Abstract
Objective:
Medical Safety Practice (MSP) is a safe procedure in medication process. It is important to investigate the use of MSP among childhood cancer patients because pediatric oncology is a high-risk area for potentially harmful adverse events. The purpose of this study is to determine the effects of the implementation of MSP in chemotherapy on the incidence of medication errors in childhood ALL patient at Dr. Sardjito Hospital, including in 1) transcribing, 2) administering, 3) monitoring, 4) the incidence of adverse drugs events. (ADEs).
Methods:
The study design is a quasi-experimental study with pre- and post-intervention without control. The sample consists of ALL patients who are taken care of at an academic hospital in Indonesia from 2012 to 2013. The sample was consecutively collected during the period of study. The data were collected through medical records, research form, observation, and discussion with the nurse. The intervention given is training and implementation of medical safety practice in chemotherapy.
Result:
Based on the analysis of the effect of the implementation of MSP (75 and 106 medical records of pre- and post-intervention), it is obtained: 1) the adherence of chemotherapy transcribing post-intervention increases significantly compared to pre-intervention (p<0.05), 2) the adherence of chemotherapy administering increases significantly in almost every aspect (p<0.05), except in preparing drugs by two different health worker, patient’s confirmation of ADEs management, and verification of drug’s expired date, 3) The adherence of chemotherapy monitoring improved significantly post-intervention (p<0.05), 4) Adverse Drug Events (ADE) decreased significantly post-intervention (p<0.05), from 52.1% to 30.5%.
Conclusion:
The implementation of MSP decreased the incidence of medication errors in ALL patients at Dr. Sardjito Hospital in ordering, dispensing, transcribing, administering, and monitoring chemotherapy. It also reduced the incidence of ADEs related to chemotherapy. Specific training for nurses are needed in order to improve the knowledge and skills, especially for medication error and skill in patients’ care.
Keywords: Children ALL-standard chemotherapy, medication errors
Introduction
The incidence of childhood cancer is about 250,000 annually, in which 200,000 of those cases are from developing countries (Kellie and Howard, 2008). The survival rate of Acute Lymphoblastic Leukemia (ALL) in developing countries is only around 25% (Wilimas and Ribeiro, 2001), compared to developed countries which are more than 80 % (Pui and Evans, 2006). According to a research by Supriyadi (2011), the most common cases of leukemia In the Special Region of Yogyakarta were ALL (L1 L2) of 68.9%, followed by AML (23.6%), CML (4.2%) and ALL (L3) (3.3%).
A study showed that the management of pediatric cancer has improved with the advancement of technology and communication and also adequate treatment. On the other hand, with adequate treatment, mortality rates in childhood cancer are still high due to treatment toxicity (Howard et al., 2008). Another study showed that the complex combinations and protocols of chemotherapy result in higher incidence of adverse events. The administration of chemotherapy in pediatric cancer patients should be clearly processed to minimize risks that may lead to adverse events (Branowicki et al., 2003; Leonard et al., 2006).
Two most common medication errors (ME) in pediatric chemotherapy were in the administering phase of medication delivery and in the drug-dispensing phase. Performance deficit, equipment and medication delivery devices, communication, knowledge deficit and written order errors were found to be the most common causes of medication errors (Rinke et al., 2007).
Pediatric oncology is a high-risk area for potentially harmful adverse events due to lack of competence from health personnel, management issues, and because supporting infrastructure is not optimal. Thus, the use of Medication Safety Practice (MSP), especially among ALL patients receiving chemotherapy, is important to be investigated. With the implementation of these standards, the patient is expected to avoid adverse events which resulted in a better outcome.
This research is conducted to determine the effect of MSP implementation on the administration of chemotherapy for patients in Dr. Sardjito hospital. The MSP which is referred in this study is safe procedures and policies in chemotherapy treatment ranging from clinical information review and chemotherapy regimen selection, treatment plan and informed consent, drug preparation, treatment regulation, chemotherapy administration and monitoring, and monitoring of drug response and side effects.
