Abstract
Background:
Caspases proteins are protease enzymes involved in the initiation and execution of apoptosis process. Regulation of apoptosis process plays an important role in the normal biological events and development. In addition to developmental abnormalities, dysregulated apoptosis system may lead to tumorigenesis, autoimmunity, and other serious health problems. Aberrant regulation of apoptosis may also be the paramount cause of chemoresistance during cancer therapy. It is aimed through this study to evaluate the transcript levels of Caspase 3, 8, and 9 in tumoral tissues from patients with colorectal cancer (CRC) and compare it with normal marginal tissues.
Methods:
Fifty tumor tissues and their matched marginal tissues, as control group, were obtained from CRC patients. Total mRNA of all tissue samples was extracted and cDNA was synthesized. Using SYBR Green PCR master mix and Real-time gene expression technique, the transcript level of target genes was quantified.
Results:
Experiments indicated that mRNA expressions of caspase 9 and 3 were downregulated in tumoral tissues from CRC patients in comparison to marginal tissues. In contrast, tumoral tissues expressed mRNA of caspase 8 higher than normal marginal tissues. Modified transcript levels of caspase 3, 8, and 9 were correlated with the clinical manifestations of the patients.
Conclusions:
Alteration in the mRNA level of caspase genes may be involved in the development of CRC.
Keywords: Colorectal cancer, caspase, gene expression, mRNA level
Introduction
Colorectal cancer (CRC) is one of the most prevalent and lethal cancers throughout the world. Similar to other cancers, it appears that both environmental factors such as obesity, physical activity, smoking and inflammation, and genetics play important roles in initiation and development of CRC. Alongside with environmental contributing factors, genetic variations also participate in CRC development, therefore approximately 10% of CRC cases originate from various hereditary impairments (Eberhart et al., 1994; Suzuki et al., 2004; Lanza et al., 2007; Haggar and Boushey, 2009; Asadi et al., 2017).
Apoptosis is one of the most important players, which is involved in etiopathology of several malignancies and most therapeutic ways of cancer like chemotherapy and radiotherapy are trying to induce apoptosis in cancer cells in order to kill them (Herr and Debatin, 2001; Johnstone et al., 2002). There are two pathways of apoptosis in cells, namely intrinsic and extrinsic pathways (Walczak and Krammer, 2000). Caspase 8 is an important molecule in extrinsic pathway. Activation of caspase 8, which is induced by external signals, leads to apoptosis (Eberhart et al., 1994; Lanza et al., 2007). However in intrinsic pathway, releasing of cytochrome c from mitochondria, causes cell death via activation of caspase 9 and some other apoptosis mediators (Suzuki et al., 2004). Both apoptotic pathways are merged in one point at the end in an apoptotic cell and both caspase 9 and caspase 8 activate caspase 3 at the end (Kumar, 1997; Sternberg et al., 1999; Zheng et al., 2003). In this point, cleavage of inactive caspase 3 is occurred by other apoptosis mediators, resulting in activation of caspase 3, which is a critical event in apoptosis initiation (Hsia et al., 2003; Kim et al., 2011).
Previous studies have shown that caspase 3, 8 and 9 expression levels are useful prognostic factors in digestive system related cancers, especially in CRC (de Heer et al., 2007; de Oca et al., 2008; Koelink et al., 2009; Sträter et al., 2010; Kim et al., 2011; Hector et al., 2012; Noble et al., 2013). However, most of previous studies have evaluated these genes separately and did not reach a clear conclusion. In this study, we try to fill this gap by evaluation of caspase 3, 8 and 9 expression levels together in order to better identify the caspase family’s role in CRC.
Materials and Methods
Sampling
This study was designed and performed according to the institutional bioethical guidelines by the Ethical Committee of Tabriz University of medical sciences. Fifty tumor tissues and their matched marginal tissues as control group were gathered from patients referred to Imam Reza Hospital of Tabriz University of Medical Sciences during surgery. Written informed consent was obtained from all the patients. Clinical and pathological characteristics of patients are summarized in table 1. All tissue samples were immediately transferred to RNAase inhibitor solation (Qiagen, Cat No. 76104) and stored in -80 ºC till RNA extraction procedures.
