Abstract
Platelets play a crucial role in the survival of metastatic cells in the blood circulation. The interaction of tumour cells with platelets leads to the production of plethoric factors among which our review will focus on lysophosphatidic acid (LPA), because platelets are the highest producers of this bioactive lysophospholipid in the organism. LPA promotes platelet aggregation, and blocking platelet function decreases LPA signalling and leads to inhibition of breast cancer cell metastasis. Autotaxin (ATX), a lysophospholipase D responsible for the basal concentration of LPA in blood, was detected in platelet α‐granules. Functionally, active ATX is eventually released following tumour cell‐induced platelet aggregation, thereby promoting metastasis. Megakaryocytes do not express ATX but respond to LPA stimulation. Whether LPA‐primed megakaryocytes contribute to the recently reported negative action of megakaryocytes on cancer metastasis is not yet known. However, an understanding of the ATX/LPA signalling pathways in platelets, cancer cells and megakaryocytes opens up new approaches for fighting cancer metastasis.
Abbreviations
- ATX
autotaxin
- LPA
lysophosphatidic acid
- LPC
lysophospatidylcholine
- NSAIDs
nonstreroidal anti‐inflammatory drugs
- TCIPA
tumour cell induced platelet aggregation
- TEP
tumour‐educated platelets
- TPO
thrombopoietin
Introduction
Platelets are well known allies of cancer cells during their transit in the blood circulation, supporting their survival in flux and successful implantation in secondary sites (Gay and Felding‐Habermann, 2011; Leblanc and Peyruchaud, 2016). Over the past 40 years, the involvement of platelets in this process has been firmly demonstrated, and many essential pro‐tumoural and pro‐metastatic platelet‐derived factors have been discovered and characterized (Gay and Felding‐Habermann, 2011). A series of large randomized trials of daily aspirin (≥75 mg daily) versus control for the prevention of vascular events in the United Kingdom were used for evaluating the frequency of distant metastasis in patients who developed cancer during these trials (Rothwell et al., 2012). This meta‐analysis revealed that under such regimens, aspirin prevents distant metastasis, providing the support that including aspirin in cancer therapies might be beneficial in some cancers because of its preventative effect on distant metastasis (Rothwell et al., 2012).
Whether such a characteristic of aspirin is shared with other nonsteroidal anti‐inflammatory drugs (NSAIDs) remains to be determined. Aspirin inhibits http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1375 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376 and consequently the production of inflammatory molecules (prostanoids) like other NSAIDs. This was considered to be the essential anti‐tumoural mechanism of aspirin, through which it blocks the self‐perpetuating inflammatory process during tumour growth (Zha et al., 2004). More specifically, aspirin has a unique capacity to induce COX‐2 acetylation, which increases the accumulation of potent anti‐inflammatory molecules such as specialized proresolving lipid mediators derived from n‐3 (ω‐3) fatty acids or from a series of 15‐epimers of lipoxin, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1034, known as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3933 (Goh et al., 2003; Barden et al., 2015). However, the functional involvement of these molecules in the anti‐tumoural action of aspirin requires experimental proof.
Despite this knowledge, targeting platelets has not yet been established as a standard of care for preventing metastasis in cancer patients. This is likely due to the vital role of platelets in haemostasis. Long‐term depletion of platelet function may lead to an elevated risk of haemorrhage. This is one of the reasons why our laboratory and others are interested in discovering new therapeutic targets, with the perspective of blocking the pro‐tumoural activity of blood platelets while not interfering with their physiological functions in haemostasis. Included among recently identified targets that may fall into this category are the interactions between podoplanin and C‐type lectin‐like receptor 2 (CLC2), and high‐mobility group box 1 (HMGB1) with http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754 (TLR4), whose functions have recently been addressed (Leblanc and Peyruchaud, 2016; Menter et al., 2017). Our present review focuses on the intimate connections established between cancer cells and platelets involving the lysophosphatidic acid (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2906)/autotaxin (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2901) signalling pathways during the metastasis process.
