Abstract
Codeine-containing cough syrups (CCS) have become one of the most popular drugs of abuse in young population worldwide. However, the neurobiological mechanisms underlying CCS-dependence are yet ill-defined. Therefore, understanding the brain abnormalities in chronic users of CCS is crucial for developing effective interventions. The present study depicted the intrinsic dysconnectivity pattern of whole-brain functional networks at the voxel level in chronic users of CCS. In addition, the degree centrality (DC) changes were correlated to the Barratt Impulsiveness Scale (BIS-11) total score, dose, duration of CCS use, and the age at first use of cough syrups. The current study included 38 chronic CCS users and 34 matched control subjects. All patients were evaluated using the BIS-11. Next, resting-state functional magnetic resonance imaging (rs-fMRI) datasets were acquired from these CCS users and controls. Whole-brain connectivity was analyzed using a graph theory approach: degree centrality (DC). CCS-dependent individuals exhibited low DC values in the left inferior parietal lobule and the left middle temporal gyrus, while high DC values were noted in the right pallidum and the right hippocampus (P < 0.01, AlphaSim corrected). Also, significant correlations were established between average DC value in the left inferior parietal lobule and attentional impulsivity scores and the age at first CCS use. The rs-fMRI study suggested that the abnormal intrinsic dysconnectivity pattern of whole-brain functional networks may provide an insight into the neural substrates of abnormalities in the cognitive control circuit, the reward circuit, and the learning and memory circuit in CCS-dependent individuals.
Keywords: Addiction, Codeine-containing cough syrups, Degree centrality, fMRI, Impulsivity
Highlights
-
•
The abuse of CCS has gained a severe foothold among young individuals worldwide.
-
•
DC is one of the more reliable and compelling measures among several nodal network metrics.
-
•
The present study depicted intrinsic dysconnectivity pattern of whole-brain functional networks in CCS-dependent individuals.
-
•
CCS-dependent individuals showed altered DC in the right pallidum, right hippocampus, left IPL and left middle temporal gyrus.
-
•
Significant correlations were established between average DC value in the left IPL and attentional impulsivity scores and the age at first CCS use.
1. Introduction
Codeine-containing cough syrup (CCS)-dependent individuals refer to long-term and sustained administration of CCS (in which, codeine and ephedrine are intended for cough and analgesic function, respectively) for pleasure and hallucination (Mattoo et al., 1997; Vree et al., 2000). As CCS is cheap, convenient, and legal to purchase, the abuse of the drug has gained a severe foothold among young individuals worldwide and is considered as an increasing concern in modern society (Shek and Lam, 2006). According to a Report of the International Narcotics Control Board for 2012, the abuse of prescription and over-the-counter (OTC) drugs has continued to spread worldwide since 2009, which poses severe health and social challenges in several countries. In the USA, prescription drug abuse is more prevalent than any other internationally controlled substance except for cannabis (Agnich et al., 2013; Martins et al., 2010). In China, Zhou (2010) demonstrated that CCS is the maximally abused drug (50% of all documented abused substances) in adolescents. Previous studies have confirmed that mental functioning, behavior, and personality were abnormal in CCS-dependent individuals (Mattoo et al., 1997; Yang and Yuan, 2008); these characteristics were similar to those expressed by heroin- and other illicit substances-dependent individuals. However, accumulated evidence has demonstrated that illicit drugs, including heroin, have an effect on brain functional changes as measured by functional magnetic resonance imaging (fMRI) (Jiang et al., 2011; Zhang et al., 2011; Zhang et al., 2015) Furthermore, the abnormality of impulsive decision making in illicit drug-dependent individuals may be related to the deficient function of the orbitofrontal cortex and the right inferior parietal lobe. Compared to the illicit drug addiction of the so-called “street drugs,” less attention has been directed to the illicit use of drugs such as CCS (Ma et al., 2011; Ma et al., 2010; Qiu et al., 2014).