Materials and Methods
This is a quasi-experimental study with pre- and post-intervention design without control. The intervention given is the implementation of standardized Medication Safety Practice (MSP) in chemotherapy, including the training beforehand. The study took place in the pediatric cancer ward, Dr. Sardjito Hospital, Yogyakarta, Indonesia. This study was conducted for 8 months, from September 2012 to May 2013.
Data collection and procedures
The data collected includes secondary data obtained from medical records. These data include the incidence of MEs which is contained from the medical record examination of child cancer patients during the year 2012 and 2013.
The data collection procedure is as follows:
a) Determining the helpers in the research process, i.e. hematologists-oncologists / doctors, data analysis personnel and administrative staff;
b) Establish research subjects based on inclusion and exclusion criteria;
c) Conducting data of ME number based on medical record data, related research form of child cancer patient in 2012 which become research subject, and direct observation to related officer;
d) Data collection characteristics of practitioners of chemotherapy services of pediatric patients in Dr. Sardjito through a questionnaire;
e) Determine and establish MSP policies to be applied to the services of pediatric cancer patients at Dr. Sardjito;
f) Conduct observations on the implementation of chemotherapy at an early stage before the implementation of standard chemotherapy procedures;
g) Conducted observations on the implementation of chemotherapy after the implementation of standard chemotherapy procedures;
h) Record the incidence rate of ME based on medical records data on pediatric patients with ALL diagnosis in 2013 and direct observation.
MSP regarding chemotherapy that is used in this study regulates: 1) chemotherapy ordering process related to staffs, 2) general chemotherapy ordering standard, 3) transcribing chemotherapy, 4) prescribing chemotherapy, 5) labeling chemotherapy, 6) administering chemotherapy, and 7) monitoring chemotherapy. The training about MSP was provided before implementing MSP, with doctors, nurses and pharmacist related to pediatric oncology ward as the participants.
Description of the intervention
a) Pre-intervention activities
Prior to the intervention of both pre and post-test, some of the activities carried out were the development of guidebooks, policies and standard operating procedures regarding MSP Standard for cancer patients in Dr. Sardjito General Hospital.
Pre-test. Questionnaires concerning the Implementation of Safe Standard of Chemotherapy Procedures are distributed to medical personnel who are involved in chemotherapy in the ward of the Dr. Sardjito Hospital RSUP Yogyakarta. Questionnaires are given twice in September 2013, hereinafter referred to as pre-test stage, and in June 2014 hereinafter referred to as posttest stage. Post-test observations were conducted after intervention in the form of oncology nursing training, policy socialization, guidebook, implementation of several new medical record forms, prescription, drugs mixed, and monitoring the side effects of chemotherapy in January-February 2014. Besides the policy-making and the guidebook, is the development and manufacture of dispensing prescription drug form as well as monitoring the side effects of chemotherapy.
b) Stage of intervention
After the pre-test stage, the policy and the manual are completed, intervention was given to medical personnel working in Pediatric Oncology Ward in Dr. Sardjito General Hospital collaborated in the study. The author, together with the standard team of Care of Patients (COP), Medical Management and Use (MMU), and related units conducted several socialization steps to carry out the task of serving as well as part of the research process with forum group discussion. The intervention was given in the form of chemotherapy training to all nurses oncology ward Dr. Sardjito RSUP and socialization of MSP Guidebook in pediatric chemotherapy service in Dr. Sardjito to all Estella I and II ward nurses through forum group discussions.
