistance (MDR) in cancer cells responding to EPI (Cunningham et al., 2006).
MDR is typically associated with the overexpression of the efflux pump ABCB1, also known as P-glycoprotein (Gottesman et al., 2002). ABCB1 is a member of the ATP-binding cassette (ABC) transporter family encoded by the ABCB1 gene. This protein considerably influences drug efficacy because increased drug efflux lowers intracellular drug concentrations (Ueda et al., 1986; Richter et al., 2006). Modulating MDR to reverse tumor cell chemoresistance to improve cancer chemotherapy has shown good results. However, adverse side effects can arise when the modulator is not selective (Borowski et al., 2005).
Fortunately, the overexpression of drug-transporter proteins such as ABCB1 can be specifically disrupted using RNA interference (RNAi) mechanism (Wu et al., 2003; Duan et al., 2004; Peng et al., 2004; Yagüe et al., 2004; Nieth et al., 2003). RNAi, an intrinsic mechanism utilized in all multicellular eukaryotes, is a powerful and specific posttranscriptional gene silencing process (Elbashir et al., 2001; Mittal, 2004). This process can be induced by 21–23 nt-long double stranded RNAs molecules, known as short interfering RNAs (siRNAs). These small regulatory RNAs trigger endonucleolytic degradation of complementary target mRNA (Dillin, 2003; Pai et al., 2006).
We recently reviewed the latest advances concerning the promising application of RNAi in GC treatment (Felipe et al., 2014a). In support of this technology, Yang and Zhang suggested that RNAi therapy might represent a novel approach to specifically target MDR genes and reverse chemoresistance in cancer cells (Yang and Zhang, 2012). In this respect, Zhu et al., (2013) recently showed that ABCB1 knockdown increases adriamycin sensitivity in the drug-resistant human GC cell subline SGC7901. The objective of our present study was to attempt the reversal of MDR by silencing ABCB1 expression by using siRNAs in the EPI-resistant gastric cancer cell subline AGS/EPI. In this way, we aimed to reduce MDR, and thus increase EPI sensitivity to improve the efficacy of chemotherapy.
Materials and Methods
Cell lines and culture
The human gastric adenocarcinoma cell line (AGS) was purchased from Rio de Janeiro Cell Bank (BCRJ ID 0311, Brazil). The EPI-resistant cell subline (AGS/EPI) were established from parental AGS cells by culturing them with increasing concentrations of EPI (Accord Healthcare Ltd, Middlesex, Wielka Brytania, UK) up to a final dose of 1 μM. EPI-resistant cells demonstrated elevated ABCB1 mRNA expression (Felipe et al., 2014b). The EPI-sensitive gastric cancer cell subline (AGS-siRNA) was developed using ABCB1 mRNA knockdown in AGS/EPI cells. All cell lines were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine at 37ºC in a humidified incubator containing 5% CO2 (MCO-5M, Panasonic, Tokyo, Japan).
siRNA design, synthesis, and transfection
siRNA sequences targeting ABCB1 mRNA were designed using BLOCK-iTTM RNAi Designer Software and were purchased from Ambion/Molecular Probes/Life Technologies (Carlsbad, CA, USA). These sequences were given inventory identification numbers (IDs): s10418(#1), sense: 5‘-GGCUUGCUGUAAUUACCCAtt, antisense: 5‘-UGGGUAAUUACAGCAAGCCtg-3‘ and ID: s10419(#2); sense: 5‘- CGAUACAUGGUUUUCCGAUtt; antisense: 5‘-AUCGGAAAACCAUGUAUCGga-3‘). To assess transfection efficiency, a non-targeting siRNA (The Silencer select control) was used as a negative control and BLOCK-iT™ Alexa Fluor® (555-labeled) Red Fluorescent Control as a positive control for siRNA transfection. siRNA transfections were performed following the manufacturer’s protocol. In brief, AGS/EPI cells (1 × 104 cells/well) were seeded in 96-well plates to a final volume of 120 μL, and transfections were performed using Lipofectamine RNAi-MAX (Invitrogen/Life Technologies, Carlsbad, CA, USA) and OPTI-MEM I reduced serum medium (Invitrogen/Life Technologies). AGS/EPI cells were transfected with pooled siRNAs containing two siRNAs (s10418 and s10419) specific for ABCB1 mRNA. The final siRNA concentration was 50 nM. The number of transfected cells per transfection (% positive cells) was counted using a Guava EasyCyte HT Flow Cytometry System (Millipore-Guava Technologies). Gene silencing was verified 72 h after transfection by measuring ABCB1 mRNA.