Table 1.
Clinicopathological Data of the CRC Patients
| Feature | Grouping | Score |
|---|---|---|
| Sex | Male | 32 |
| Female | 18 | |
| Age | >60 | 26 |
| <60 | 24 | |
| Distant metastasis | pM0 | 42 |
| pM1 | 8 | |
| Tumor stage | 1 | 4 |
| 2 | 18 | |
| 3 | 22 | |
| 4 | 6 | |
| smoking | Yes | 31 |
| No | 19 | |
| Tumor location | Rectum | 13 |
| Right colon | 21 | |
| Left colon | 16 | |
| Differentiation | Poor | 11 |
| Moderate | 26 | |
| Well | 13 |
RNA isolation and cDNA synthesis
Tripure isolation reagent (Roche, Cat No.11667165001) was used in order to isolate total RNA from tissue samples, considering the company’s manual. To determine the quality and quantity of the extracted RNAs, optical density (OD) of all the samples were measured by Nanodrop device. Afterwards, complementary DNA (cDNA) synthesis was done by TAKARA cDNA syntheses kit (TAKARA Cat No. 6130) according to the manufacture’s protocol.
Quantitative real time PCR
Quantitative analysis was carried out by StepOne Plus Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Relative quantification of mRNA expression level of caspase 3, 8, and 9 from tumoral and marginal tissue samples was performed using gene-specific primers and SYBR Green master mix (TAKARA Cat No. RR820W). Expression level of GAPDH (housekeeping gene) was used in order to normalize expression level of target genes. Primers (Table 2) were designed using Oligo 7 software, and for spasticity and accuracy all primers were blasted in NCBI website. Average score of duplicated Ct values was measured for each sample and comparative Ct method was used to determine the relative expression level of target genes (Pfaffl, 2001).
Table 2.
Primer Sequence and Characteristics Used in the Quantification of the Target Genes
| Gene | Forward primer | Annealing temp (°C) |
|---|---|---|
| Caspase 3-Forward | 5'-ATGGTTTGAGCCTGAGCAGA-3' | 59.5 |
| Caspase 3-Reverse | 5'-GGCAGCATCATCCACACATAC-3' | 58.5 |
| Caspase 8- Forward | 5'-ACCTTGTGTCTGAGCTGGTCT-3' | 59.5 |
| Caspase 8- Reverse | 5'-GCCCACTGGTATTCCTCAGGC-3' | 59 |
| Caspase 9- Forward | 5'-GCAGGCTCTGGATCTCGGC-3' | 60.5 |
| Caspase 9- Reverse | 5'-GCTGCTTGCCTGTTAGTTCGC-3' | 59.5 |
| GAPDH- Forward | 5'-CAAGATCATCAGCAATGCCTCC-3' | 59 |
| GAPDH- Reverse | 5'-GCCATCACGCCACAGTTTCC-3' | 59 |
Statistical analysis
Statistical analysis was performed using the Graph Pad Prism 6 (Graph Pad Software Inc. San Diego, CA, USA). Kolmogorov-Smirnov’s normality test was applied for evaluating the normal distribution of data. Independent sample t-test was conducted to compare target gene expression level between CRC tissues and their paired marginal tissues. Cross tab (Eta) analysis was conducted to evaluate relationship between clinical features of the patients with relative mRNA expression of caspase genes. All results were expressed as mean ± standard deviation (SD). Statistical significance level for all P value was less than 0.05.
Results
Our experiments confirmed revealed downregulation of mRNA expression level of caspase 3 in tumoral samples in comparison to normal marginal colon tissues (Fold change = 0.32, P = 0.021; Figure 1). Tumor stage (Eta = 0.453, P = 0.037) and differentiation (Eta = 0.621, P = 0.038) were the clinical manifestations of the CRC patients with statistically significant relation with the transcript level of caspase 3. Table 3 summarizes the relation between caspase 3 mRNA expression level and clinicopathological manifestations of the patients.
