Abstract
Background:
The efficiency of radiotherapy for tumors can be enhanced with different radiosensitizers. Previous studies have shown that electroporation (EP) can sensitize some cancer cell lines to ionizing radiation (IR). HT-29 is a radiation resistant colorectal cancer cell line, representative of a cancer type which is the second cause of cancer mortalities in developed countries. The present study aimed to evaluate radiosensitizing effects of EP on HT-29 cells in vitro exposed to 6 MV X-ray photon beams.
Methods:
HT-29 cells were exposed to a 6 MV X-ray photon beam as the control or to a combination of electroporation and irradiation. The response of cells was evaluated by colony formation assay and survival curves.
Results:
The survival fraction of the HT-29 cells was significantly decreased by electroporation prior to radiotherapy. A single electric pulse increased colorectal HT-29 cancer cell sensitivity to mega-voltage radiation by a factor of 1.36.
Conclusion:
Our findings showed that EP before radiotherapy can significantly enhance tumor cell sensitivity. This combined treatment modality should be assessed for its applicability in clinic settings for employment against radioresistant cancers. However, to facilitate achieving this goal, many different tumors with a broad range of radiosensitivities should be evaluated.
Keywords: Electroporation, irradiation, radiosensitizing, colon cancer
Introduction
Colorectal cancer is a common malignancy and second leading cause of cancer mortalities in United States and other developed countries (Azadeh et al., 2007; Siegel et al., 2012). Surgery and chemotherapy are currently the primary treatment options for colorectal cancer and radiotherapy serves as a complementary therapeutic option (Chen et al., 2010). Radiotherapy has the ability to shrink and kill cancer cells by bombardment of them with ionizing radiation and causing DNA damage by direct action or through production of reactive oxygen species (ROS) (Hall and Giaccia, 2006). In order to induce sufficient damages in targeted cancer cells, it is crucial to increase the radiation dose (Hendee, 2006). However, radiations can also induce biological damages in normal tissues due to little discrimination of ionizing radiations between normal and malignant tissues (Hainfeld et al., 2008; Anijdan et al., 2013; Cooper et al., 2014). Therefore, the doses must be limited below the curative level to protect normal surrounding tissue (Hainfeld et al., 2008). For this reason, the recurrence of colorectal cancer is observed in more than 50% of cases (Arab-Bafrani et al., 2015). Using appropriate radiosensitizer is one of the interesting approaches to increase radiation dose only at the site of tumor. Electroporation (EP) technique can be used to induce radiosensitivity in tumor cells (Serša et al., 2000; Kranjc et al., 2005; Shil et al., 2006). EP is a physical process to increase the permeability of cell membrane in response to short high-voltage electric pulses (Gehl, 2003; Yarmush et al., 2014; Robert et al., 2015). This technique has been used to transport different molecules such as chemotherapeutic drugs, proteins, DNA, and dyes through cell membranes (Gothelf et al., 2003; Davalos et al., 2005; Miklavcic et al., 2010; Kotnik et al., 2015; Lamichhane et al., 2015; Meglic et al., 2015; Takahashi et al., 2015; Bianchi et al., 2016; Rezaee et al., 2017). Moreover, delivering short intense electric pulses ranging nano- to micro-second pulse duration to cell leads to the production of ROS in the electroporated side of the membrane (Gabriel and Teissie, 1994). ROS can sensitize the cells to ionization radiation. Therefore, EP with its noninvasive nature can be employed prior to radiotherapy to selectively enhance of dose in the targeted tumor cells. Previous studies have shown different protocols of EP can induce radiosensitizing effects in different tumor cells. The common protocols of EP comprise of microsecond pulse duration in the frequencies ranging 1 to 100 Hz applied in the intensities of 1 to 1.5 kV/cm. There is no published study that investigated the radiosensitizing effect of single microsecond electric pulse on colorectal cancer cells. Therefore, the present study was aimed to investigate the radiosensitizing effect of a single microsecond electric pulse (100-µs pulse duration) in colorectal cancer cells using colony formation assay through survival curve. The sensitizer enhancement ratios of HT-29 cell lines irradiated with 6 MV X-ray photons under two irradiation conditions ionizing radiation alone and EP at 10 min prior to ionizing radiation were comparatively investigated.
Materials and Methods
Cell culture: Human colorectal (HT-29) cells were purchased from National Cell Bank of Pasteur Institute of Iran (NCBI, C466) and cultured in Roswell park memorial institute (RPMI) 1640 medium (BIO-IDEA, B11031) supplemented with 10% Fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Bio-Idea, Iran). The cells were routinely subcultured every 4 days and kept at 37 °C in a humidified atmosphere with 5 % CO2 in incubator (RS Biotech Galaxy R).
