Abstract
Background:
Curcumin was shown to reduce epithelial-mesenchymal transition (EMT) markers in previous short term studies. This study was aimed to investigate the potential of curcumin in the prevention of EMT activation in MCF-7 cells induced by endoxifen.
Methods:
MCF-7 breast cancer cells were treated with Endoxifen 1000 nM+beta-estradiol 1 nM with or without curcumin (8.5µM or 17 µM). Cells treated with dimethyl sulfoxide (DMSO) 0.001% were used as negative control. After 8 weeks of continuous treatment, the cells were counted, analyzed for mRNA E-cadherin, vimentin, TGF-β expression, total reactive oxygen species (ROS) and observed for morphological changes using confocal microscope and transmission electron microscope.
Result:
MCF-7 cell viability was increased in endoxifen + β-estradiol group. Cell viability was significantly decreased in curcumin 17 µM, but not in curcumin 8.5 µM group. Analysis of EMT markers at week 8 indicates that there were increase in vimentin and TGF-β mRNA expressions, while E-cadherin mRNA expressions and TGF-β1 protein concentrations were shown to decrease. The results showed that administration of curcumin in all the dose administered were incapable improving the expressions of vimentin, TGF-β1 and E-cadherin. There was a decrease in ROS concentration in curcumin treated cells (8.5 µM) while in curcumin 17 µM, ROS concentration was increased. Morphological observation using confocal microscope and TEM showed the presence of mesenchymal cells and adherens junction.
Conclusion:
endoxifen treatments for eight weeks resulted in upregulation of EMT markers and changes in morphology of MCF-7 breast cancer cells. The addition of curcumin did not prevent the activation of EMT.
Keywords: Curcumin, endoxifen, epithelial-mesenchymal transition (EMT), vimentin, TGF-β1, E-cadherin
Introduction
Resistance of cancer cells to anticancer drug is a serious obstacle in the treatment of cancer. At the beginning of therapy, many types of cancer cells is sensitive to chemotherapy. However, over time the cancer cells become resistant. Current knowledge of resistance mechanism includes drug inactivation, changes in drug targets, drug efflux from cancer cells, DNA damage repair and epithelial-mesenchymal transition process (Housman et al., 2014).
Epithelial-mesenchymal transition (EMT) is a biological process of epithelial cells, which normally interacts with the basement membrane through the basal surface, then undergo various changes that allow cells having mesenchymal phenotypes such as increasing the capacity of the migration, invasion, and resistant to apoptosis (Kalluri and Weinberg, 2009; Crook et al., 2009). At molecular level, EMT transition is marked by a series of changes include downregulation marker of epithelial and upregulation mesenchymal marker, which resulted in a number of phenotypic changes such as polarity and adhesion cell disruption, increasing of ability to migrate and invasion (Kalluri and Weinberg, 2009). EMT requires the cooperation of various signaling pathways and regulators. In the process of EMT, potential targets are divided into 3 groups: effector (E-cadherin, vimentin), the regulator (transcription factors such as Zeb, Snail and Twist) and inducers (TGF-β, growth factors (FGF, HGF, EGF, and IGF1)) (Pasquier J et al., 2015).
EMT phenomenon is found also on tamoxifen, which is still a first-line drug for endocrine-responsive breast cancer (Viedma-Rodriquez et al., 2014). Endoxifen is an active metabolite of tamoxifen, which have a more potent activity than tamoxifen (Wu et al., 2009). Previous study showed that continuous exposure of endoxifen 1,000 nM in MCF-7 breast cancer cells for 15 months leads to cancer cell resistance through the occurrence of epithelial-mesenchymal transition (EMT), which were characterized by the loss of expression of E-cadherin, upregulation of vimentin and TGF-β expressions (Hawse et al. 2010). In addition, another study in MCF-7 cells were treated 17β-estradiol 10 nM and endoxifen 100 nM for 48 hours showed a decrease in the expression of the protein E-cadherin and increased expression of snail (Lymperatou et al., 2013). Another study suggested that exposure endoxifen for 4 weeks in MCF-7 cells has not shown mesenchymal morphology. However, there is an increment of vimentin mRNA expression (Paramita et al., 2016).