The chemotherapy training was conducted on 11-21 February 2014, number 343 from various units, including Estella ward, one daycare (ODC) nurse in lecture hall 6 of fourth-floor Training Building of Dr. Sardjito lasts for 1 day (gradually in series). This activity contains some material that is 1) basic concept of cancer, 2) concept of chemotherapy and medication safety in chemotherapy, 3) prescription and chemotherapy drug management, 4) personal protective equipment usage, 5) policy and standard operating procedures of chemotherapy, 6) patient chemotherapy patients, and 7) practice of chemotherapy. All trainees were given a certificate as proof of legality allowing participants to work together with chemotherapy medical teams in their respective units. In addition to training, socialization was done directly to Estella I and II nurses and the provision of guidebooks to Estella I and II nurses, pharmacy staff, and several adult oncology nurses.
Formal Control policy is granted by the administrative subdivision to all related units
Population target of this study was all childhood ALL patients in Dr. Sardjito Hospital, Yogyakarta, Indonesia from 2012 to 2013. Minimum sample calculations were estimated using the hypothesis test formula against relative risk. Considering medical error prevalence in pediatric cancer was 18.8% from the previous study (Walsh et al., 2009), type I error was 5% and type II error was 20%, resulting in 75 patients as a minimum sample for each category, pre- and post-intervention.
The sampling was done consecutively, in which medical records of all pediatric patient who had been diagnosed with ALL during the study period were obtained until the minimum sample was fulfilled. The inclusion criteria were: 1) diagnosed with ALL; 2) registered between 2012 and 2013; 3) receive chemotherapy protocol; 4) following ALL chemotherapy protocol. Patients who had incomplete medical records and who dropped out during the study period were excluded from this study.
Statistical analysis
The data collected was collected by data cleaning, coding, tabulation and then digitalized. The data were then analyzed using Stata 8.0 Statistical Software and the significance of trends of categorical variables was tested using chi-square tests with nominal independent variables and dependent variables of a nominal scale, e.g. proportion (Tumbelaka et al., 2014). All of the statistical significance analyses mentioned before are 2-sided. This study has obtained Ethical Clearance from Ethical Committee, Faculty of Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Patient’s consent was obtained after they were given clear information about this study.
The independent variable in this study is the presence/absence or before/after the implementation of the MSP while the dependent variable is the number of ME incidence. The ME events are further classified as medical errors in ordering, dispensing, transcribing, administering and monitoring of drugs and each of these events is analyzed by the chi-square test. Data analysis with hypothesis testing based on significance level p <0.05.
The units analyzed in this study are the adherence of the health personnel to the standard in chemotherapy transcribing, chemotherapy administering and chemotherapy monitoring, and the incidence of adverse drug events, pre- and post-intervention. Topics related to chemotherapy transcribing process are: verification of diagnosis, documentation of cancer status on the first meeting, measurement of weight and height, physical examination of body organ related to its function, documentation patient’s history of allergy, documentation of used regimen comprehensively, documentation of psychosocial status, documentation of chemotherapy planning (dose, regimen, protocol), and documentation of oral chemotherapy schedule. Chemotherapy administering process includes preparation of drugs by two personnel, calling the name and number of medical records in preparation, confirming patient’s name and drug’s name, confirming drugs‘ route of administration, confirmation of adverse drug events management to patient, verification of the drugs (name, route of administration, dose, volume, period of administering, and expired date), giving signature after verification, documenting extravascular management, and documenting that there is a capable health personnel on site while chemotherapy is being administered. Chemotherapy monitoring process related to availability of emergency protocol, assessment and documentation done by staff in each visitation or daily, policy to review patient’s adherence to oral chemotherapy in first 2 weeks and plan to manage the identified problems, documentation and patient’s follow up procedure, monitoring during visitation and/or chemotherapy, drug toxicity documentation, standardized chemotherapy monitoring based on literature or guideline (laboratory evaluation, radiology examination), standardized report of medication error or near miss (report to be reviewed). The incidence of adverse drug events (ADE) is obtained through medical record, including the type of the event and the chemotherapy phase when an event is happening.
Results
From September 2012 to May 2013, 75 pre-intervention samples and 106 post-intervention samples were collected. Basic characteristic of the sample is shown in Table 1.