Electroporation Setup
Following trypsination, the cells were centrifuged and resuspended in the growth media. A 30 µl of cell suspension containing known cell number (100, 200, 400, 1,000 and 2,000 cells in accordance with radiation doses of 0, 2, 4, 6, and 8 Gy) was added into a 1-mm gap EP cuvette. A single square pulse with electric field intensity of 1200 V/cm and pulse duration 100 µs was delivered to the sample using a Bio-Rad Gene Pulser Xcell™ EP system. Then, the samples were transferred to a 6-well plate and the fresh medium was added to each well. Finally, the HT-29 cells were exposed to ionization radiation after 10 min.
Irradiation Setup and Colony Formation Assay
Exposure of cells to 6 MV X-ray photons were performed at the room temperature using Varian 2100 C/D linear accelerator (LINAC, Golestan hospital, Ahvaz, Iran) with a dose rate of 3 Gy/min. the cells were exposed to individual total doses 0, 2, 4, 6, and 8 Gy with a field size of 20×20 cm2. 1.5 cm thickness of a Plexiglass sheet (water equivalent) was placed on top of the six wells-plate and five centimeters of a Plexiglass sheet was utilized under the bottom of plate as a source of backscatters. After completion of irradiation, the cells were incubated for 14 days at 37°C in humidified 5% CO2 atmosphere. Then, the cells were fixed and stained by 0.4% crystal violet and visible colonies with more than 50 cells were counted. The plating efficiency (PE) was calculated based on the survival of non-treated group (0 Gy) and survival fraction (SF) of treatment group was obtained by following formula: SF = colony number/ (plating cell number × PE). The survival curve was estimated by linear quadratic model with equation of SF = exp (−αD−βD2) using MATLAB software. The sensitizer enhancement ratio (SER) was calculated by ratio of radiation dose that resulted in 50% cell survival (LD50) in the absence or the presence of EP.
Statistical Analysis
Each of our experiments was repeated at least three times and results were expressed as mean ± standard error of mean (SEM). All data were examined for normality of distribution by Kolmogorov Smirnove test. Statistical analyses were assessed by t-test and SigmaStat statistical software (SPSS Inc.). The P-values level of less than 0.05 was set as significant.
Results
Effect of electroporation on HT-29 cell Survival Curve and sensitizer enhancement ratio
In order to evaluate the radiosensitizing effect of EP, HT-29 cells were treated either with irradiation in the presence and absence of EP. The survival curve between these two groups was significantly different and the combination of EP with irradiation resulted in greater decreases in survival fraction (p-value<0.05) (Figure 1). Moreover, applying electric pulse prior to irradiation increased both α and β parameters of survival curve. The value of LD50 was decreased from 3.97 Gy in radiation alone group to 2.9 Gy in tumors were received EP before irradiation. Finally, the sensitizer enhancement ratio (SER) of 1.36 was obtained (Table 1).
Figure 1.
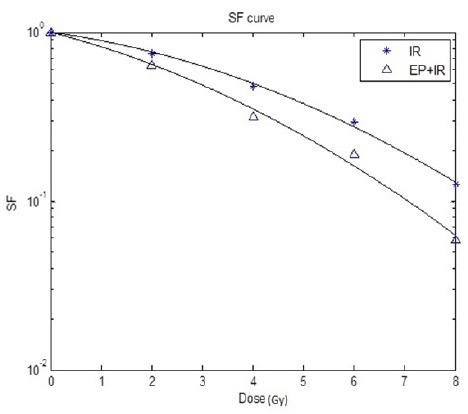
Survival Curve of HT-29 Cell Treated with Radiation only (IR) or both Electroporation and Radiation (EP+IR)
Table 1.