LPA: sources and cancer involvements
LPA is the simplest natural lysophospholipid (Figure 1). Platelets were originally defined as major sources of LPA in the organism since LPA concentrations in plasma increase more than 10‐fold in serum (from ~0.1 to >1 μM) (Eichholtz et al., 1993). The serum levels of LPA are determined by a mechanism that involves diverse phospholipase pathways (Aoki et al., 2002). However, knockout animal studies revealed that ATX (ectonucleotide pyrophosphatase/PDE2), a secreted glycosylated enzyme also present in blood, is responsible for basal levels of LPA in plasma (van Meeteren et al., 2006). Due to its unique lysophospholipase D activity, ATX catalyses the production of LPA from a series of lysophospholipid precursors, including lysophosphatidylcholine (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2508), which is the most abundant in plasma (~200 μM), and also from lysophosphatidylserine and lysophosphatidylethanolamine (Aoki et al., 2002). The outer membrane and microvesicles released from platelets were also found as sources of LPA downstream of the action of secreted http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=275 (Fourcade et al., 1995). Mildly oxidized low‐density lipoproteins are additional sources of LPA in the context of atherosclerosis (Siess and Tigyi, 2004).
Figure 1.
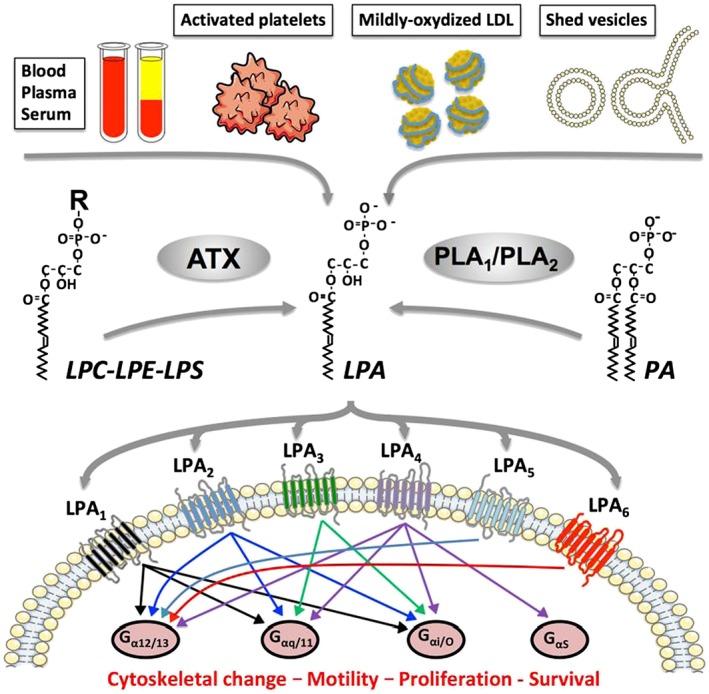
Summary of LPA origins and signalling pathways activated by six LPA receptors. R, choline, serine or ethanolamine; LPS: lysophosphatidylserine; LPE: lysophosphatidylethanolamine; PA, phosphatidic acid; G, heterotrimeric GTPase.
LPA activates six different GPCRs (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=36) (Mutoh et al., 2012) (Figure 1). Most eukaryotic cells express various combinations of LPA receptors that share multiple intracellular signalling pathways dependent on heterotrimeric G‐proteins, including Gαi, Gα12/13, Gαq and Gαs (Noguchi et al., 2009). Therefore, the pleiotropic activities of LPA (i.e. induction of cell survival, proliferation, cytoskeleton rearrangement, motility, cytokine secretion, cell differentiation) are likely the consequences of complex co‐activation signals from multiple LPA receptor signalling pathways resulting in either redundant or opposite effects. This implies that in the context of developing efficient therapies using specific antagonists, potential LPA receptor redundancy should be carefully considered according to specificity of the pathological situation.