Repeated treatments with chemotherapeutic agents can cause resistance via activation of EMT events (Al-Saleh and Luqmani, 2010). Therefore, it is important to identify substances that can inhibit the resistance process in cancer cells.
Curcumin is a natural diphenolic compound derived from turmeric root (Curcuma longa), which is a prime candidate for cancer disease (Yallapu et al., 2012). Previous study showed that curcumin was able to decrease the expression of vimentin, increasing the expression of E-cadherin in breast cancer cells induced by doxorubicin (Chen et al., 2013). Another study showed curcumin can inhibit TGF-β signaling that contribute to the occurrence EMT (Li et al., 2013). Up to date, it is not yet known whether curcumin can be used for the prevention of EMT activation in breast cancer cells treated with endoxifen. Therefore, this study was aimed to analyze the potential of curcumin in the prevention of EMT activation in MCF-7 cells induced by endoxifen.
Materials and Methods
Materials
Curcumin was purchased from Plamed Green Science Limited (China). Endoxifen was purchased from Tocris Bioscience (USA). Beta estradiol, glutaraldehyde, poly-L-lysine were purchased from Sigma-Aldrich Ltd (Singapore). Dimethylsulfoxide (DMSO) was purchased Vivantis (Malaysia), DMEM-high glucose, FBS heat-inactivated, Penicillin/Streptomycin, Fungizone, were purchased from Biowest (USA). MTS assay kit was purchased from Promega (USA). High Pure RNA isolation kit, Transcriptor First Strand cDNA Synthesis kit, FastStart DNA master SYBR Green I were purchased from Roche (USA). Primers for E-cadherin, TGF-β1, vimentin and β-actin were purchased from First-base (Singapore), Human TGF-β1 ELISA kit and Coomassie Plus (Bradford) Assay Kit were purchased from invitrogen (USA).
Cell culture
MCF-7 human breast carcinoma cell lines, was kindly provided by Makmal Terpadu Laboratory, Faculty of Medicine, Universitas Indonesia. The cells were maintained at 37 oC in a humidified atmosphere of 5% CO2 in DMEM-high glucose supplemented with 10% fetal bovine serum (FBS), 10 U/ml penicillin, 100 µg/ml streptomycin, 2.5 µg/ml fungizone. 10,000 cells were seeded into triplicate wells of 6-well plates. Passages were done every seven days following tripsinization and counting. Afterwards, 10,000 cells were re-plated in complete medium for 72 hours prior to the addition of drugs. Stocks solutions of curcumin were prepared in dimethyl sulfoxide and used at a final concentration 8.5 µM and 17 µM. Final concentrations of DMSO used in the experiments were not exceeding 0.01%. All the following experiments were carried out in four groups: 1) control (DMSO 0.001%), 2) endoxifen 1,000 nM+β-estradiol 1 nM (positive control), 3) endoxifen 1,000 nM+β-estradiol 1 nM+curcumin 8.5 μM, 4) endoxifen 1,000 nM+β-estradiol 1 nM+curcumin 17 μM. Treatments were performed 3 times a week for 8 weeks.
Cell viability assay
Cell viability assay was performed by using trypan blue dye exclusion assay. The number of viable cells were counted by trypan blue dye exclusion by using an automated cell counter LunaTM (Logosbio, Korea). The results were expressed as percentage relative to the control cells.
Quantitative realtime PCR analysis
Total cellular RNA was extracted from cell pellets using the High Pure RNA isolation kit (Roche, USA) according to the manufacturer’s protocol, and 1 µg (in 20 µl) converted to cDNA using a Transcriptor First Strand cDNA Synthesis kit (Roche, USA). Quantitative realtime PCR was performed on 100 ng cDNA using a FastStart DNA master SYBR Green I kit (Roche, USA), according to manufacturer’s protocol with β-actin as housekeeping gene. PCR primers used in this study were designed as described, summarized in Table 1, and synthesized in Integrated DNA Technologies (Singapore). A total of 20 µl reactions were performed in a 32-well plate on an qRT-PCR Light Cycler Nano RocheTM by incubation at 95 oC for 10 min, followed by 45 cycles of 95 oC for 20 s and annealing temperature of 55-62 oC for 1 min. The raw quantification cycle (Cq) values were analysed by relative quantification using Livak method to determine normalized expression ratios of target genes.
Table 1.