Comparison between α, β, LD50 and Sensitizer Enhancement Ratio (SER) for Irradiation alone (IR) or Combined with Electroporation (EP+IR)
| Group | α (Gy-1) | β (Gy-2) | LD50(Gy) | SER |
|---|---|---|---|---|
| IR | 0.0928 | 0.0203 | 3.97 | -- |
| EP+IR | 0.1755 | 0.0213 | 2.9 | 1.36 |
Discussion
In the present study, the radiosensitizing effect of EP was investigated in HT-29. Our results confirmed the previous studies (West, 1992; Kranjc et al., 2005). The first study on radiosensitizing effect of EP has been reported by West (1992). They used one exponential decaying electric pulse to sensitize CHO cells to 137Cs- gamma radiation. Their results revealed that electric pulse can change the α and β parameters and sensitize cells by factor of 1.19 (West, 1992). Similarly, we delivered one electric pulse but with square pulse shape. One square electric pulse could sensitize HT-29 cells by factor of 1.36. The probably mechanism of radiosensitization is generation of ROS in electroporated site of membrane (Bonnafous et al., 1999). When the cell is exposed to electric pulse, the oxidative jump is induced in electroporated sites of membrane and ROS is generated (Gabriel and Teissie, 1994; Bonnafous et al., 1999). ROS generation is restricted to the electroporated part of membrane (Gabriel and Teissie, 1995) and can enhance the effect of ionizing radiation. Later, the level of generated ROS in the Ehrlich Ascites Carcinoma (EAC) cells after delivering electric pulse has measured by Shil et al., (2006). ROS level in the case of electroporation and irradiation was significantly higher than irradiation alone group. In vivo study that has been performed by Shil et al., (2006) demonstrated that the average tumor volume of group that were treated by electroporation and irradiation was significantly (51%) smaller than this volume in irradiation alone group. In another in vivo study, electroporation decreased the tumor blood flow reversibly and induced tumor hypoxia. However, electroporation could improve response of tumor to irradiation by factor of 1.25 due to generation of ROS during the electroporation procedure (Kranjc et al., 2005).
Kranjc et al., (2005) and Sersa et al., (2000) have reported that the effect of 220KV radiation can be improved by electric pulses. Indeed, Previous studies have used orthovoltage unit to deliver 220KV x-rays (Serša et al., 2000; Kranjc et al., 2005) or radioisotope sources of Cs137 (West, 1992) and Co60 (Shil et al., 2006) to generate γ-radiation. But, because of the extensive uses of MV photons to treat deep seated tumors as well as spare skin of patients in clinic, we used a LINAC as a radiation source to exposure mega-voltage x-ray.
We suggest that electroporation can be combined with radiosensitizing drug such as gold (Jain et al., 2011) and silver (Liu et al., 2013) nanoparticles, chemotherapeutic agents (Kranjc et al., 2009), and drugs with low toxicity such as melatonin (Najafi et al., 2017a; Najafi et al., 2017b), metformin (Koritzinsky, 2015) and celecoxib (Gore, 2004). In this way, EP as a drug delivery system can increase the uptake of drugs and due to its intrinsic radiosensitizing effect, synergistic effect is seen.
In conclusion, based on our result electric pulse can sensitize colorectal HT-29 cancer cell to mega-voltage radiation and has a potential to use as a physical radiosensitizer for treatment of radio resistance tumor cells.
Acknowledgements
This study was financially supported by Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran (Grant No: CMRC-9428).
References
- 1.Anijdan SHM, Mahdavi SR, Shirazi A, et al. Megavoltage X-ray dose enhancement with gold nanoparticles in tumor bearing mice. Int J Mol Cell Med. 2013;2:118–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Arab-Bafrani Z, Saberi A, Birgani MJT, et al. Gold nanoparticle and mean inactivation dose of human intestinal colon cancer HT-29 cells. Jundishapur J Nat Pharm Prod. 2015;10:1–6. [Google Scholar]
- 3.Azadeh S, Moghimi-Dehkordi B, Fatem S, et al. Colorectal cancer in Iran:an epidemiological study. Asian Pac J Cancer Prev. 2007;9:123–6. [PubMed] [Google Scholar]
- 4.Bianchi G, Campanacci L, Ronchetti M, et al. Electrochemotherapy in the treatment of bone metastases:a phase II Trial. World J Surg. 2016;40:3088–94. doi: 10.1007/s00268-016-3627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnafous P, Vernhes M-C, Teissié J, et al. The generation of reactive-oxygen species associated with long-lasting pulse-induced electropermeabilisation of mammalian cells is based on a non-destructive alteration of the plasma membrane. Biochim Biophys Acta (BBA)-Biomembr. 1999;1461:123–34. doi: 10.1016/s0005-2736(99)00154-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen W-S, Lee Y-J, Yu Y-C, et al. Enhancement of p53-mutant human colorectal cancer cells radiosensitivity by flavonoid fisetin. Int J Radiat Oncol Biol Phys. 2010;77:1527–35. doi: 10.1016/j.ijrobp.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DR, Bekah D, Nadeau JL. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front Chem. 2014;2:1–13. doi: 10.3389/fchem.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davalos R, Mir L, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–31. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel B, Teissie J. Generation of reactive-oxygen species induced by electropermeabilization of Chinese hamster ovary cells and their consequence on cell viability. Eur J Biochem. 1994;223:25–33. doi: 10.1111/j.1432-1033.1994.tb18962.x. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel B, Teissie J. Spatial compartmentation and time resolution of photooxidation of a cell membrane probe in electropermeabilized Chinese hamster ovary cells. Eur J Biochem. 1995;228:710–8. doi: 10.1111/j.1432-1033.1995.tb20314.x. [DOI] [PubMed] [Google Scholar]