The rapid development of neuroimaging techniques has provided many new methods for the detection of early changes in the brain in several neuropsychological diseases. Diffusion-tensor imaging (DTI), a non-invasive tool for probing the microanatomical organization of human brain white matter (WM) in vivo, has been used to investigate the properties of WM in CCS-dependent individuals (Qiu et al., 2015). Positron-emission tomography (PET) has also been employed for examining the drug-addicted individuals. Botelho et al. (2006) found that the perfusion of the orbitofrontal region, the occipital, and the temporal lobes was decreased in heroin addicts; these regions were responsible for the control of attention, motor speed, memory, and visual-spatial processing. Volkow et al. (2005) demonstrated that the metabolic responses of the right medial orbital prefrontal cortex significantly increased in cocaine-addicted subjects. Qiu et al. (2013) used the regional homogeneity (ReHo) analysis method and found that ReHo was diminished in the bilateral medial orbitofrontal cortex (mOFC) and the left dorsal striatum in CCS-dependent individuals. These abnormalities were speculated to be related to the dysfunction of learning and memory in CCS-dependent individuals. Using the voxel-based morphometry (VBM) analysis method, Qiu et al. (2014) also found significantly decreased grey matter (GM) volume in CCS users in the bilateral ventral medial prefrontal cortex (vmPFC). The study further explored the changes in spontaneous functional connectivity of the vmPFC-related circuitry and observed reduced integration in CCS users between the bilateral vmPFC and the bilateral hippocampal complex and between the bilateral vmPFC and the bilateral inferior parietal lobule (IPL). Enhanced functional connectivity was observed in CCS users between the bilateral vmPFC and the right insula (extending to the right dorsal striatum) and between the bilateral vmPFC and the right dorsolateral prefrontal cortex (dlPFC). These results implied that the vmPFC plays a major role in chronic CCS abuse that might lead to disordered impulsive control. Using independent component analysis (ICA), Qiu et al. (2017) investigated the intrinsic brain network abnormalities in CCS-dependent males via resting-state fMRI (rs-fMRI). Compared to the healthy control group, the CCS-dependent individuals presented aberrant intrinsic connectivity within the default mode network (DMN), executive control network (ECN), and salience network (SN) (P < 0.05, AlphaSim corrected). However, the limitations of the study included the spontaneous functional connectivity of abnormal brain regions in a subnet of individuals. In addition, the spontaneous functional connectivity of abnormal brain regions in the whole-brain network is unknown. Among the graph-based methods, DC is recently gaining attention (Wang et al., 2011). It counts the number of direct connections for a given voxel in a network and reflects the functional connectivity within the brain network. It is one of the more reliable and compelling measures among several nodal network metrics and measures the centrality or importance of individual elements in the brain (for example, brain regions) by capturing their relationships with the entire brain network at the voxel level (Zuo et al., 2012). Furthermore, DC has been used in several studies where the dorsal and ventral precuneus, anterior and posterior cingulate gyrus, ventromedial prefrontal cortex, and inferior parietal lobes showed high DC values in normal individuals, which was found to be in agreement with DMN (Wang et al., 2013; Li et al., 2013; van den Heuvel and Sporns, 2013). Voxel-wise centrality maps provided novel insights into the patterns and complexity of functional connectivity throughout the huge human functional network that has been widely used in brain network studies (Di Martino et al., 2013; Tomasi and Volkow, 2010). In the other studies (Qiu et al., 2013, Qiu et al., 2014, Qiu et al., 2015, Qiu et al., 2017), the DC measure was not calculated in CCS-dependent individuals. However, the analysis level in the present study using DC is based on voxels. Therefore, the present study aimed to depict the intrinsic dysconnectivity pattern of whole-brain functional networks at the voxel level, focusing on network architecture, in CCS-dependent individuals. In addition, we also correlated the DC changes to the BIS-11 total score, dose, duration and the age at first use of CCS. Therefore, we speculated that in CCS-dependent individuals, abnormal activity of some brain regions associated with learning, memory, and executive control might exist. The potential brain changes in impaired cognition provided an in-depth insight of the level of functional integration.
2. Materials and methods
2.1. Participants
This prospective study was approved by the Ethics Committee of Guangdong Second Provincial General Hospital. Written informed consent was obtained from all subjects. A total of 72 right-handed participants, including 34 control subjects and 38 CCS-dependent individuals, were enrolled in this study. The CCS-dependent individuals were recruited from the patients seeking treatment at the Addiction Medicine Division of the Guangdong Second Provincial General Hospital. All the patients were screened based on the DSM-IV criteria from medical history and underwent a urine test and an interview on the same day. All participants regularly smoked cigarettes but did not use any type of psychotropic agents prior to the rs-fMRI scan. The exclusion criteria for all participants included central nervous system diseases, schizophrenia, bipolar disorder, prior significant head trauma, positive human immunodeficiency virus (HIV) status, diabetes, hepatitis C, other major medical illness, and left-handedness. The inclusion criteria for the control subjects was the absence of diagnosis of any type of substance abuse or dependence.
2.2. Impulsivity assessment
BIS-11 was used for assessing the impulsivity. This 30-item self-rated scale has three oblique factors: attentional/cognitive that measures the toleration for cognitive complexity and persistence; motor that estimates the tendency to act on the spur of the moment; and non-planning impulsivity, which evaluates the lack of sense of the future. Items are rated from 1 (rarely/never) to 4 (almost always/always). To determine the overall impulsiveness scores, all items are summed; high scores indicate remarkable impulsivity (Patton et al., 1995). BIS-11 is a valid and reliable instrument for healthy Chinese and psychiatric populations (Yao et al., 2007).
2.3. Data acquisition
All MRI datasets were obtained using a Philips Achieva 1.5 T Nova dual MR scanner at the Department of Medical Imaging, Guangdong Second Provincial General Hospital. These datasets included rs-fMRI dataset and T1-weighted structural images. The rs-fMRI dataset [22 axial slices, repetition time (TR)/echo time (TE) = 2000 ms/50 ms, matrix = 64 × 64, field of view (FOV) = 230 × 230 mm2, slice thickness = 4.5 mm without gap, flip angle = 90°] was acquired using an echo planar imaging (EPI) sequence. Each functional run contained 240 volumes (8 min) [the slices were approximately along the anterior commissure-posterior commissure (AC-PC) line and covered approximately 230 to 60 in the inferosuperior direction], and the subjects were asked to lie quietly with their eyes closed and avoid eye movement or thinking of anything specific or falling asleep while in the scanner, during the rs-fMRI data acquisition. Sagittal structural images (160 sagittal slices, TR/TE = 25 ms/4.1 ms, thickness = 1.0 mm, no gap, in-plane resolution = 231 × 232, FOV = 230 × 230 mm, flip angle = 30°) were acquired using a fast field echo (FFE) three-dimensional (3D) T1-weighted sequence.