Quantitative real-time reverse-transcription PCR (qRT-PCR)
Total RNA isolation and cDNA synthesis was performed using the TaqMan Gene Expression Cell-to-CT Kit (Ambion) following the manufacturer’s protocol. Expression assays were performed using TaqMan Gene Expression Assays for human ABCB1 (Hs00184491_m1) and endogenous control human ACTB (Hs01060665_g1) with TaqMan Gene Expression Master Mix according to the manufacturer’s protocol (Applied Biosystems, Carlsbad, CA, USA). Reverse transcriptase reactions and real-time PCR were performed using the StepOnePlus Real-Time PCR System (Applied Biosystems). Briefly, the reactions were incubated in a 96-well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. In each experiment, triplicate reactions were performed per sample. Finally, the relative expression per sample was calculated as the ratio of ABCB1 to ACTB. Specifically, relative ABCB1 mRNA content per sample was calculated as 2−ΔΔCT, where ΔCT = (mean of triplicate CTABCB1 − mean of triplicate CTACTB) and ΔΔCT = (ΔCT – mean ΔCT of all the samples).
Laser-scanning confocal microscopy analysis
ABCB1 protein localization and EPI intracellular fluorescence intensity in transfected and untransfected AGS/EPI cells were detected by confocal microscopy by using a Leica TCS SP8 microscope (Leica Microsystems, Wetzlar, Germany). Briefly, glass coverslips (Helmut Sauer, Reutlingen, Germany) were autoclaved, and cells were grown to approximately 50% confluence on these coverslips. The cells were stained with CellMask™ Deep Red plasma membrane stain (1:1000; Invitrogen, Molecular Probes) in phosphate-buffered saline (PBS) containing 10% normal bovine serum for 40 min at 22°C and washed three times in PBS. The cells were then permeabilized with 0.1% saponin in PBS containing 10% normal bovine serum for 30 min at 22°C and then stained with a combination of fluorescent dyes. The cells were immunostained with ABCB1-FITC 488 (green) (Santa Cruz Biotechnology, Santa Cruz, CA). Nuclei were counterstained with the blue fluorescent DNA stain 4′,6-diamidino-2-phenylindole (DAPI, 1:1000; Invitrogen, Molecular Probes) for 10 min at 22°C. The cells were imaged using 40× or 100× oil immersion objective lens. EPI is an autofluorescent compound. Accordingly, untreated cells showed minimal autofluorescence and this condition was used to adjust the background to zero. Images were captured and EPI was measured using the microscopic image analysis software Leica Application Suite, LAS version 3.1.0 (Leica Microsystems, Wetzlar, Germany).
Cell viability assay
All GC cell lines were treated with different concentrations of EPI for 48 h, and seeded in 96-well U-bottom culture plates for an additional 24 h at 37°C. Following overnight culture, 20 μL of 2-(4,5-dimethyltriazol-2yl)-2,5-diphenyltetrazoliumbromide (MTT; 5 mg/mL) was added to each well and incubated for an additional 4 h under the same conditions. Formazan crystals were dissolved in 100 μL of acid-isopropanol (0.04 M HCl in isopropanol). Cell viability based on mitochondrial inactivity was evaluated by measuring optical density at 570 nm, with a reference wavelength of 630 nm, by using an Elx800 microplate reader (BIO-TEK Instruments, Inc., Winooski, VT, USA). Viability was measured as the ratio of the absorbance measured for EPI-treated and untreated cell samples. EPI resistance ratio and Reversal ratio were obtained from the following equations:
EPI resistance ratio (fold change) = (IC50 of AGS/EPI cells) / (IC50 of AGS cells)
Reversal ratio (fold change) = (IC50 of AGS/EPI cells) / (IC50 of AGS-siRNA cells)
Statistical analysis
Measurements were performed in triplicate and data are expressed as mean ± standard deviation (SD). Pearson’s correlation analysis was used to determine the relationship between mRNA expression and EPI resistance. All other statistical differences were determined with unpaired Student’s t-test or one-way ANOVA, followed by the Tukey test for multiple comparisons. P values less than 0.05 were regarded as statistically significant. Statistical analyses were performed using Minitab V16.0 software (State College, PA).