List of Primers Used in This Study
| Target Gene | Primer sequence | Product size (bp) | Annealing temperature (0C) |
|---|---|---|---|
| E-cadherin | Fwd: 5’-CAG CAC GTA CAC AGC CCT AA-3’ | 227 | 55 |
| Rev: 5’-CCC CGT GTG TTA GTT CTG CT-3’ | |||
| Vimentin | Fwd: 5’-GAC AAT GCG TCT CTG GCA CGT CTT-3’ | 236 | 60 |
| Rev: 5’-TCC TCC GCC TCC TGC AGG TTC TT-3’ | |||
| TGF-β1 | Fwd: 5’-TGA ACC GGC CTT TCC TGC TTC TCA TG-3’ | 152 | 62 |
| Rev: 5’-GCG GAA GTC AAT GTA CAG CTG CCG C-3’ | |||
| β-actin | Fwd: 5’-GCT GGA AGG TGG ACA GCG A-3’ | 613 | 55 |
| Rev: 5’-GGC ATC GTG ATG GAC TCC G-3’ |
Transmission Electron Microscopy
Confluent cultured breast cancer cells were scraped from wellplate. Samples rinsed once with cold PBS, fixed with 2.5% glutaraldehyde, followed by embedding in Spurr’s resin. Adherens junctions were observed with a JEOL JEM-1010 transmission electron microscope using an acceleration voltage of 80.0 kV.
Confocal Microscopy
Coverslip was coated using a poly-L-lysine. Coverslips were placed in each well of a six well cell culture plate. Cells (104) were seeded into each well of a 6-well plate. After 72-hour incubation to allow cells to adhere, cells were treated with endoxifen+β-estradiol with or without curcumin or DMSO every 48 hours until the 60% of cell confluency. Coverslips were washed and mounted on glass slide. All images were captured on Olympus Fluoview FV1,200 Confocal Laser Scanning Microscope (Olympus, Japan) using a 40× lens. Images were captured using FV10-ASW ver 4.1 and Cellsens ver 1.14 imaging software (Olympus, Japan).
TGF-β1 concentrations in medium culture
Cell supernatants were harvested after 8-weeks of treatments, followed by centrifugation at 1,000 × g for 10 min. TGFβ1 concentrations in MCF-7 cell culture supernatants were determined with sandwich ELISA using a human TGF-β1 ELISA kit (Invitrogen, USA) according to manufacturer’s guidelines. The protein levels were calibrated on a microplate reader at 450 nm. All standards, controls and samples were run in duplicate. TGF-β1 concentration was calculated in mg protein concentration. Protein concentration was performed using Coomassie Plus (Bradford) Assay Kit.
Assay of Cellular Reactive Oxygen Species
Dichlorofluorescein (DCF)-sensitive cellular ROS in MCF-7 cells on week 8 were measured by a fluorimetric assay with dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay. In brief, 5,000 cells in 96-well plates were incubated with DMSO, endoxifen+β-estradiol with or without curcumin (8.5; 17 μM) for seven days. Then, the cells were incubated with 25 mM carboxy-H2DCFDA for 30 min at 37 oC and measured at 485 nm excitation and 535 nm emissions.
Statistical Analysis
All data were presented as mean ± standard error (SEM). Statistical analyses were performed with one-way ANOVA or Kruskal-Wallis followed by Tukey’s multiple comparison method or Mann-Whitney. Differences were considered statistically significant at p<0.05. All of the statistical analysis and graphs presented were calculated and drawn using GraphPad software (GraphPad Prism v.7.0, USA).
Results
Figure 1 showed that in the positive control group, cells sensitivity started to decrease at week 2 to week 8. While, in the group End + BE + cur 8.5 cells viability decreased in the first week, began to rise in week 3 to weeks 5 and were higher than the positive control group at week 8. Whereas in the group End + BE + cur 17 decreased cell viability in the first week, began to rise in week 3 and viability settled until week 8 compared to the positive control group.
Figure 1.