The role of LPA in cancer emerged in the late 1990s because of its aberrant production in several cancers, including ovarian and prostate cancers (Mills and Moolenaar, 2003). The expression of LPA receptors and LPA‐dependent signalling pathways is altered in certain cancerous contexts. In colon cancer, specific inactivation of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=273 or http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=274 receptors markedly affects LPA‐induced cell proliferation dependent on the β‐catenin pathway, suggesting a particular role for these receptors in the growth of intestinal tumours (Yang et al., 2005). In contrast to the hormone‐insensitive PC3 prostate cancer cells that express http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=272 receptors, the hormone‐sensitive LnCap cells do not express this receptor and are incapable of generating xenograph tumours in mice unless co‐injected with fibroblasts. Remarkably, de novo expression of LPA1 receptor in LnCap cells confers self‐autonomous growth and progression of xenograph tumours, suggesting that LPA1 receptor expression may contribute to the hormonal switch during the progression of prostate cancers (Guo et al., 2006). In the context of ovarian cancers, the expression levels of the EDG LPA receptors (LPA2 and LPA3 receptors) may function as rheostats. Overexpression and down‐regulation strategies on SKOV‐3 and OVCAR‐3 cells showed direct correlations both in vitro to cell proliferation, migration and cytokine secretions and in vivo to tumour xenograph growth and metastasis. These receptors may play a critical role in the carcinogenesis and aggressiveness of ovarian cancer (Yu et al., 2008). MMTV‐driven overexpression of ATX or each LPA receptor (LPA1, LPA2 and LPA3 receptors) in mammary epithelium of transgenic mice was sufficient to induce carcinogenesis and metastasis of mammary cancer, demonstrating that ATX and LPA receptors can contribute to the carcinogenesis and progression of breast cancers (Liu et al., 2009).
The concentrations of LPA and ATX are remarkably elevated in the context of nonmalignant inflammation and fibrosis where the LPA1 receptor plays a central role in disease pathogenesis (Bourgoin and Zhao, 2010). Therefore, efforts were made to develop pharmacological molecules to target the LPA1 receptor and/or ATX that were validated in preclinical animal models of fibrosis (idiopathic pulmonary fibrosis, dermal fibrosis, kidney fibrosis, systemic sclerosis) and inflammation (rheumatoid arthritis, asthma) (Table 1). An update on the IC50 values and specificities of a large series of LPA receptor blockers developed by the University of Memphis and different pharmaceutical companies was recently reviewed in detail by Llona‐Minguez and co‐workers (2015). Inflammation and fibrosis are also prevalent during cancer progression, supporting the role of LPA and ATX as important mediators in the tumour micro‐environment (Benesch et al., 2017). The efficacy of pharmacological drugs targeting LPA receptors and/or ATX has already been validated in multiple preclinical animal models of primary tumour growth and metastasis (Table 1).
Table 1.
List of pharmacological molecules targeting autotaxin and LPA receptors validated in preclinical animal models
| Targets | Drug names | Diseases | References |
|---|---|---|---|
| LPA1 receptor | AM966 | Idiopathic pulmonary fibrosis | Swaney et al. (2010) |
| LPA1 receptor | AM095 | Dermal fibrosis | Castelino et al. (2011) |
| Lung fibrosis | Swaney et al. (2011) | ||
| Kidney fibrosis | Swaney et al. (2011) | ||
| LPA1 receptor | LA‐01 | Rheumatoid arthritis | Miyabe et al. (2013) |
| LPA2 receptor | DBIBB | Asthma | Knowlden et al. (2016) |
| LPA2 receptor | GRI977143 | Resistance to radiation | Kiss et al. (2013) |
| LPA5 receptor | H2L‐5765411 | Thrombosis | Williams et al. (2009) |
| LPA1, LPA3 receptors | VPC‐12249 | Renal ischaemia–reperfusion | Okusa et al. (2003) |
| LPA1, LPA3 receptors | Ki16425 | Rheumatoid arthritis | Orosa et al. (2014) |
| Hydrocephalus | Yung et al. (2011) | ||
| Renal interstitial fibrosis | Pradere et al. (2007) | ||
| Cancer: | |||
| Breast cancer bone metastasis | Boucharaba et al. (2004) | ||
| Renal cell carcinoma | Su et al. (2013) | ||
| LPA1, LPA3 receptors | Debio 0719 | Osteoporosis | David et al. (2014) |
| Cancer: | |||
| Breast cancer liver metastasis | Marshall et al. (2012) | ||
| Breast cancer lung metastasis | Marshall et al. (2012) and | ||
| David et al. (2012) | |||
| Breast cancer bone metastasis | David et al. (2012) | ||
| LPA1, LPA3 receptors | Ki16198 | Cancer: | |
| Pancreatic cancer | Komachi et al. (2012) | ||
| Pancreatic cancer lung, liver and brain metastases | Komachi et al. (2012) | ||