2.4. Data pre-processing and DC calculation
Data Processing Assistant for Resting-State Functional MR Imaging toolbox (http://www.restfmri.net.sci-hub.cc/forum/DPARSF, Yan and Zang, 2010) was used for the post-processing of the imaging data. Before pre-processing, the first 10 volumes were discarded for each subject so that magnetization reached a steady state and subjects adapted to the MRI scanning noise. Then, the remaining 230 fMRI images were obtained with slice time correction for the acquisition time delay and realignment of the head motion correction. One patient and two control subjects, who showed head motion >1.5 mm or 1.5° during image acquisition, were excluded in subsequent analysis. All realigned images were normalized to the standard Montreal Neurological Institute (MNI) template by applying the echo planar imaging (EPI) template at a 3 × 3 × 3 mm3 resolution. The normalized functional image was subjected to spatial smoothing [6 mm half high full-width (FWHM) Gaussian kernel]. Finally, the imaging data were processed to remove the linear trends and filtered using typical temporal bandpass (0.01–0.08 Hz) to reduce the low-frequency drift and high-frequency noise.
For calculating the DC, we performed voxel-wise voxel-based whole-brain correlation analysis on the pre-processed fMRI data, as described previously (Li et al., 2016). The time course of each voxel in each brain was correlated to every other voxel time course in the GM mask. Thus, we could acquire a n × n matrix of Pearson's correlation coefficients between any pair of voxels, where n is the voxel number of the GM mask. Next, we transformed the Pearson's correlation data into normally distributed Fisher Z-scores and constructed the whole-brain functional network by thresholding each correlation at r > 0.25 (Buckner et al., 2009). The threshold was the default setting while constructing the DC map. Only positive Pearson's correlation coefficients were considered in the present study. For a given voxel, the DC was calculated as the sum of the significant connections at the individual level. As described by previous studies, DC represents the number of direct connections for a given voxel in the voxel-based graphs. It has been widely used to represent the node characteristics of large-scale brain intrinsic connectivity networks.
2.5. Statistical analysis
The differences in age, education, and nicotine use between the CCS-dependent and control groups were assessed by two-sample two-tailed t-tests using SPSS statistical software. A two-tailed Pearson's chi-square test was performed to determine the differences in the gender between the two groups. To examine the between-group differences in the DC using zDC maps, a two-sample t-test was performed between the two groups using REST. A correction for multiple comparisons was performed by Monte Carlo simulation using the AlphaSim program, (http://afni.nimh.nih.gov) (Ledberg et al., 1998). The AlphaSim program was implemented in the REST software by combining the height threshold of P < 0.001 (Eklund et al., 2016) and an extent threshold of P < 0.01, which corresponded to a corrected P < 0.01. The average DC values of all voxels in the significantly different regions demonstrated by voxel-based analysis were extracted independently using the extract time series in REST and entered into SPSS. Then, a Pearson's correlation analysis was performed to clarify the relationships between abnormal DC values of significantly different regions and clinical characteristics in CCS-dependent patients, including the duration of CCS usage, age at first CCS use, and BIS-11 scores; the significance levels were set at P < 0.05 (two-tailed).
3. Results
3.1. Demographics and clinical characteristics
No significant differences were observed in age (t = 4.232, P = 0.084), gender distribution (χ2 = 0.842, P = 0.359), formal years of education (t = 0.862, P = 0.392), number of cigarettes smoked per day (t = −1.592, P = 0.116), and head motion (t = 1.905, P = 0.061) between the CCS-dependent individuals and the control subjects. Details of the demographic data and corresponding clinical characteristics are presented in Table 1.
Table 1.
Demographic and clinical characteristics of the Codeine-containing Cough Syrup (CCS)-dependent individuals and control subjects.
| Characteristic | CCS-dependent individuals (n = 37) | Controls (n = 32) | P-value |
|---|---|---|---|
| Age (years) | 24.081 (3.252) | 26.218 (6.131) | 0.084 |
| Education (years) | 12.432 (3.287) | 13.250 (4.557) | 0.392 |
| Nicotine (no. of cigarettes/day) | 16.243 (8.597) | 13.218 (6.926) | 0.116 |
| Cough syrups use (years) | 5.872 (range: 1–15) | N/A | – |
| Age at the first use of cough syrups | 18.148 (range: 13–24) | N/A | – |
| Mean dose (mL/d) | 446.486 (range: 60–1800) | N/A | – |
| Total BIS scores | 71.621 (4.872) | 58.812 (5.127) | 0.000⁎ |
| Attentional impulsivity | 18.487 (5.670) | 16.469 (1.502) | 0.044 |
| Motor impulsivity | 23.838 (5.781) | 21.188 (3.533) | 0.023 |
| Nonplan impulsivity | 29.297 (2.644) | 20.812 (2.620) | 0.000⁎ |
| Head motion (mean FD_Power) | 0.203166 (0.115205) | 0.159498 (0.072997) | 0.061 |
Unless otherwise indicated, data are means ± standard deviations. NA, not applicable.