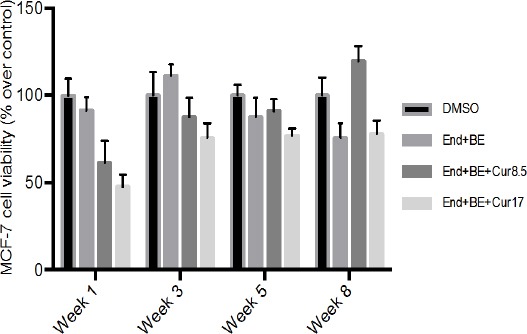
Effect of Endoxifen with or without Curcumin on MCF-7 Cells Viability. The number of viable cells were counted using trypan blue dye exclusion method in an automated cell counter. Cell viability are expressed as percentage relative to control (cells exposed to DMSO). Results are presented as mean of three experiments. Drug treatments were: control (DMSO); End+BE: Endoxifen 1000 nM+ β-estradiol 1 nM; End+BE+Cur8.5: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 8.5 μM; End+BE+Cur17: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 17 μM.
In the group of DMSO appeared MCF-7 cells still retain morphological cobblestone appearance. While the positive control group, curcumin (8.5 and 17 μM) exhibited cells with elongated and slim size like fibroblasts.
Observations via confocal microscope showed curcumin accumulates in the cytoplasm of the cell. Increased levels of curcumin was linear with the number in the cell, it showed from the more curcumin in the cell in the group End+BE+cur 17 compared End+BE+cur 8.5. Curcumin in the cell could be observed because they have green fluorescence (Figure 2, indicated by black arrow).
Figure 2.
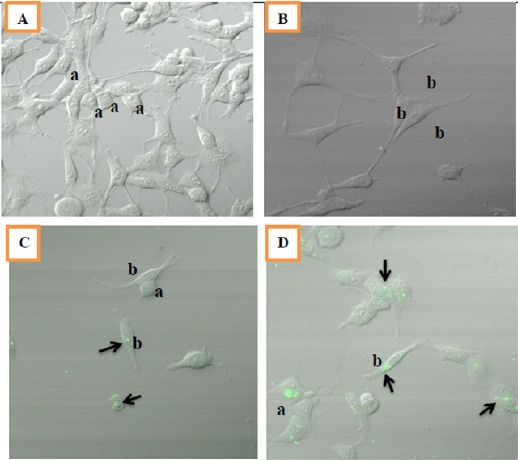
Morphology of MCF-7 Cells after 8 Weeks of Treatments. Cells were seeded at density of 104 viable cells/ml per well. Observations were done using confocal fluorescent microscope at 40x magnification. A: control (DMSO), B: End+BE, C: End+BE+Cur 8.5, D: End+BE+Cur17. Green fluorescence indicated the location of curcumin in a cell (black arrow); a: cobblestone appearance; b: fibroblast-like appearance
We observed adherens junction like-structures after 8-weeks of drug treatment in Figure 3. The control group seem that adherens junction was intact, cell stick together properly. While, in group endoxifen with/without curcumin seem that junction disrupt and distance between cells was clearly founded.
Figure 3.
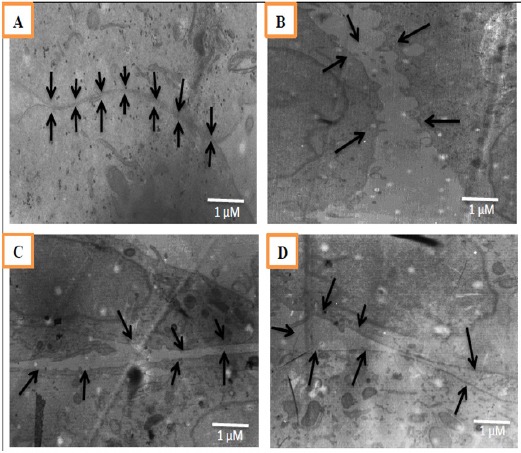
Adherens Junction-Like Structures Observed between Adjacent cells of MCF-7 Cells after 8-Weeks of Treatment. Observations were done using Transmission Electron Microscope at 8000x magnification. The black arrows in figures showed the junctions and gaps between two cells. A: control (DMSO), B: End+BE, C: End+BE+Cur 8.5, D: End+BE+Cur 17.
Figure 4 showed that the addition of curcumin led to the expression of E-cadherin mRNA lower than DMSO at week 8. The addition of curcumin did not seem able to increase the expression of E-cadherin mRNA compared to a positive control.
Figure 4.