Results
ABCB1 protein localization and siRNA efficiency
A non-targeting control siRNA and two ABCB1-specfic siRNAs were transfected into AGS/EPI cells. AGS/EPI cells were visualized by laser-scanning confocal microscopy after transfection to evaluate the effect of siRNA on ABCB1 expression. The ABCB1 glycoprotein was predominantly localized in the transmembrane region in epirubicin-resistant gastric cancer cell subline and labeled with FITC, as shown in Figure 1 A-D. The levels of BLOCK-iT™ Alexa Fluor® (555-labeled) fluorescence of transfected and untransfected AGS/EPI cells was measured after siRNA treatment by flow cytometry to confirm transfection and to determine the proportion of transfected cells (% positive cells), as shown in Figure. 1E.
Figure 1.
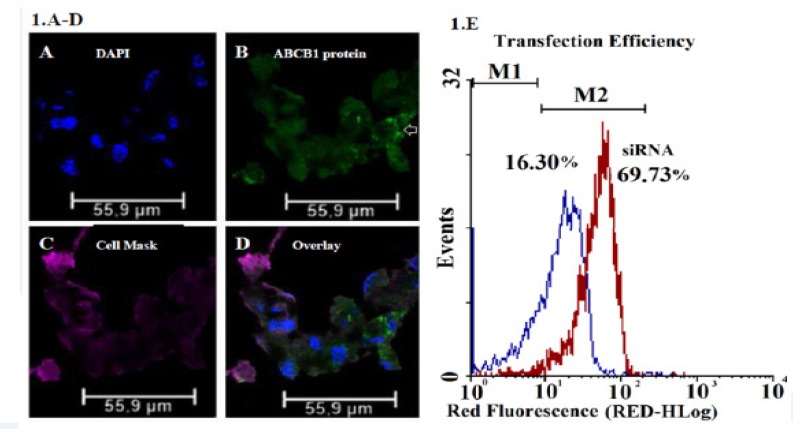
Confocal Microscopy Images Characteristic of AGS/EPI Cancer Gastric Cells. (A) Nuclei stained with DAPI, (B) ABCB1 proteins labeled with FITC, (C) plasma membranes labeled with Cell Mask™ deep red dye, (D) and overlay. (E) AGS/EPI cells in log phase were divided into two groups: one group was transfected with the Silencer® Select Negative Control M1 (negative control group) and the second was transfected with the Silencer® Select ABCB1 Positive Control siRNA M2 (positive control group). Group M1 was used to measure the basal levels of ABCB1 mRNA. After 72 h, the number of transfected cells per sample (% positive cells) was measured by flow cytometry. This method demonstrated that the transfection efficiency was nearly 70%.
EPI localization and fluorescence in AGS/EPI cells
EPI was added to AGS/EPI cell cultures and its localization was detected by laser-scanning confocal microscopy, as indicated in Figure 2 A-D. The spectral properties of EPI are shown in Figure 2E.
Figure 2.