P < 0.05.
The comparison of average BIS-11 scores from the chronic CCS-dependent individuals and control group is shown in Table 1. We observed that the CCS-dependent individuals had significantly higher total scores, attentional impulsivity, motor impulsivity, and non-plan impulsivity than the control group (P < 0.05).
3.2. DC analysis
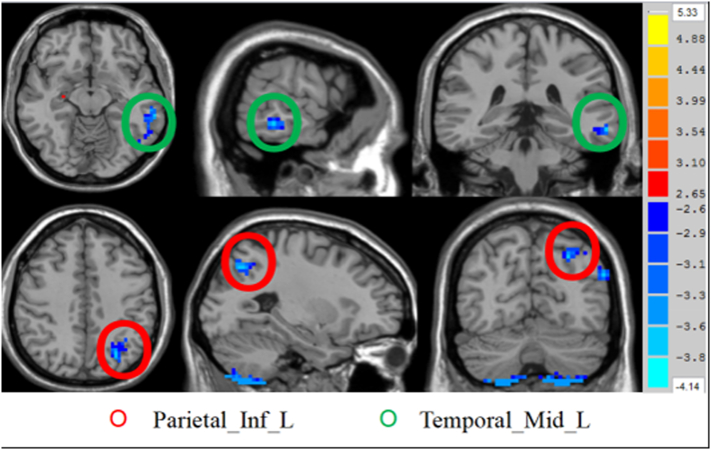
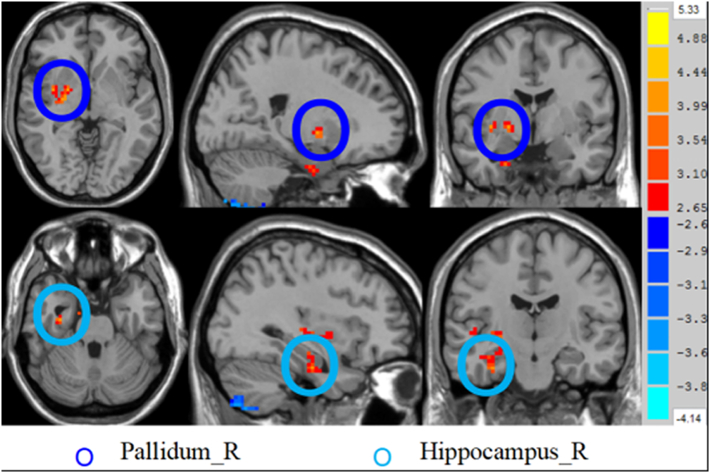
The two-sample t-test analysis revealed significantly increased DC values within the right hippocampus and right pallidum and significantly decreased DC values within the left IPL and left middle temporal gyrus in CCS-dependent individuals as compared to the control group (P < 0.01, AlphaSim corrected) (Table 2 and Fig. 1, Fig. 2).
Table 2.
Brain regions showing differences in the degree between control subjects and CCS-dependent patients.
| Brain regions |
MNI coordinates |
Number of Voxels |
T value |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Hippocampus_R | 33 | −15 | −27 | 19 | 4.0148 |
| Temporal_Mid_L | −63 | −36 | −12 | 37 | −3.856 |
| Pallidum_R | 21 | −3 | −3 | 24 | 4.2402 |
| Parietal_Inf_L | −27 | −69 | 42 | 35 | −3.853 |
Fig. 1.
Brain regions showing decreased degree of differences in CCS-dependent group as compared to the HC group. The regions showing decreased degree of differences in axial (Z = −12, Z = 42 mm), coronal (X = −63, X = −27), and sagittal maps (Y = −36, Y = −69).
Fig. 2.
Brain regions showing increased degree of differences in CCS-dependent group as compared to the HC group. The regions showing increased degree of differences in axial (Z = −3, Z = −27 mm), coronal (X = 21, X = 33), and sagittal maps (Y = −3, Y = −15).
3.3. Correlation results
A decreased average DC value of the left IPL observed in CCS-dependent individuals correlated negatively with the subscale of attentional impulsivity (P = 0.002, PBonferroni < 0.0021) and positively with age at the first use of CCS (Fig. 3).
Fig. 3.