Table 1.
Basic Characteristic of Correspondents
| No | Characteristics | PRE | % | POST | % | p value |
|---|---|---|---|---|---|---|
| (N=75) | (N=106) | |||||
| 1 | Sex | 0.082 | ||||
| Male | 50 | 67% | 57 | 54% | ||
| Female | 25 | 33% | 49 | 46% | ||
| 2 | Group of Age | 0.438 | ||||
| <1 years old | 0 | 0% | 1 | 1% | ||
| 1-10 years old | 50 | 67% | 77 | 73% | ||
| 10-18 years old | 25 | 33% | 28 | 26% | ||
| 3 | Inward | <0.001* | ||||
| Estella 1 | 32 | 43% | 51 | 48% | ||
| Estella 2 | 30 | 40% | 55 | 52% | ||
| ODC | 13 | 17% | 0 | 0% | ||
| 4 | Main Diagnostic | <0.001* | ||||
| ALL HR | 66 | 88% | 52 | 49% | ||
| ALL SR | 9 | 12% | 54 | 51% | ||
| 5 | Chemotherapy phase | 0.096 | ||||
| Induction | 33 | 44% | 45 | 41% | ||
| Consolidation | 25 | 33% | 32 | 30% | ||
| Reinduction | 0 | 0% | 9 | 9% | ||
| Maintenance | 17 | 23% | 20 | 20% |
p<0.05 is found to be statistically significant
Chemotherapy Transcribing
Comparison of chemotherapy transcribing pre and post-intervention is shown in Figure 1. Measurement of height, body surface area, physical examination of functional-related organ, documenting patient’s history of allergy, psychosocial status, chemotherapy protocol and schedule reports post-intervention increased significantly, compared to pre-intervention (p <0.05). Other topics of chemotherapy transcribing observed not mentioned above reached more than 90%, and some of them reached 100% in standard-based adherence.
Figure 1.
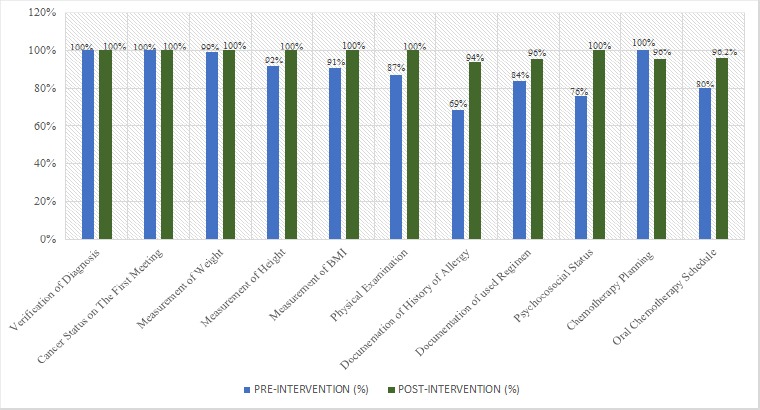
Chemotherapy Transcribing before and after Implementation of Medication Safety Practices
Chemotherapy Administering
Figure 2 shows the data of chemotherapy administering observed pre- and post-intervention. All of the variables related to chemotherapy administering post-intervention significantly increased (p<0.05), except in preparing drugs by two different health worker done independently, patient’s confirmation of ADEs management, and verification of drug’s expiration date.
Figure 2.