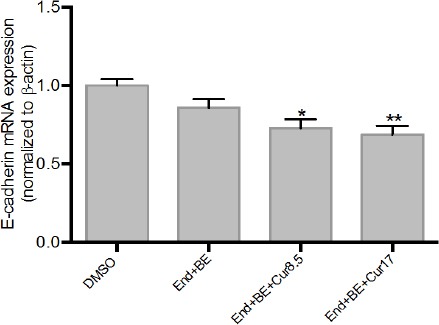
Effects of Curcumin on E-Cadherin mRNA Expressions in Endoxifen-Induced MCF-7 Cells for 8 Weeks. Data were normalized to β-actin as reference gene. The results are expressed as the mean ± SD of the four experiments in three trials. DMSO (control); End+BE: Endoxifen 1000 nM+ β-estradiol 1nM; End+BE+Cur 8.5: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 8.5 μM; End+BE+Cur 17: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 17 μM. *p<0.05 or **p<0.01 versus control.
Vimentin mRNA expression was increased significantly in all groups compared to DMSO after 8-week drug treatment. The addition of 8.5 μM curcumin looked better in reducing the expression of vimentin mRNA compared with curcumin 17 μM (Figure 5).
Figure 5.
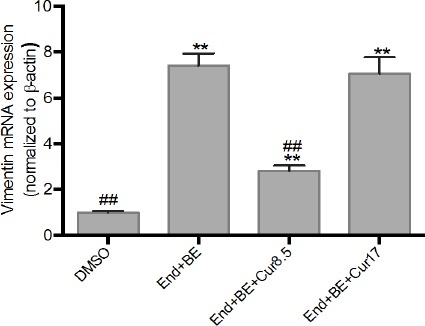
Effects of Curcumin on Vimentin mRNA Expressions in MCF-7-Induced Endoxifen for 8 Weeks. Data were normalized to β-actin as reference gene. The results are expressed as the mean ± SD of the four experiments in three trials. DMSO (control); End+BE: Endoxifen 1000 nM+ β-estradiol 1nM; End+BE+Cur8.5: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 8.5 μM; End+BE+Cur17: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 17 μM. **p<0.01 versus control, ##p<0.01 versus End+BE.
Compared to DMSO, TGF-β1 mRNA expressions were increased significantly in all groups. The addition of curcumin (8.5 and 17 μM) did not able to decrease expression of TGF-β1 mRNA significantly compared to the positive control group (Figure 6).
Figure 6.
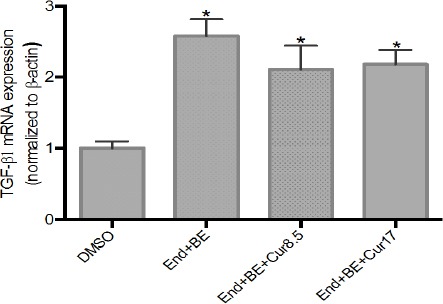
Effects of Curcumin on TGF-β1 mRNA Expressions in MCF-7-Induced Endoxifen for 8 Weeks. Data were normalized to β-actin as reference gene. The results are expressed as the mean ± SD of the four experiments in three trials. DMSO (control); End+BE: Endoxifen 1000 nM+ β-estradiol 1nM; End+BE+Cur8.5: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 8.5 μM; End+BE+Cur17: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 17 μM. *p<0.05 versus control.
Figure 7 showed that there were increment of TGF-β1 concentration in the positive control group and End+BE+cur 8.5 μM compared to DMSO. While the opposite result was found in curcumin 17 μM. The addition of curcumin (8.5 and 17 μM) able to decrease TGF-β1 concentration significantly compared to the positive control group.
Figure 7.
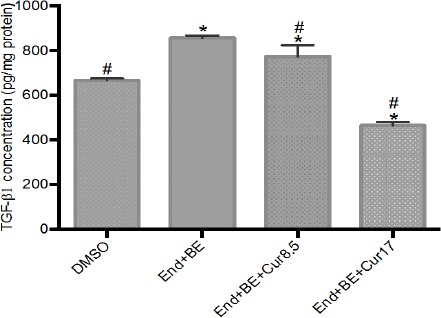
TGF-β1 Concentration in Cell Culture Medium after 8 Weeks of Treatments. The results are expressed as the mean ± SD of the four experiments in three trials. DMSO (control); End+BE: Endoxifen 1000 nM+ β-estradiol 1 nM; End+BE+Cur8.5: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 8.5 μM; End+BE+Cur17: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 17 μM. *p<0.05 versus control; #p<0.05 versus End+BE.