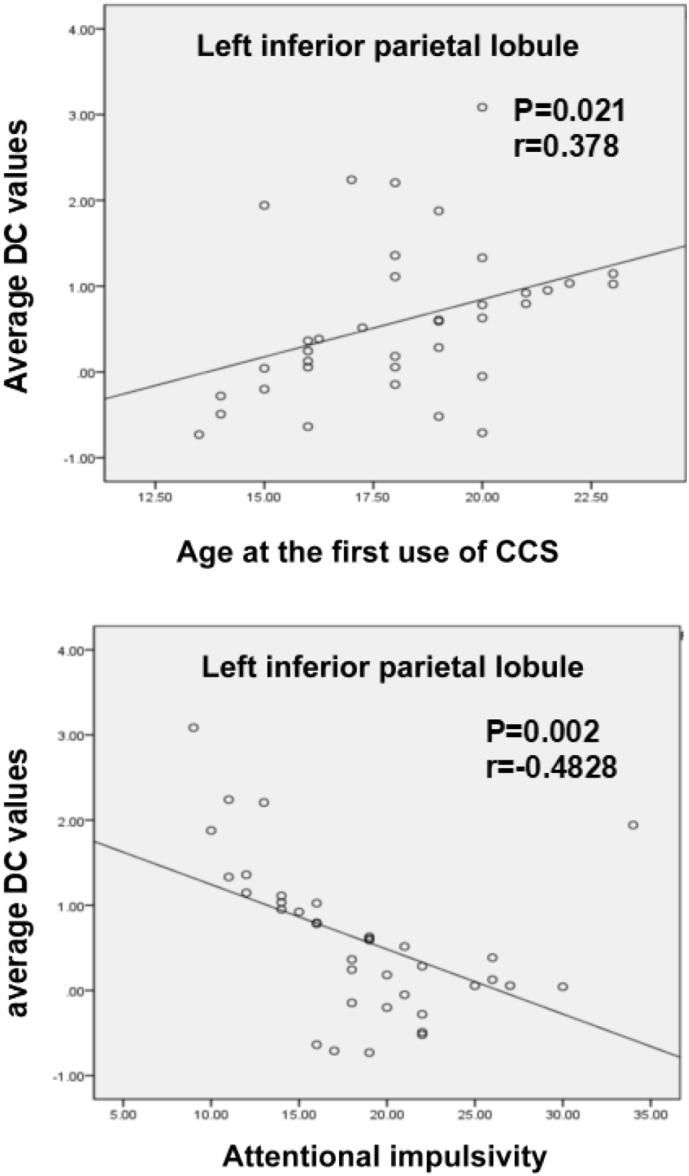
Scatter plot of the mean DC values in the left inferior parietal lobule changing with the age at first use of codeine-containing cough syrups and attentional impulsivity scores, respectively.
4. Discussion
In the present study, we used the DC analysis method to depict the intrinsic dysconnectivity pattern of the whole-brain functional networks at the voxel level in CCS-dependent individuals. An increased degree value represents an increased number of direct connections and also reflects the increased centrality or importance of a specific voxel in the brain network. However, significantly increased DC values within the right hippocampus and the right pallidum and significantly decreased DC values within the left IPL and the left middle temporal lobe were found in CCS-dependent individuals as compared to the control group. Furthermore, a significantly negative correlation was established between the decreased average DC value of the left IPL and the subscale of attentional impulsivity, and a positive correlation between the decreased average DC value of the left IPL and the age at the first use of CCS.
An important finding in the current study was that CCS-dependent individuals showed increased degree values in the right globus pallidus (ventral pallidum includes the portions of the globus pallidus), which is a key region involved in the reward circuit (Haruno and Kawato, 2006). Previous studies have demonstrated that rats can eliminate a preference for the food reward, craving, and learning ability by the selective destruction of the ventral pallidum (VP), whereas to excite or disinhibit the VP neuron activity, they could promote food craving (Hubner and Koob, 1990). The current results suggested that the number of direct connections of the right globus pallidus increased within the brain network in CCS-dependent individuals, indicating the increased activity of the brain region. Thus the increased DC value of the globus pallidus is speculated to promote the craving for addictive drugs in CCS-dependent individuals. Consecutively, the dysfunction of the globus pallidus may indicate the dysfunction of the striatum in CCS-dependent individuals as it is a part of the striatum. The striatum is considered to be associated with stressful experiences (Pruessner et al., 2004; Shaham et al., 2000). The PET studies revealed that acute stress exposure increased the amount of dopamine release in the striatum (Pruessner et al., 2004). Furthermore, Shalev et al. (2003) found that stress could induce reinstatement of heroin-seeking behavior following a test for food deprivation-induced reinstatement in rats. Liu et al. (2009) employed rs-fMRI and found that the connectivity strength was overall high in the striatum in heroin-addicted patients. Furthermore, the study considered that such a dysregulation of the brain regions might lead to a deficit in stress regulatory processes during the resting state. Thus, increased degree values in the globus pallidus in the current study could be correlated to a deficit in stress regulatory processes in drug-addicted patients during the resting state; this postulate can probably provide novel approaches for customized therapies for the treatment of CCS-dependent patients. Moreover, the increased average DC value of the right globus pallidus in CCS-dependent individuals did not show a distinct correlation with the BIS-11 total score. However, Qiu et al. (2017) found that BIS-11 total scores were correlated with the functional connectivity of the bilateral striatum within the DMN in CCS-dependent individuals as assessed by the independent component analysis method. The opposite result may be attributed to the differences arising due to the selected subjects and processing methods.