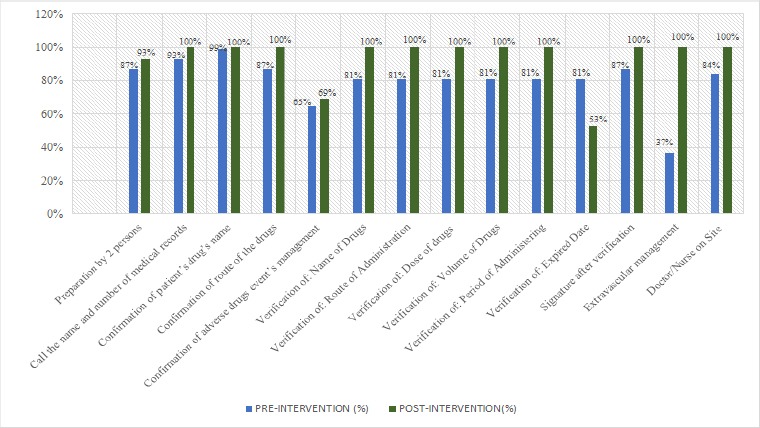
Chemotherapy Administering before and after Implementation of Medication Safety Practices
Chemotherapy Monitoring
Comparison of chemotherapy monitoring pre- and post-intervention is shown in Table 2. There was a statistically significant improvement found in chemotherapy monitoring. Assessment and documentation done by staff in each visitation, documentation, and patient’s follow up procedure, monitoring during visitation and/or chemotherapy, drug toxicity documentation, standardized chemotherapy monitoring based on literature or guideline significantly increased post-intervention compared to pre-intervention (p<0.05).
Table 2.
Chemotherapy Monitoring before and after Implementation of Medication Safety Practices
| NO | Monitoring | PRE | % | POST | % | p value |
|---|---|---|---|---|---|---|
| n=75 | n=106 | |||||
| 1 | Emergency Protocol | 75 | 100% | 106 | 100% | ns |
| 2 | Assessment and Documentation Done by Staff in Each Visitation or Daily | 63 | 84% | 106 | 100% | 0.001 |
| 3 | Policy to Review Patient’s Adherence to Oral Chemotherapy in First 2 Weeks, and Plan to Manage the Identified Problems | 75 | 100% | 106 | 100% | ns |
| 4 | Documentation and Patient’s Follow Up Procedure, Monitoring During Visitation and/or Chemotherapy | 40 | 53% | 106 | 100% | 0.001* |
| 5 | Drug Toxicity Documentation | 11 | 15% | 84 | 79% | 0.001* |
| 6 | Standardized chemotherapy monitoring based on Literature or Guideline (laboratory evaluation,radiology examination) | 37 | 49% | 106 | 100% | 0.001* |
| 7 | Standardized Report of Medication Error or Near Miss. the Report to be Reviewed | 0 | 0% | 106 | 100% | 0,001* |
p<0.05 is found to be statistically significant
Adverse Drug Events
The number of ADEs had lowered significantly post-intervention, as shown in Table 3 (p<0.05). Pre-intervention ADEs was at 52.1% meanwhile post-intervention ADEs had been reduced to 30.5%. The sign and symptoms of ADEs found in correspondents did not differ significantly in post-intervention compared to pre-intervention. The most common ADEs found in correspondents was fever, followed by pruritic skin, vomitus, and epistaxis, respectively.
Table 3.