Figure 1.
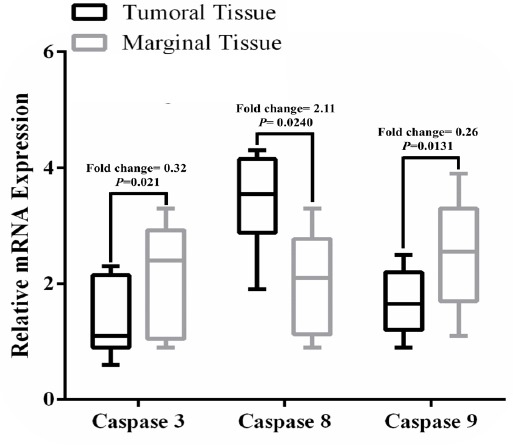
Box and Whisker Plot Demonstrates the Relative mRNA Expression Levels of Caspase 3, 8, and 9 in Tumoral Tissues of the CRC Patients in Comparison to Healthy Marginal Tissues.
Table 3.
Association of Demographic and Clinical Characteristics of the Studied CRC Population with the Transcript Levels of Caspase 3, 8, and 9 (Data are shown as P values).
| Characteristic | Caspase 3 | Caspase 8 | Caspase 9 | |||
|---|---|---|---|---|---|---|
| Eta | P value | Eta | P value | Eta | P value | |
| Sex | 0.211 | 0.397 | 0.081 | 0.451 | 0.32 | 0.351 |
| Age | 0.321 | 0.346 | 0.225 | 0.371 | 0.287 | 0.246 |
| Smoking | 0.021 | 0.764 | 0.201 | 0.453 | 0.168 | 0.841 |
| Tumor stage | 0.453 | 0.037 | 0.01 | 0.121 | 0.461 | 0.041 |
| Distant metastasis | 0.354 | 0.078 | 0.551 | 0.043 | 0.601 | 0.034 |
| Tumor location | 0.178 | 0.341 | 0.022 | 0.578 | 0.208 | 0.436 |
| Differentiation | 0.621 | 0.038 | 0.538 | 0.037 | 0.722 | 0.021 |
*, Values in bold demonstrate significant p values.
On the other hand, it was found that mRNA expression level of caspase 8 was upregulated significantly in tumoral samples compared with normal marginal colon tissues (Fold change = 2.11, P = 0.0240; Figure 1). Lack of expression of caspase 8 mRNA was observed in just one sample. Considering the clinical data, caspase 8 expression level was associated with distant metastases (Eta = 0.551, P = 0.043) and differentiation stage (Eta = 0.538, P = 0.037) of the CRC patients (Table 3).
It was observed that caspase 9 expression level was downregulated significantly (Fold change = 0.26, P = 0.0131; Figure 1) in comparison to normal marginal colon tissues. However, in 4 samples we did not identify expression of caspase 9. Expression level of caspase 9 was associated significantly with some of the clinicopathological features of the patients, including tumor stage (Eta = 0.461, P = 0.041), distant metastases (Eta = 0.601, P = 0.034), and differentiation stage (Eta = 0.722, P = 0.021). But no significant association was seen between expression level of caspase 9 and age, sex, smoking, tumor location, and differentiation (Table 3).
Discussion
Achieving approaches toward prevention and early diagnosis of CRC requires better understanding of genetic and molecular pathways involved in the disease etiopathogenesis (Bodmer et al., 1994). Caspase 3, 8 and 9 are important molecules in apoptotic pathways which play key roles in cancer development and progression (Fearnhead et al., 1998). Due to important functions of caspase proteins in cancers, we evaluated expression levels of them in CRC tissues and compared them with normal marginal tissues. It was also aimed to reveal if there was any relation between mRNA expression level of these molecules and clinical features of the patients.