In the present study, increased degree values in the right hippocampus were found in CCS-dependent individuals. The hippocampus plays a critical role in the formation of the pathological memory of addiction (Šlamberová et al., 2014). In the case of long-term withdrawal of addiction patients, drug-related stimulus conditions could still strongly recall the experience of using drugs, which is known as “addiction memory” (Dunbar and Taylor, 2016). The pathological memory is closely related to acquiring drug addiction (Torregrossa et al., 2011; Hyman et al., 2006). In an experimental mouse model of addiction, Jasinska et al. (2014) found that stimulating the hippocampus could lead to a strong craving for drugs. The blockade of beta-adrenergic receptors in the dorsal hippocampus of the rats would reduce drug seeking and induce the relapse of the cue (Otis et al., 2014). Several studies associated with cocaine-dependent participants noted that participants exposed to cocaine cues showed strong craving for drugs while an increased activity of the hippocampus was observed (Castilla-Ortega et al., 2016; Goldstein and Volkow, 2002). Fotros et al. (2013) used a fallypride (18F) tracer with a high-resolution PET to assess the ability of drug cues in order to induce dopamine (DA) release in the hippocampus of cocaine-dependent participants, and the results showed a dopaminergic response to cocaine cues in the hippocampus. Importantly, these cocaine-dependent participants showed a high degree of desire, leading the investigators to speculate that increased hippocampal activity was related to the memory pathways of cocaine stimulation. Furthermore, in the current study, the abnormal degree values in the right hippocampus demonstrated that the vitality of this region of the brain was increased among learning and memory circuits in CCS-dependent individuals, which contributed to the formation of the addiction memory. However, any significant correlations of the mean degree values in the right hippocampus with the BIS-11 total scores and the duration of CCS use were not observed.
In addition, we also found decreased degree values in the left inferior parietal lobule and left middle temporal gyrus were related to the cognitive function in the CCS-dependent individuals. A previous study demonstrated that the left inferior parietal lobule, one of the crucial brain regions of the attentional control network, was involved in executive function (Monge et al., 2017). The current results suggested that such abnormal intrinsic connectivity may indicate cognitive and executive deficits in CCS-dependent individuals. Garavan et al. (2000) demonstrated that the frontal-parietal working memory circuits in the cocaine-dependent participants were involved in the enhancement of attention towards drug cues, which were related to the initiation and maintenance of craving. Previous studies also found that the mirror-neuron of the inferior parietal cortex in monkeys was activated during the execution and observation of the identical actions (Molenberghs et al., 2012; Plata-Bello et al., 2017). This finding provided new evidence in order to elucidate the mechanism underlying craving formation and the role of IPL in the process. Therefore, decreased degree values in the left inferior parietal lobule and the left middle temporal gyrus in our study may be related to the deficit of attention and cognition in drug cue response in the addicts. Thus, decreased degree values in both the regions in CCS-dependent patients, based on the functional network integrity perspective, postulate that the function of cognitive control-related regions was weakening. Then, a significantly negative correlation was observed between the decreased average DC value of the left IPL and the subscale of the attentional impulsivity. Thus, we hypothesized that a deficit in cognitive control regulatory processes might occur in CCS-dependent patients during the resting state. When the patients were influenced by drug-related cues, they exhibit a rather intense craving, putatively leading to poor decision making and repeated drug use. Qiu et al. (2017) also found that the attentional impulsivity correlated with the right IPL within the ECN in CCS-dependent patients; this finding coincides with our results. We also found a significantly positive correlation between the decreased average DC value of the left IPL and the age at the first use of CCS. Previous studies (Yuan et al., 2010; Yuan et al., 2009) have shown that longer duration of heroin use was associated with more damage to the brain and that these changes might be cumulative. Nevertheless, the current results might further confirm the presence of brain damage in CCS-dependent individuals.
Since the causes of CCS abuse are complex, we cannot absolutely assert the mechanisms underlying these changes. Thus, additional rigorous experimental designs are essential in future studies. Nonetheless, the present study had several limitations. First, as a cross-sectional study, DC can only find the brain regions showing abnormal functional connectivities and is unable to provide detailed information about the functional connection. Thus, in future studies, it will be interesting to trace the brain regions to which the abnormal functional connectivities are linked. Second, the sample size was relatively small in the present study, which might influence the statistical power to detect specific effects. Thus, additional studies with a large sample size of CCS-dependent patients are needed to improve the power of the statistical analysis of our results. Third, there were 10 brands of CCS reported to be used by volunteers enrolled in the present study, and for one volunteer, one to three brands of CCS were used simultaneously. Therefore, a rigorous experimental study is imperative to exclude the influence of different CCS brands.
5. Conclusion
In the current study, we applied the DC approach, derived from the rs-fMRI technique, to examine the intrinsic dysconnectivity pattern of the whole-brain functional networks in CCS-dependent patients. We also correlated the DC changes to the BIS-11 total score, dose, duration of CCS use, and the age at first CCS administration. The current results showed increased connectivity in the reward, learning, and memory circuit-related regions in the right hippocampus and the right pallidum. In addition, decreased functional connectivities were found in the cognitive control of circuit-related regions, including the left inferior parietal lobule and the left middle temporal gyrus. Moreover, a significant correlation was established between the average DC value in the left inferior parietal and the subscale of attentional impulsivity, dose, duration and the age at the first CCS use. Taken together, these results suggested a novel approach for exploring the complex disorder of addiction. This rs-fMRI study advanced our understanding of CCS dependency towards an intrinsic dysconnectivity pattern of whole-brain functional networks.
Conflict of interest
The authors have no relevant conflicts of interest to disclose.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Numbers: 81471639, 81771807, and 81701111), the Natural Science Foundation of Guangdong (Grant Numbers: 2015A030313723, 2016A020215125, 201607010056, and 2017A020215077), and the Science Foundation of Guangdong Second Provincial General Hospital (Grant number: YQ2015-11).