Adverse Drugs Events Related to Chemotherapy Before and After Implementing Medication Safety Practice
| No | Variable | PRE | POST | p-value |
|---|---|---|---|---|
| N=94 | N=82 | |||
| 1 | Incidence of Adverse Drugs Events | 49 (52%) | 25 (31%) | 0.004* |
| 2 | Sign/symptoms of adverse drugs events | 0.09 | ||
| a) Cough | 2 (4%) | 1 (4%) | ||
| b) Angioedema | 1 (2%) | 0 (0%) | ||
| c) Skin rash | 0 (0%) | 1 (4%) | ||
| d) Fever | 12 (25%) | 4 (16%) | ||
| e) Diarrhea | 3 (6%) | 1 (4%) | ||
| f) Epistaxis | 4 (8%) | 0 (0%) | ||
| g) Skin pruritus | 6 (12%) | 0 (0%) | ||
| h) Constipation | 1 (2%) | 0 (0%) | ||
| i) Nausea | 2 (4%) | 3 (12% | ||
| j) Vomit | 5 (10%) | 3 (12%) | ||
| k) Local pain related to infection | 0 (0%) | 2 (8%) | ||
| l) Myalgia | 2 (4%) | 1 (4%) | ||
| m) Abdominal pain | 2 (4%) | 0 (0%) | ||
| n) Low back pain | 0 (0%) | 1 (4%) | ||
| o) Odynophagia | 1 (2%) | 0 (0%) | ||
| p) Epigastric Pain | 1 (2%) | 0 (0%) | ||
| q) Loss of appetite | 3 (6%) | 6 (24%) | ||
| r) Oral bleeding | 1 (2%) | 0 (0%) | ||
| s) Bleeding on injection site | 0 (0%) | 1 (4%) | ||
| t) Petechiae | 1 (2%) | 0 (0%) |
Discussion
The incidence rate of medication error varies with various study method and definition. Most medication errors were found as the result of prescribing error and incompetent health workers (Williams, 2007). According to a previous research, nurses’ understanding about Medication Safety Practice is quite good and it is getting better with training intervention (Mulatsih et al., 2016), but not sufficient regarding the source of medication error (Mulatsih et al., 2015). According to Watts (2013), the most common type of medication errors is chemotherapy medication error, 42% of their findings, followed by roadmap errors with 26%, supportive care errors with 15%, timing errors with 12%, pharmacy errors with 4%, and clerical errors with 1%. Roadmap error defined with using an outdated and/or incorrect roadmap, incorrect sequencing of therapy phases, or any deviation of drug administration from the scheduled one.
On chemotherapy transcribing aspects, there was a significant increase post-intervention, e.g. in measurement of height, measurement of body mass index and physical examination of related organ, documenting history of allergy, regimen, psychosocial status, and chemotherapy planning. A few aspects such as documenting chemotherapy regimen and chemotherapy schedule did not meet the standard of 100%. The unmet standard finding is in line with a systematic review by Allard (2002) which stated that out of 49% MEs drug ordering stage, 11% are done during drug transcription, which frequency, routes or time deviation are included.
Some aspect of chemotherapy administering post-intervention had met 100% of the criteria (Figure 2), which contained the patient’s identity, name of the drugs, dose of the drugs, volume of the drugs, route of administration, and calculated dose. This finding is in line with a previous study, which found that approaching institution through a multidiscipline system in implementing medication patient safety reduces medication error in giving chemotherapy, e.g. dose, in inpatients wards, from 6.2/1000 to 1.0/1000 (Womer et al., 2002). Adherence of health practitioners to fill in drug label increased post-intervention. A significant difference post-intervention was also found in completing medical record number and the date of drugs preparation, compared to pre-intervention.
This study found that chemotherapy preparing by two different health workers that carried out independently had not conformed to the standard of 100% due to the lacking of the number of nurses compared to patients and resulted in lack of double-checking of chemotherapy drugs. On the contrary, a study included 274 nurses in Switzerland found that double checking of chemotherapy drugs by two different independent nurses is a common thing (69%) and it is believed to prevent medication error effectively (Schwappach et al., 2016). Through observation, it was found that drug verification post-intervention increased significantly, except in verification of expiration date (53%), compared to pre-intervention. It clearly is important to avoid this medical error in every aspect, since medication error happen 3% of every medication orders for any patient regardless their age, meanwhile, 60% of those medication errors potentially lead to ADEs (Gandhi et al., 2005).
This study found that chemotherapy monitoring had gotten better post-intervention compared to pre-intervention (Table 2). This is an important finding, since children who receive more than 1 drug whose age less than 5 years are related to a higher medication error in chemotherapy administering (Lemer et al., 2009). A significant difference was found in documentation and assessment done by staff each patient’s visitation, procedure documentation and patient follow up in a daily basis, drug toxicity documentation, standard monitoring based on literature/guideline, and documentation of medical error/near-miss. A study found that 94% medication error with low harm potential and 60% of near-miss medication error happen in prescribing process, most likely due to the usage of inappropriate abbreviation, dosing error, and in legality aspect (Kaushal et al., 2010).