In the group that received 8.5 μM curcumin seemed to decrease ROS levels compared to DMSO group and positive control group. While the increase in ROS levels seen in the group that received curcumin 17 μM (Figure 8).
Figure 8.
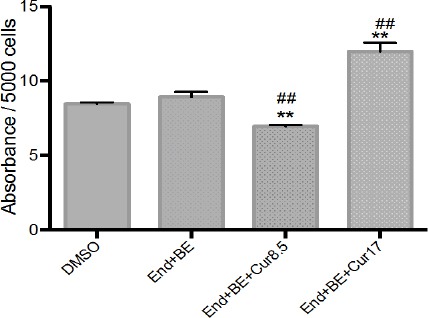
Dichlorofluorescein (DCF)-sensitive cellular reactive oxygen species (ROS) on MCF-7 cell treated with endoxifen in the presence and absence of curcumin. The results are expressed as the mean ± SD of the four experiments in three trials. DMSO (control); End+BE: Endoxifen 1000 nM+ β-estradiol 1 nM; End+BE+Cur8.5: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 8.5 μM; End+BE+Cur17: Endoxifen 1000 nM+ β-estradiol 1 nM+ curcumin 17 μM. *p<0.05 or **p<0.01 versus control; #p<0.05 or ##p<0.01 versus End+BE.
Discussion
EMT process is characterized by the loss of apical-basal polarity and cells adhesion such as E-cadherin in adherens junctions, occludin and claudin on tight junction, desmoplakin in desmosomes. Moreover during EMT occurs decline in cytokeratin, vimentin increment, reorganization of the cytoskeleton that cause the cells have a spindle-like morphology, and resistant to apoptosis (Crook et al., 2009). This study aimed to assess the potency of curcumin as an inhibitor of EMT activation in MCF-7 cells chronically exposed to endoxifen, were curcumin is given simultaneously since the first day of treatment. We chose curcumin due to the fact that prior study proved that curcumin was able to decrease the expression of vimentin, TGF-β1 and increase the expression of E-cadherin in cells that have undergone EMT in various cell lines (Chen et al., 2013; Li et al., 2013; Hu et al., 2010).
Our study used breast cancer cells chronically treated with endoxifen. Previous study by JR Hawse et al., 2010, showed that the exposure of endoxifen for 15 months activated EMT in MCF-7 cells. In the present study, endoxifen was used to trigger an increase in cell viability through EMT, while β-estradiol was given as to resemble estrogen levels in breast cancer patients (Folkerd et al., 2014). Previous studies had shown that estradiol also induced EMT, particularly in cells with the loss of ER- expressions (Bouris et al., 2016). Since prior studies on endoxifen-induced EMT were limited, we have conducted a study that showed that in MCF-7 cells treated with endoxifen, the fold increase of vimentin expressions are in greater magnitude than beta estradiol, and the highest increase was in the cells treated with endoxifen and beta estradiol, and the highest increase was in the cells treated with endoxifen and estradiol (Paramita et al., 2016). Therefore, we thought that estradiol might exaggerate the endoxifen-induced EMT induction.
The results of cell viability analysis showed that the group that received endoxifen and β-estradiol indicates that endoxifen lost its activity in suppressing cell growth as early as one week of treatment, while concomitant treatment with curcumin tend to decrease cell viability on the first week. However, unlike at the higher dose, the lower dose of curcumin started to decrease in sensitivity from the third week and continued to decline until the 8th week. To the best of our knowledge, there were no previous study has been done to show the effect of curcumin on cell viability in endoxifen-induced breast cancer cells. However, numerous studies have shown that curcumin alone is a potent anticancer due to its numerous molecular targets in breast cancer cells (Huang et al., 2013). Shao et al., (2002), reported that curcumin was able to suppress the effects of cell growth caused by estrogen and able to reduce the expression of ERα. However, these two studies done in just a short time (up to 72 hours of drug treatment).