References
- Agnich L.E., Stogner J.M., Miller B.L. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict. Behav. 2013;38:2445–2449. doi: 10.1016/j.addbeh.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Botelho M.F., Relvas J.S., Abrantes M., Cunha M.J., Marques T.R., Rovira E., Fontes Ribeiro C.A., Macedo T. Brain blood flow SPET imaging in heroin abusers. Ann. N. Y. Acad. Sci. 2006;1074:466–477. doi: 10.1196/annals.1369.047. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla-Ortega E., Serrano A., Blanco E., Araos P., Suárez J., Pavón F.J., Rodríguez De Fonseca F., Santín L.J. A place for the hippocampus in the cocaine addiction circuit: potential roles for adult hippocampal neurogenesis. Neurosci. Biobehav. Rev. 2016;66:15–32. doi: 10.1016/j.neubiorev.2016.03.030. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O. Network hubs in the human brain[J] Trends Cogn. Sci. 2013;17(12):683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Zuo X.N., Kelly C., Grzadzinski R., Mennes M., Schvarcz A., Rodman J., Lord C., Castellanos F.X., Milham M.P. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74(8):623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar A.B., Taylor J.R. Inhibition of protein synthesis but not β-adrenergic receptors blocks reconsolidation of a cocaine-associated cue memory. Learn. Mem. 2016;23(8):391–398. doi: 10.1101/lm.042838.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotros A., Casey K.F., Larcher K., Verhaeghe J.A., Cox S.M., Gravel P., Reader A.J., Dagher A., Benkelfat C., Leyton M. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [18F]fallypride study in cocaine dependent participants. Neuropsychopharmacology. 2013;38(9):1780–1788. doi: 10.1038/npp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Pankiewicz J., Bloom A., Cho J.K., Sperry L., Ross T.J., Salmeron B.J., Risinger R., Kelley D., Stein E.A. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatr. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M., Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J. Neurophysiol. 2006;95(2):948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Hubner C.B., Koob G.F. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508(1):20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- Hyman S.E., Malenka R.C., Nestler E.J. Neural mechanisms of addiction: the role of reward—related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jasinska A.J., Stein E.A., Kaiser J., Naumer M.J., Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci. Biobehav. Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G.H., Qiu Y.W., Zhang X.L. Amplitude low-frequency oscillation abnormalities in the heroin users: a resting state fMRI study. NeuroImage. 2011;57(1):149–154. doi: 10.1016/j.neuroimage.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Ledberg A., Akerman S., Roland P.E. Estimation of the probabilities of 3D clusters in functional brain images. NeuroImage. 1998;8(2):113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Li S., Wang B., Xu P., Lin Q., Gong G., Peng X. Increased global and local efficiency of human brain anatomical networks detected with FLAIR-DTI compared to non-FLAIR-DTI. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ma X., Huang R., Li M., Tian J., Wen H., Lin C., Wang T., Zhan W., Fang J., Jiang G. Abnormal degree centrality in neurologically asymptomatic patients with end-stage renal disease: a resting-state fMRI study. Clin. Neurophysiol. 2016;127(1):602–609. doi: 10.1016/j.clinph.2015.06.022. [DOI] [PubMed] [Google Scholar]
- Liu J., Liang J., Qin W., Tian J., Yuan K., Bai L., Zhang Y., Wang W., Wang Y., Li Q., Zhao L., Lu L., von Deneen K.M., Liu Y., Gold M.S. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci. Lett. 2009;460(1):72–77. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Ma N., Liu Y., Li N., Wang C.X., Zhang H., Jiang X.F., Xu H.S., Fu X.M., Hu X., Zhang D.R. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49(1):738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Liu Y., Fu X.M., Li N., Wang C.X., Zhang H., Qian R.B., Xu H.S., Hu X., Zhang D.R. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S.S., Keyes K.M., Storr C.L. Birth-cohort trends in lifetime and past-year prescription opioid-use disorder resulting from nonmedical use: results from two national surveys. J. Stud. Alcohol Drugs. 2010;71:480–487. doi: 10.15288/jsad.2010.71.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S.K., Basu D., Sharma A., Balaji M., Malhotra A. Abuse of codeine-containing cough syrups: a report from India. Addiction. 1997;92(12):1783–1787. [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. Brain regions with mirror properties: a meta-analysis of 125 human FMRI studies. Neurosci. Biobehav. Rev. 2012;36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Monge Z.A., Geib B.R., Siciliano R.E., Packard L.E., Tallman C.W., Madden D.J. Functional modular architecture underlying attentional control in aging. NeuroImage. 2017;155:257–270. doi: 10.1016/j.neuroimage.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis J.M., Fitzgerald M.K., Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J. Neurosci. 2014;34(17):6057–6064. doi: 10.1523/JNEUROSCI.4980-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Exp. Neuropsychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Plata-Bello J., Modroño C., Hernández-Martín E., Pérez-Martín Y., Fariña H., Castañón-Pérez A., Marcano F., González-Mora J.L. The mirror neuron system also rests. Brain Struct. Funct. 