Caspase 9 expression level was downmodulated in tumoral tissues compared with normal marginal colon samples, which had previously been shown by other studies (Fearnhead et al., 1998; Shen et al., 2010). Moreover, association of mRNA expression level of caspase 9 and clinical manifestations of CRC patients was found in this study, as it was in accordance with another study (Shen et al., 2010). A number of studies demonstrated that caspase 8 had upregulation in tumoral tissues of CRC patients (Heijink et al., 2007; Xu et al., 2008; Chen et al., 2015). In the present study, there was also an upregulation of caspase 8 mRNA expression level in CRC patients. This upregulation may be related to other roles of caspase 8, especially in cell migration and cell-cell interaction (Helfer et al., 2006; Finlay and Vuori, 2007; Heijink et al., 2007; Senft et al., 2007; Barbero et al., 2009). In the present study, caspase 3 expression level was downregulated in tumoral tissues of CRC patients. Previous studies have also indicated downregulation of this gene in CRC patients (de Oca et al., 2008; Koelink et al., 2009; Meyer et al., 2009; Guan et al., 2012).
In conclusion, modifications in molecular markers throughout the onset and progression of malignancies can underpin the designing of much effective therapeutics and diagnostic tools and may prevent cancer development in early diagnosed cases. These molecular signatures can then be applied as a target of novel therapeutic strategies. In the ongoing investigation, we observed an aberrant mRNA expression level of caspase 3, 8, and 9 in tumoral tissues of CRC patients in relation to normal marginal tissues. Furthermore, the altered expression level of these genes was related to some of clinicopathological specifications of the patients. It is essential to perform further studies to confirm caspase molecules as therapeutic or diagnostic tool in CRC patients.
Disclosure of conflict of interests
None.
Acknowledgements
We are deeply thankful of our patients for their contribution. This study was supported by a grant from Tabriz University of Medical Sciences.
References
- 1.Asadi M, Shanaehbandi D, Zarintan A, et al. TP53 gene Pro72Arg (rs1042522) single nucleotide polymorphism as not a risk factor for colorectal cancer in the Iranian Azari Population. Asian Pac J Cancer Prev. 2017;18:3423–7. doi: 10.22034/APJCP.2017.18.12.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbero S, Mielgo A, Torres V, et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755–63. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodmer W, Bishop T, Karran P. Genetic steps in colorectal cancer. Nat Genet. 1994;6:217–9. doi: 10.1038/ng0394-217. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Yang X, Feng Z, et al. Prognostic value of Caspase-3 expression in cancers of digestive tract:a meta-analysis and systematic review. Int J Clin Exp Med. 2015;8:10225. [PMC free article] [PubMed] [Google Scholar]
- 5.de Heer P, de Bruin EC, Klein-Kranenbarg E, et al. Caspase-3 activity predicts local recurrence in rectal cancer. Clin Cancer Res. 2007;13:5810–5. doi: 10.1158/1078-0432.CCR-07-0343. [DOI] [PubMed] [Google Scholar]
- 6.de Oca J, Azuara D, Sanchez-Santos R, et al. Caspase-3 activity, response to chemotherapy and clinical outcome in patients with colon cancer. Int J Colorectal Dis. 2008;23:21–7. doi: 10.1007/s00384-007-0362-3. [DOI] [PubMed] [Google Scholar]
- 7.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology-Orlando. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 8.Fearnhead HO, Rodriguez J, Govek E-E, et al. Oncogene-dependent apoptosis is mediated by caspase-9. Proc Natl Acad Sci U S A. 1998;95:13664–9. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay D, Vuori K. Novel noncatalytic role for caspase-8 in promoting SRC-mediated adhesion and Erk signaling in neuroblastoma cells. Cancer Res. 2007;67:11704–11. doi: 10.1158/0008-5472.CAN-07-1906. [DOI] [PubMed] [Google Scholar]
- 10.Guan J, Zhao R, Zhang X, et al. Chicken skin mucosa surrounding adult colorectal adenomas is a risk factor for carcinogenesis. Am J Clin Oncol. 2012;35:527–32. doi: 10.1097/COC.0b013e31821dedf7. [DOI] [PubMed] [Google Scholar]