Studies had shown that EMT is related to cancer cell progression. The failure of curcumin in decreasing cell viability in endoxifen-induced cells might be in part due to the its inability to suppress markers of EMT process induced by endoxifen and estradiol.
E-cadherin is an important transmembrane adhesion molecules, which binds to the cytoskeleton through catenin and influence the properties of adhesion, motility and morphology of cells. Some studies show that losing E-cadherin contributes to the increasing cell invasion and metastasis in carcinoma (Lu et al., 2012). Maeda et al., (2005), observed that cadherin turnover is more meaningful to increase cell motility compared to morphological changes in EMT.
Our results showed that long exposure of endoxifen and estradiol did not affect E-cadherin mRNA expressions. The addition of curcumin were also have no effect on E-cadherin expressions. These results are in contrast to studies conducted by Huang et al., (2013). That study suggested that 24-hour of curcumin treatment can increase E-cadherin mRNA expression in MCF-7 cells, by inhibiting the Snail in breast cancer cells induced by lipopolysaccharides. Another study stated that curcumin can restore E-cadherin by inhibiting β-catenin translocation into nucleus in breast cancer stem cells (Mukkherjee et al., 2014). The contrast in our results mostly is due to the difference in the period of treatment.
Vimentin is an intermediate filament protein that is only expressed by mesenchymal cells, while in the epithelial cells expressed cytokeratin. During EMT, cytokeratin is replaced by vimentin. Therefore, a lot of research on the EMT use vimentin as a mesenchymal marker (Mendez et al., 2010; Micallizzi et al. 2010). In the present study, lower dose of curcumin, not the higher dose was shown to suppress the mRNA expression of vimentin. This is likely to be caused by the relatively high dose of curcumin, which might be linked to the increase of ROS production.
TGF-β acts as a major inducer of the EMT through Smad dependent and Smad independent signaling pathways. Our study showed endoxifen exposure for 8 weeks activated EMT, characterized by the increase in TGF-β1 mRNA expressions. The addition of curcumin tend to decrease in TGF-β1 mRNA expressions when compared to endoxifen-estradiol only treatment. Other study by Li et al. (2013), curcumin given for 12 hours in renal tubular epithelial cells curcumin is able to inhibit EMT induced by TGF-β1 through PPAR-γ pathway.
Curcumin has been known as an antioxidant, but may also act as a prooxidant. Bhaumik et al., 1999 reported that at concentrations of over 25 μM, curcumin acts as prooxidant and produce ROS that contribute to apoptosis. Another study conducted by Palanikumar et al., (2011), showed curcumin at a concentration of 20 µg/mL has antimutagenic property, while concentrations above 30 µg/mL have clastogenic property. This is similar to our results in which lower dose of curcumin was shown to reduce ROS levels, while higher dose seemed to act as prooxidant.
The link between ROS with EMT was supported by Kristic et al., (2015), which showed that ROS can increase synthesis of mRNA TGF-β and activation of latent TGF-β complex. TGF-β binds to its receptor and carry out biological functions so it is assumed that the more ROS produced more TGF-β is bound to the receptor. Therefore, there were reduced levels of TGF-β released in culture medium.
In theory, EMT activation will alter epithelial cuboidal shape of cells which have good contact between cells into elongated, scattered mesenchymal cells with cell-cell contact disruption. In the present study, morphological observation after eight week showed that in the endoxifen-induced, breast cancer cells change shape toward the mesenchymal cells. The duration of exposure drugs given have clearly played a significant role in activating the EMT. While the cells treated with curcumin also, the cell shape still maintained mesenchymal cells.
Adherens junction observation using TEM showed that all in endoxifen-treated groups, with or without curcumin, showed disruption of adherens-like structure, where there is a gap between the two cells. This suggests that exposure endoxifen for 8 weeks can lead to EMT marked by the destruction of adherens junctions between cells. The addition of curcumin were not able to fix disruption of adherens junctions.
In conclusion, the results of this study showed curcumin reduces breast cancer cell viability in long-term use but does not prevent the activation of EMT in cancer cells chronically treated with endoxifen.
Statement confict of interest
The authors declare no conflict of interest.