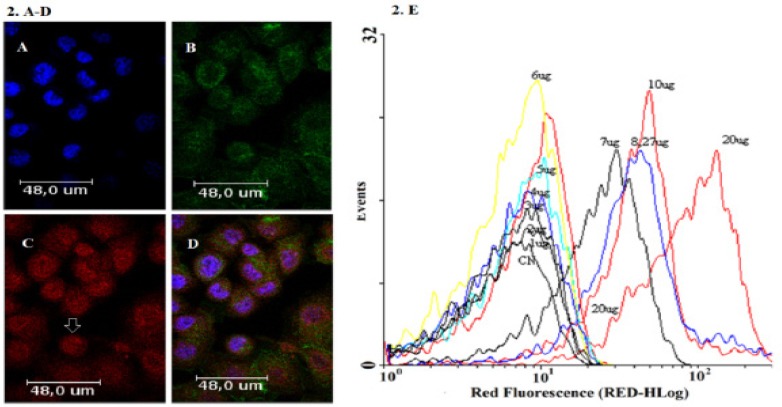
Confocal Microscopy Images Characteristic of AGS Cancer Gastric Cells Treated with EPI. (A) Nuclei stained with DAPI, (B) cytoskeleton stained with phalloidin 488, (C) EPI distribution with increased fluorescence intensity near the nucleus (arrow), and (D) overlay. (E) Spectrum of EPI fluorescence intensities by flow cytometry at different EPI concentrations (1–20 µg/mL).
Suppressed ABCB1 mRNA expression using siRNA technology
Transfected cells (AGS/EPI-siRNA), untransfected cells (AGS/EPI), and AGS cells were cultured for 72 h after transfection. ABCB1 and ACTB (normalization control) mRNAs were measured by qRT-PCR and the relative expression was calculated as the ratio of ABCB1 to ACTB for each sample. ABCB1 mRNA expression in AGS/EPI-siRNA cells was significantly reduced (P < 0.05) compared with that in AGS-EPI and AGS cells (Figure 3A). Pearson’s correlation demonstrated that ABCB1 mRNA expression and EPI sensitivity were closely correlated, suggesting that ABCB1 downregulation sensitized cells to EPI, as shown in Figure. 3B.
Figure 3.
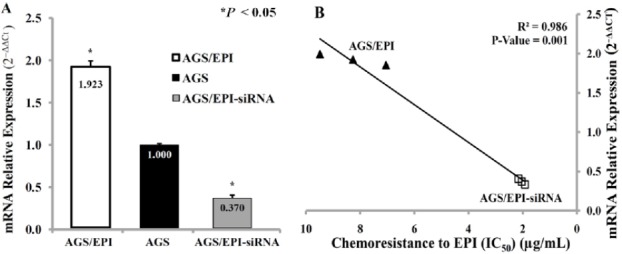
ABCB1 mRNA Expression Levels in AGS, AGS/EPI, and AGS/EPI-siRNA GC cells. (A) The 2−ΔΔCt value for AGS/EPI cells was 1.923-fold higher than that for AGS cells, and the AGS/EPI-siRNA cell subline was 0.370-fold lower than that of the AGS cells. (B) Correlation between ABCB1 mRNA expression and EPI chemoresistance (IC50) in AGS/EPI and AGS/EPI-siRNA CG cells. ABCB1 mRNA levels 72 h post-transfection were reduced by approximately 80%, demonstrating effective ABCB1 silencing by siRNA. ABCB1 mRNA expression was significantly correlated with EPI sensitivity.
siRNA-mediated ABCB1 silencing and EPI sensitization in AGS/EPI cells
AGS/EPI, AGS, and AGS/EPI-siRNA were cultured to measure viability following EPI treatment by MTT colorimetric assay. Cell viabilities were used to determine EPI IC50 values for each cell line Figure 4A. AGS/EPI-chemoresistant cells showed low sensitivity to EPI, whereas AGS/EPI-siRNA cells demonstrated significantly high sensitivity to EPI, as shown in Figure 4B.
Figure 4.