- 11.Haggar FA, Boushey RP. Colorectal cancer epidemiology:incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hector S, Conlon S, Schmid J, et al. Apoptosome-dependent caspase activation proteins as prognostic markers in Stage II and III colorectal cancer. Br J Cancer. 2012;106:1499–505. doi: 10.1038/bjc.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heijink D, Kleibeuker J, Jalving M, et al. Independent induction of caspase-8 and cFLIP expression during colorectal carcinogenesis in sporadic and HNPCC adenomas and carcinomas. Cell Oncol. 2007;29:409–19. doi: 10.1155/2007/564605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helfer B, Boswell BC, Finlay D, et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006;66:4273–8. doi: 10.1158/0008-5472.CAN-05-4183. [DOI] [PubMed] [Google Scholar]
- 15.Herr I, Debatin K-M. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–14. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 16.Hsia J-Y, Chen C-Y, Chen J-T, et al. Prognostic significance of caspase-3 expression in primary resected esophageal squamous cell carcinoma. Eur J Surg Oncol. 2003;29:44–8. doi: 10.1053/ejso.2002.1338. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis:a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim MA, Lee HE, Lee HS, et al. Expression of apoptosis-related proteins and its clinical implication in surgically resected gastric carcinoma. Virchows Arch. 2011;459:503–10. doi: 10.1007/s00428-011-1150-6. [DOI] [PubMed] [Google Scholar]
- 19.Koelink P, Sier C, Hommes D, et al. Clinical significance of stromal apoptosis in colorectal cancer. Br J Cancer. 2009;101:765–73. doi: 10.1038/sj.bjc.6605220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S. The apoptotic cysteine protease CPP32. Int J Biochem Cell Biol. 1997;29:393–6. doi: 10.1016/s1357-2725(96)00146-x. [DOI] [PubMed] [Google Scholar]
- 21.Lanza G, Ferracin M, Gafà R, et al. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Mol Cancer. 2007;6:1. doi: 10.1186/1476-4598-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer A, Merkel S, Brückl W, et al. Cdc2 as prognostic marker in stage UICC II colon carcinomas. Eur J Cancer. 2009;45:1466–73. doi: 10.1016/j.ejca.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Noble P, Vyas M, Al-Attar A, et al. High levels of cleaved caspase-3 in colorectal tumour stroma predict good survival. Br J Cancer. 2013;108:2097–105. doi: 10.1038/bjc.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45–e. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senft J, Helfer B, Frisch SM. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res. 2007;67:11505–9. doi: 10.1158/0008-5472.CAN-07-5755. [DOI] [PubMed] [Google Scholar]
- 26.Shen XG, Wang C, Li Y, et al. Downregulation of caspase-9 is a frequent event in patients with stage II colorectal cancer and correlates with poor clinical outcome. Colorectal Dis. 2010;12:1213–8. doi: 10.1111/j.1463-1318.2009.02009.x. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg MJ, Bates PA, Kelley LA, et al. Progress in protein structure prediction:assessment of CASP3. Curr Opin Struct Biol. 1999;9:368–73. doi: 10.1016/S0959-440X(99)80050-5. [DOI] [PubMed] [Google Scholar]
- 28.Sträter J, Herter I, Merkel G, et al. Expression and prognostic significance of APAF-1, caspase-8 and caspase-9 in stage II/III colon carcinoma:Caspase-8 and caspase-9 is associated with poor prognosis. Int J Cancer. 2010;127:873–80. doi: 10.1002/ijc.25111. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Watkins DN, Jair K-W, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 30.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 31.Xu B, Zhou Z-G, Li Y, et al. Clinicopathological significance of caspase-8 and caspase-10 expression in rectal cancer. Oncology. 2008;74:229–36. doi: 10.1159/000151392. [DOI] [PubMed] [Google Scholar]
- 32.Zheng H-C, Sun J-M, Wei Z-L, et al. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol. 2003;9:1415–20. doi: 10.3748/wjg.v9.i7.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]