Obtaining consent and family education had also increased post-intervention. One aspect that did not escalate in post-intervention compared to pre-intervention was the aspect of giving the patient or family emergency number for selected chemotherapy drugs, due to unavailability of the emergency number itself on the informed consent form and form of family education related to the illness. Obtaining consent after clear information given and giving sufficient education is important because communication is found as an important aspect to lower the number of medication error (Walsh et al., 2009).
Using multiple chemotherapy drug in a single patient leads to an increased chance of ADEs (Silva, 2013), as such using a tool reflecting Medical Safety Practice to reduce medication error should be implemented. The number of ADEs (Table 3) had lowered significantly post-intervention (30.5%) compared to pre-intervention (52.1%); with fever being the most common sign/symptoms of ADEs that is found in childhood ALL patient receiving chemotherapy. The proportion of ADEs in each chemotherapy phase pre- vs post-intervention did not differ significantly: 44% vs 41% in induction phase, 33% vs 30% in consolidation phase, 0% vs 9% in re-induction phase, and 23% vs 20% in maintenance phase. Post-intervention’s ADEs most commonly found in the induction phase (53.5%) followed by re-induction phase (40%), consolidation phase (34.7%), and maintenance phase (30.8%). This finding is related to the chemotherapy drugs administered in each phase. As indicated by Cohen (2005), by applying a simple medical safety program derived by the findings of local adverse drug events, they are able to reduce patient harm due to ADEs.
In conclusion, application of Medication Safety Practice guideline in term of ordering, dispensing, transcribing, administering and monitoring of chemotherapy decreases the number of medication error and adverse drug events found in childhood ALL patient. In the future, multidiscipline intervention to implement MSP should be done to have a better outcome in a long term.
Limitation of this study
The limitation of this study is its’ short-term of observation period during the MSP implementation. There should be a periodical review on the implementation of MSP to know its impact related to medication error and adverse drugs events and the adherence of health workers in a long term. Other factors such as infection could also contribute as a bias related to adverse drugs events, thus the observation of ADEs should be further investigated.
Funding Statement
This study was not financially funded by any organization.
Statement conflict of Interest
This manuscript does not have any conflict of interest.
Acknowledgements
We would like to thank Dr. Sasri and dr Adnina from Faculty of Medicine UGM for the contribution to this manuscript.
References
- 1.Allard J, Carthey J, Cope J, et al. Medication errors:causes, prevention and reduction. Br J Haematol. 2002;116:255–65. doi: 10.1046/j.1365-2141.2002.03272.x. [DOI] [PubMed] [Google Scholar]
- 2.Branowicki P, O'Neill J, Dwyer J. Improving complex medication systems:an interdisciplinary approach. J Nurs Admin. 2003;33:199–200. doi: 10.1097/00005110-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MM, Kimmel NL, Benage MK, et al. Medication safety program reduces adverse drug events in a community hospital. Qual Saf Health Care. 2005;14:169–74. doi: 10.1136/qshc.2004.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi TK, Bartel SB, Shulman LN, et al. Medication safety in the ambulatory chemotherapy setting. Cancer. 2005;104:2477–83. doi: 10.1002/cncr.21442. [DOI] [PubMed] [Google Scholar]