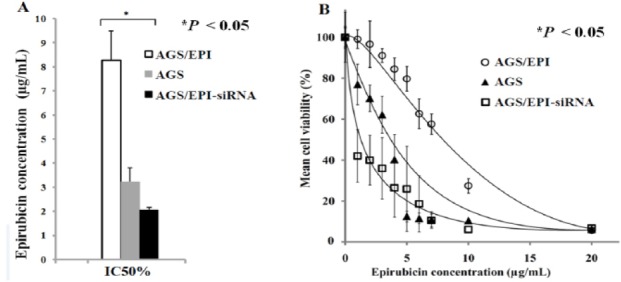
Effects of EPI on Cell Viability in Three Cancer Cell Lines. (A) EPI half-maximal inhibitory concentrations (IC50) in AGS, AGS/EPI, and AGS/EPI-siRNA cells differed significantly. (B) EPI sensitivities in AGS, AGS/EPI, and AGS/EPI-siRNA cells differed significantly at all EPI concentrations. ABCB1 knockdown reversed EPI chemoresistance and potentiated EPI effects on AGS cells. Unpaired Student’s t-test or one-way ANOVA, followed by the Tukey test for multiple comparisons, shows statistically significant differences (*P < 0.05).
Discussion
The main obstacle for effective GC chemotherapy is the prevalence of MDR strains. In several cases of MDR, cells responded to drug exposure by upregulating drug efflux pumps, cellular detoxification processes, and DNA repair mechanisms. These responses enable the development of undesirable MDR phenotypes in GC cells (Gottesman, 2002).
Overexpression of efflux pump proteins such as ABCB1 has been directly linked to drug resistance and represents a major obstacle for effective chemotherapy. Consequently, ABCB1 knockdown has been frequently investigated in vitro in a variety of human cancers, including breast cancer (Wu et al., 2003), ovarian cancer (Duan et al., 2004), leukemia (Peng et al., 2004; Yagüe et al., 2004), pancreatic cancer, and gastric carcinoma (Nieth et al., 2003). In this study, we investigated the reversal of MDR by silencing ABCB1 in EPI-resistant gastric cancer cells to increase the efficacy of EPI treatment.
We verified that both siRNAs effectively downregulated ABCB1 expression in AGS/EPI cells. Specifically, RT-qPCR failed to detect ABCB1 transcripts in non-target group cells. Moreover, we observed a high percentage of transfected cells in our transfection studies. Collectively, these data demonstrate effective ABCB1 knockdown using siRNAs. We compared our transfection efficiency (70%) with reports from other investigators, which showed 90% efficiency in the human GC cell subline SGC7901; our findings agreed well with those of the previous studies (Zhu et al., 2013).
We found that EPI is less cytotoxic to AGS/EPI cells compared to AGS cells. However, ABCB1 knockdown significantly decreased chemoresistance of AGS/EPI-siRNA cells to EPI. Additionally, gene silencing may have contributed to decreased EPI efflux observed in AGS/EPI-siRNAs cells, suggesting that ABCB1 downregulation was responsible for apoptosis.
EPI treatment in AGS/EPI-siRNA cells demonstrated an in vitro anticancer effect due to reduced EPI efflux, and therefore, chemoresistance reversal. To put our results into context, we compared our reversal ratio with one reported in a similar study featuring another anthracycline antitumor drug in a GC cell line. The reversal ratio (4-fold) in our model of EPI resistance was higher than that reported by Nieth et al., (2003) (1.42-fold) in the daunorubicin-resistant human gastric carcinoma cell subline EPG85-257P (Yang and Zhang, 2012). Importantly, these two studies reported similar knockdown efficiencies.
AGS cells demonstrated low expression of ABCB1 mRNA, while EPI-resistant AGS/EPI cells stably express high levels of ABCB1 mRNA, and ABCB1 knockdown by siRNA significantly decreased EPI resistance. However, we have clearly demonstrated MDR reversal in the AGS/EPI cells, and these results can help design future in vitro chemoresistance assays for the GC field.
Our findings suggest that ABCB1 knockdown reverses EPI resistance in ABCB1-expressing, drug-resistant cells. Based on these data, we conclude that ABCB1 knockdown using siRNA technology increases EPI sensitivity due to reduced EPI efflux in GC cells. These findings can be promisingly applied to the development of siRNA therapies to reverse EPI resistance.