2017;222(5):2193–2202. doi: 10.1007/s00429-016-1335-5. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Champagne F., Meaney M.J., Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J. Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Lv X., Su H., Jiang G., Tian J., Zhuo F., Han L., Zhang X. Reduced regional homogeneity in bilateral frontostriatal system relates to higher impulsivity behavior in codeine-containing cough syrups dependent individuals. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.W., Lv X.F., Jiang G.H., Su H.H., Yu T., Tian J.Z., Zhang X.L., Zhuo F.Z. Reduced ventral medial prefrontal cortex (vmPFC) volume and impaired vmPFC-default mode network integration in codeine-containing cough syrups users. Drug Alcohol Depend. 2014;134:314–321. doi: 10.1016/j.drugalcdep.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Qiu Y.W., Su H.H., Lv X.F., Jiang G.H. Abnormal white matter integrity in chronic users of codeine-containing cough syrups: a tract-based spatial statistics study. AJNR Am. J. Neuroradiol. 2015;36(1):50–56. doi: 10.3174/ajnr.A4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.W., Su H.H., Lv X.F., Ma X.F., Jiang G.H., Tian J.Z. Intrinsic brain network abnormalities in codeine-containing cough syrup-dependent male individuals revealed in resting-state fMRI. J. Magn. Reson. Imaging. 2017;45(1):177–186. doi: 10.1002/jmri.25352. [DOI] [PubMed] [Google Scholar]
- Shaham Y., Erb S., Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shalev U., Robarts P., Shaham Y., Morales M. Selective induction of c-Fos immunoreactivity in the prelimbic cortex during reinstatement of heroin seeking induced by acute food deprivation in rats. Behav. Brain Res. 2003;145(1–2):79–88. doi: 10.1016/s0166-4328(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Shek D.T., Lam C.M. Adolescent cough medicine abuse in Hong Kong: implications for the design of positive youth development programs in Hong Kong. Int. J. Adolesc. Med. Health. 2006;18(3):493–503. doi: 10.1515/ijamh.2006.18.3.493. [DOI] [PubMed] [Google Scholar]
- Šlamberová R., Vrajová M., Schutová B., Mertlová M., Macúchová E., Nohejlová K., Hrubá L., Puskarčíková J., Bubeníková-Valešová V., Yamamotová A. Prenatal methamphetamine exposure induces long-lasting alterations in memory and development of NMDA receptors in the hippocampus. Physiol. Res. 2014;63(4):S547–S558. doi: 10.33549/physiolres.932926. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity density mapping. Proc. Natl. Acad. Sci. U. S. A. 2010;107(21):9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa M.M., Corlett P.R., Taylor J.R. Aberrant learning and memory in addiction[J] Neurobiol. Learn. Mem. 2011;96(4):609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Ma Y., Fowler J.S., Wong C., Ding Y.S., Hitzemann R., Swanson J.M., Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J. Neurosci. 2005;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vree T.B., van Dongen R.T., Koopman-Kimenai P.M. Codeine analgesia is due to codeine-6-glucuronide, not morphine. Int. J. Clin. Pract. 2000;54(6):395–398. [PubMed] [Google Scholar]
- Wang J.H., Zuo X.N., Gohel S., Milham M.P., Biswal B.B., He Y. Graph theoretical analysis of functional brain networks: test-retest evaluation on short- and long-term resting-state functional MRI data. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0021976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Fan Y., Lu M., Li S., Song Z., Peng X. Brain anatomical networks in world class gymnasts: a DTI tractography study. NeuroImage. 2013;65:476–487. doi: 10.1016/j.neuroimage.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Zang Y.F. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yuan Q.Y. Investigation and analysis on personalities of male-codeine phosphate addicts by MMPI. Chin. J. Drug Abuse Prev. Treat. 2008;14:143–145. [Google Scholar]
- Yao S., Yang H., Zhu X., Auerbach R.P., Abela J.R., Pulleyblank R.W., Tong X. An examination of the psychometric properties of the Chinese version of the Barratt impulsiveness scale, 11th version in a sample of Chinese adolescents. Percept. Mot. Skills. 2007;104:1169–1182. doi: 10.2466/pms.104.4.1169-1182. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhu Z., Shi J. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71(3):223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Yuan K., Qin W., Liu J. Altered small-world brain functional networks and duration of heroin use in male abstinent heroin-dependent individuals. Neurosci. Lett. 2010;477(1):37–42. doi: 10.1016/j.neulet.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tian J., Yuan K., Liu P., Zhuo L., Qin W., Zhao L., Liu J., von Deneen K.M., Klahr N.J., Gold M.S., Liu Y. Distinct resting-state brain activities in heroin-dependent individuals. Brain Res. 2011;1402:46–53. doi: 10.1016/j.brainres.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Zhang R., Jiang G., Tian J. Abnormal white matter structural networks characterize heroin-dependent individuals: a network analysis. Addict. Biol. 2015;21:667–678. doi: 10.1111/adb.12234. [DOI] [PubMed] [Google Scholar]
- Zhou X. Sun Yat-Sen University; Guangzhou Province, People's Republic of China: 2010. Investigation on the Abuse of ‘Cough Syrup’ With Codeine Phosphate Among Middle School Students in Guangdong Province. [Google Scholar]
- Zuo X.N., Ehmke R., Mennes M., Imperati D., Castellanos F.X., Sporns O., Milham M.P. Network centrality in the human functional connectome. Cereb. Cortex. 2012;22(8):1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]