- 11.Gehl J. Electroporation:theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–47. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 12.Gore E. Celecoxib and radiation therapy in non-small-cell lung cancer. Oncology (Williston Park) 2004;18:10–4. [PubMed] [Google Scholar]
- 13.Gothelf A, Mir LM, Gehl J. Electrochemotherapy:results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–87. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 14.Hainfeld JF, Dilmanian FA, Slatkin DN, et al. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 2008;60:977–85. doi: 10.1211/jpp.60.8.0005. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, Physics and chemistry of radiation absorption. Philadelphia: PA:Lippincott Williams and Wilkins; 2012. pp. 8–10. [Google Scholar]
- 16.Hendee WR. Radiation physics for medical physicists. Med Phys. 2006;33:249. doi: 10.1118/1.3481669. [DOI] [PubMed] [Google Scholar]
- 17.Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79:531–9. doi: 10.1016/j.ijrobp.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koritzinsky M. Metformin:a novel biological modifier of tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:454–64. doi: 10.1016/j.ijrobp.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Kotnik T, Frey W, Sack M, et al. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015;33:480–8. doi: 10.1016/j.tibtech.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Kranjc S, Cemazar M, Grosel A, et al. Radiosensitising effect of electrochemotherapy with bleomycin in LPB sarcoma cells and tumors in mice. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranjc S, Tevz G, Kamensek U, et al. Radiosensitizing effect of electrochemotherapy in a fractionated radiation regimen in radiosensitive murine sarcoma and radioresistant adenocarcinoma tumor model. Radiat Res. 2009;172:677–85. doi: 10.1667/RR1873.1. [DOI] [PubMed] [Google Scholar]
- 22.Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm. 2015;12:3650–7. doi: 10.1021/acs.molpharmaceut.5b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Huang Z, Chen Z, et al. Silver nanoparticles:a novel radiation sensitizer for glioma? Nanoscale. 2013;5:11829–36. doi: 10.1039/c3nr01351k. [DOI] [PubMed] [Google Scholar]
- 24.Meglic SH, Marolt T, Miklavcic D. Protein extraction by means of electroporation from E. coli with preserved viability. J Membr Biol. 2015;248:893–901. doi: 10.1007/s00232-015-9824-7. [DOI] [PubMed] [Google Scholar]
- 25.Miklavcic D, Snoj M, Zupanic A, et al. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online. 2010;9:1–12. doi: 10.1186/1475-925X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Najafi M, Shirazi A, Motevaseli E, et al. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017a;9:139–48. doi: 10.1007/s12551-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Najafi M, Shirazi A, Motevaseli E, et al. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology. 2017b;25:403–13. doi: 10.1007/s10787-017-0332-5. [DOI] [PubMed] [Google Scholar]
- 28.Rezaee Z, Yadollahpour A, Rashidi S, et al. Radiosensitizing effect of electrochemotherapy:A systematic review of protocols and efficiency. Curr Drug Targets. 2017;18:1893–903. doi: 10.2174/1389450118666170622091014. [DOI] [PubMed] [Google Scholar]
- 29.Serša G, Kranjc S, Čemažar M. Improvement of combined modality therapy with cisplatin and radiation using electroporation of tumors. Int J Radiat Oncol Biol Phys. 2000;46:1037–41. doi: 10.1016/s0360-3016(99)00464-2. [DOI] [PubMed] [Google Scholar]
- 30.Shil P, Vidyasagar P, Sanghvi S, et al. Enhancement of cytotoxic effects of radiation and drug by electroporation in cancer cells:in vitro and in vivo studies. BARC Newslett. 2006;273:167–71. [Google Scholar]
- 31.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M, Kikkawa T, Osumi N. Electroporation Methods in Neuroscience, Gene transfer into cultured mammalian embryos by electroporation. New York, NY: Humana Press; 2015. pp. 141–57. [Google Scholar]
- 33.West CML. A potential pitfall in the use of electroporation:cellular radiosensitization by pulsed high-voltage electric fields. Int J Radiat Biol. 1992;61:329–34. doi: 10.1080/09553009214551011. [DOI] [PubMed] [Google Scholar]
- 34.Yarmush ML, Golberg A, Serša G, et al. Electroporation-based technologies for medicine:principles, applications, and challenges. Ann Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]