- 5.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112:461–72. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 6.Kaushal R, Goldmann DA, Keohane CA, et al. Medication errors in paediatric outpatients. Qual Saf Health Care. 2010;16:1–6. doi: 10.1136/qshc.2008.031179. [DOI] [PubMed] [Google Scholar]
- 7.Kellie SJ, Howard SC. Global child health priorities:What role for paediatric oncologists? Eur J Cancer. 2008;44:2388–96. doi: 10.1016/j.ejca.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Lemer C, Bates DW, Yoon C, et al. The role of advice in medication administration errors in the pediatric ambulatory setting. J Patient Saf. 2009;5:168–75. doi: 10.1097/PTS.0b013e3181b3a9b0. [DOI] [PubMed] [Google Scholar]
- 9.Leonard MS, Cimino M, Shasa S, et al. Risk reduction for adverse drug events through sequential implementation of patient safety initiatives in a children's hospital. Pediatrics. 2006;118:1124–9. doi: 10.1542/peds.2005-3183. [DOI] [PubMed] [Google Scholar]
- 10.Mulatsih S, Dwiprahasto I, Soetaryo Pemahaman perawat mengenai medication errors di Bangsal Perawatan Kanker Anak RSUP Dr. Sardjito. Indonesian J Cancer. 2015;9:111–7. [Google Scholar]
- 11.Mulatsih S, Dwiprahasto I, Soetaryo Pemahaman perawat mengenai medication safety practice di Bangsal. Sari Pediatri. 2016;17:463–8. [Google Scholar]
- 12.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 13.Rinke ML, Shore AD, Morlock L, et al. Characteristics of pediatric chemotherapy medication errors in a national error reporting database. Cancer. 2007;110:186–95. doi: 10.1002/cncr.22742. [DOI] [PubMed] [Google Scholar]
- 14.Schwappach DLB, Pfeiffer Y, Taxis K. Medication double-checking procedures in clinical practice:a cross-sectional survey of oncology nurses'experiences'. BMJ Open. 2016;6:1–10. doi: 10.1136/bmjopen-2016-011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva DCB, Araujo OR, Arduini RG, et al. Adverse drug events in a pediatric intensive care unit:a prospective cohort. BMJ Open. 2013;3:e001868. doi: 10.1136/bmjopen-2012-001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Supriyadi E, Widjajanto PH, Purwanto I, et al. Incidence of childhood leukemia in Yogyakarta, Indonesia. Pediatr Blood Cancer. 2011;57:588–93. doi: 10.1002/pbc.23109. [DOI] [PubMed] [Google Scholar]
- 17.Tumbelaka AR, Riono P, Sastroasmoro S, et al. Pemilihan uji hipotesis. In: Sastroasmoro S, Ismael S, editors. ‘Dasar-dasar Metodologi Penelitian Klinis’. Jakarta: Sagung Seto; 2014. [Google Scholar]
- 18.Walsh KE, Dodd KS, Seetharaman K, et al. Medication errors among adults and children with cancer in the outpatient setting. J Clin Oncol. 2009;27:891–6. doi: 10.1200/JCO.2008.18.6072. [DOI] [PubMed] [Google Scholar]
- 19.Watts RG, Parsons K. Chemotherapy medication errors in a pediatric cancer treatment center:prospective characterization of error types and frequency and development of a quality improvement initiative to lower the error rate. Pediatr Blood Cancer. 2013;60:1320–4. doi: 10.1002/pbc.24514. [DOI] [PubMed] [Google Scholar]
- 20.Wilimas JA, Ribeiro RC. Pediatric hematology-oncology outreach for developing countries. Hematol Oncol Clin North Am. 2001;15:775–87. doi: 10.1016/s0889-8588(05)70246-x. [DOI] [PubMed] [Google Scholar]
- 21.Williams DJP. Medication errors. J R Coll Physicians Edinb. 2007;37:343–6. [Google Scholar]
- 22.Womer RB, Tracy E, Soo-Hoo W, et al. Multidisciplinary systems approach to chemotherapy safety:Rebuilding processes and holding the gains. J Clin Oncol. 2002;20:4705–12. doi: 10.1200/JCO.2002.04.108. [DOI] [PubMed] [Google Scholar]