Acknowledgments
The authors would like to thank Prof. Dra. Helena Bonciani Nader for her generosity to help with confocal microscopy. Special thanks are in place for Prof. Dr. Ismael Dale Cotrim Guerreiro da Silva, for allowing us to use the Flow Cytometry System. Finally, special thanks must go to our colleagues at UNINOVE (Prof. Dr. Michel Sant’Anna de Pinho and Prof. Dr. Alfredo Ribeiro Filho) who have offered us the scientific support.
References
- 1.Borowski E, Bontemps-Gracz MM, Piwkowska A. Strategies for overcoming ABC-transporters-mediated multidrug resistance (MDR) of tumor cells. Acta Biochim Pol. 2005;52:609–27. [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Dillin A. The specifics of small interfering RNA specificity. Proc Natl Acad Sci U S A. 2003;100:6289–91. doi: 10.1073/pnas.1232238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–8. [PubMed] [Google Scholar]
- 5.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felipe AV, Oliveira J, Chang PYJ, et al. RNA interference:a promising therapy for gastric cancer. Asian Pac J Cancer Prev. 2014a;15:5509–15. doi: 10.7314/apjcp.2014.15.14.5509. [DOI] [PubMed] [Google Scholar]
- 7.Felipe AV, Moraes AA, Oliveira J, Silva TD, Forones NM. Establishment and partial characterization of an epirubicin-resistant gastric cancer cell line with upregulated ABCB1. Asian Pac J Cancer Prev. 2014b;15:6849–53. doi: 10.7314/apjcp.2014.15.16.6849. [DOI] [PubMed] [Google Scholar]
- 8.Findlay M, Cunningham D, Norman, et al. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF) Ann Oncol. 1994;5:609–16. doi: 10.1093/oxfordjournals.annonc.a058932. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer:role of ATP–dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 11.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 12.Mittal V. Improving the efficiency of RNA interference in mammals. Nat Rev Genet. 2004;5:355–65. doi: 10.1038/nrg1323. [DOI] [PubMed] [Google Scholar]
- 13.Nieth C, Priebsch A, Stege A, Lage H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi) FEBS lett. 2003;545:144–50. doi: 10.1016/s0014-5793(03)00523-4. [DOI] [PubMed] [Google Scholar]
- 14.Pai SI, Lin YY, Macaes B, et al. Prospects of RNA interference therapy for cancer. Gene Ther. 2006;13:464–77. doi: 10.1038/sj.gt.3302694. [DOI] [PubMed] [Google Scholar]
- 15.Peng Z, Xiao Z, Wang Y, et al. Reversal of P-glycoprotein-mediated multidrug resistance with small interference RNA (siRNA) in leukemia cells. Cancer Gene Ther. 2004;11:707–12. doi: 10.1038/sj.cgt.7700738. [DOI] [PubMed] [Google Scholar]
- 16.Richter M, Molnár J, Hilgeroth A. Biological evaluation of bishydroxymethyl-substituted cage dimeric 1, 4-dihydropyridines as a novel class of p-glycoprotein modulating agents in cancer cells. J Med Chem. 2006;49:2838–40. doi: 10.1021/jm058046w. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K, Cornwell M M, Gottesman MM, et al. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun. 1986;141:956–62. doi: 10.1016/s0006-291x(86)80136-x. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–9. [PubMed] [Google Scholar]
- 19.Yagüe E, Higgins CF, Raguz S. Complete reversal of multidrug resistance by stable expression of small interfering RNAs targeting MDR1. Gene Ther. 2004;11:1170–4. doi: 10.1038/sj.gt.3302269. [DOI] [PubMed] [Google Scholar]
- 20.Yang WQ, Zhang Y. RNAi-mediated gene silencing in cancer therapy. Expert Opin Biol Ther. 2012;12:1495–504. doi: 10.1517/14712598.2012.712107. [DOI] [PubMed] [Google Scholar]
- 21.Zhu CY, Lv YP, Yan DF, Gao FL. Knockdown of MDR1 increases the sensitivity to adriamycin in drug resistant gastric cancer cells. Asian Pac J Cancer Prev. 2013;14:6757–60. doi: 10.7314/apjcp.2013.14.11.6757. [DOI] [PubMed] [Google Scholar]