References
- 1.Al-Saleh S, Luqmani YA. Endocrine resistance and epithelial mesenchymal transition in breast cancer. In 'breast cancer-focusing tumor microenvironment, stem cells and metastasis. InTech. 2011:451–86. [Google Scholar]
- 2.Bhaumik S, Anjum R, Rangaraj N, et al. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–4. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- 3.Bouris P, Skandalis SS, Piperigkou Z, et al. Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol. 2016;43:42–60. doi: 10.1016/j.matbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Chen WC, Lai YA, Ling YC, et al. Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of TGF-βand PI3K/AKT signaling in triple-negative breast cancer cells. J Agric Food Chem. 2013;6:11817–24. doi: 10.1021/jf404092f. [DOI] [PubMed] [Google Scholar]
- 5.Crook ET, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:1–10. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkerd EJ, Lønning PE, Dowsett M. Interpreting plasma estrogen levels in breast cancer:caution needed. J Clin Oncol. 2014;32:1396–40. doi: 10.1200/JCO.2013.53.9411. [DOI] [PubMed] [Google Scholar]
- 7.Hawse JR, Subramaniam M, Wu X, et al. Abstract pd05-11:development, characterization, and effective in vitro treatment of an endoxifen resistant breast cancer cell line. Cancer Res. 2010;70:PD05–11. [Google Scholar]
- 8.Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer:an overview. Cancers. 2014;6:1769–92. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, Liang H, Du Y, et al. Curcumin inhibits transforming growth factor-beta activity via inhibition of Smad signaling in HK-2 cells. Am J Nephrol. 2010;31:332–41. doi: 10.1159/000287230. [DOI] [PubMed] [Google Scholar]
- 10.Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT through downregulation on NF-kB-snail signaling in breast cancer cells. Oncol Rep. 2013;29:117–24. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basic of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–28. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krstic J, Trivanovic D, Mojsilovic, et al. Transforming growth factor-beta and oxidative stress interplay:implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:1–15. doi: 10.1155/2015/654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Wang Y, Liu Y, et al. Curcumin inhibit transforming growth factor-β1-induced EMT via PPARγpathway, not Smad pathway in renal tubular epithelial cell. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0058848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Zhou D, Jiang X, et al. Loss of E-cadherin in multidrug resistant breast cancer cell line MCF-7/Adr:possible implication in the enhanced invasive ability. Eur Rev Med Pharmacol Sci. 2012;6:1271–9. [PubMed] [Google Scholar]
- 15.Lymperatou D, Giannopoulou E, Koutras AK, et al. The exposure of breast cancer cells to fulvestrant and tamoxifen modulates cell migration differently. Bio Med Res Int. 2013;2013:1–14. doi: 10.1155/2013/147514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching:essential for behavioral but not morphological changes during epithelial to mesenchymal transition. J Cell Sci. 2005;181:873–87. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 17.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–51. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer:parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–34. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukkherjee S, Mazumdar M, Chakraborty S, et al. Curcumin inhibits breast cancer stem cell migration by amplifying the E-cadherin/β-catenin negative feedback loop. Stem Cell Res Ther. 2014;5:116. doi: 10.1186/scrt506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palanikumar L, Ragunathan I, Panneerselvam N. Chromosome aberrations induced by curcumin and aloin in Allium cepa L. root meristem cells. Turk J Biol. 2011;35:145–52. [Google Scholar]
- 21.Paramita Louisa M, Nafrialdi Increased vimentin mRNA expression in MCF-7 breast cancer cell line after repeated endoxifen-treatment. Med J Indones. 2016;25:207–13. [Google Scholar]
- 22.Pasquier J, Kaoud NA, Thani HA, et al. Epithelial to mesenchymal transition in a clinical perspective. J Oncol. 2015;2015:1–10. doi: 10.1155/2015/792182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao ZM, Shen ZZ, Liu CH, et al. Curcumin exert multiple suppresive effects on human breast carcinoma cells. Int J Cancer. 2002;98:234–40. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 24.Viedma-Rodríguez R, Baiza-Gutman L, Salamanca-Gómez F, et al. Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review) Oncol Rep. 2014;32:3–15. doi: 10.3892/or.2014.3190. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Hawse JR, Subramaniam M, et al. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor αfor degradation in breast cancer cells. Cancer Res. 2009;69:1722–7. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 26.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations:a future nanomedicine for cancer. Drug Discov Today. 2012;17:71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