| All LPA receptors and autotaxin | BrP‐LPA | Rheumatoid arthritis | Nikitopoulou et al. (2013) |
| Cancer: | |||
| Breast cancer | Zhang et al. (2009) | ||
| Glioma | Schleicher et al. (2011) | ||
| Autotaxin | VPC8a202 | Cancer: | |
| Breast cancer lung metastasis | Peyruchaud et al. (2013) | ||
| Autotaxin | ONO‐8430506 | Cancer: | |
| Breast cancer | Benesch et al. (2014) | ||
| Breast cancer lung metastasis | Benesch et al. (2014) | ||
| Autotaxin | PF‐8380 | Inflammation | Gierse et al. (2010) |
| Cancer: | |||
| Glioblastoma | Bhave et al. (2013) | ||
| Autotaxin | 4PBPA | Cancer: | |
| Melanoma lung metastasis | Gupte et al. (2011) | ||
| Autotaxin | S32826 | Glaucoma | Iyer et al. (2012) |
| Autotaxin | BMP‐22 | Cancer: | |
| Breast cancer bone metastasis | Leblanc et al. (2014) | ||
| Autotaxin | Gintonin | Cancer: | |
| Melanoma lung metastasis | Hwang et al. (2013) | ||
| Autotaxin | GWJ‐A‐23 | Idiopathic pulmonary fibrosis | Oikonomou et al. (2012) |
| Asthma | Park et al. (2013) |
Effect of LPA on platelets and megakaryocytes
The bioactive fraction of LPA in the circulation is bound to albumin and gelsolin (Meerschaert et al., 1998). This may have an important impact on its bioavailability and capacity to activate specific receptors (Goetzl et al., 2000). LPA concentration in blood is tightly buffered, which involves highly dynamic processes of formation/degradation (Morris et al., 2009). However, the biological significance and pathophysiological roles of circulating LPA are still largely unrecognised. Circulating LPA might control different biological functions depending on animal species; it may control blood pressure as an i.v. injection of synthetic LPA causes hypertension in rats and guinea pigs but hypotension in cats and rabbits (Tokumura et al., 1978).
LPA may also control platelet aggregation since it acts directly as a mild agonist on the aggregation of human platelets. In platelets from rabbits and dogs, LPA enhances http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1712‐induced aggregation, and after these platelets had been primed with a low dose of ADP, LPA is itself effective in stimulating aggregation (Gerrard et al., 1979). In striking contrast, in murine platelets, LPA inhibits agonist‐induced activation of these platelets (Pamuklar et al., 2009). These remarkable functional differences dependent on animal species should be carefully taken into account when addressing the biological functions of LPA, at least with regard to its contribution as a systemic factor in blood and more specifically on platelet functions.
Among the healthy population, platelets obtained from 20% of human donors fail to aggregate in response to LPA (Pamuklar et al., 2008). Since the first identification of the existence of a cell surface LPA receptor based on platelet studies (Watson et al., 1985) and cloning of Vzg1/Edg‐2 as the first LPA receptor (known now as LPA1 receptor) (Hecht et al., 1996), all six LPA receptor mRNAs (LPA1–6 receptors) have been found in human platelets (Rowley et al., 2011). Interestingly, an overexpression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=94 was detected in platelets from unresponsive patients (Pamuklar et al., 2008). LPA4 has the unique ability to link to GαS, resulting in the activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257 and consequently causing an increase in http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2352 levels in cells; whereas other LPA receptors activate Gα that at the opposite end inhibits adenylate cyclase (Figure 1). An increase in intracellular levels of cAMP inhibits platelet aggregation (Noe et al., 2010). Therefore, the unresponsiveness of human platelets to LPA is likely the consequence of predominant activation of the Gαs rather than the Gαi pathways promoting adenylate cyclase activation and increasing cAMP levels. As judged by RNA‐seq analyses, the repertoire of LPA receptor mRNAs differs between human and murine platelets. LPAR5 mRNA is remarkably prevalent in human platelets but totally absent in murine platelets (Rowley et al., 2011). The http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=124 receptor has unique ligand selectivity to alkyl‐LPAs, whereas acyl‐LPAs are the most common ligands for all other LPA receptors. Intriguingly, alkyl‐LPAs revealed higher potencies than acyl‐LPAs in activating platelets (Tokumura et al., 2002). Moreover, silencing, individually, each LPA receptor in Meg‐01 and Dami megakaryocyte cell lines revealed that only LPA5 receptor knockdown significantly inhibited LPA‐induced shape change (Khandoga et al., 2011). These results provide strong evidence that the LPA5 receptor is the functionally active LPA receptor in platelets (Williams et al., 2009) (Figure 2).
Figure 2.
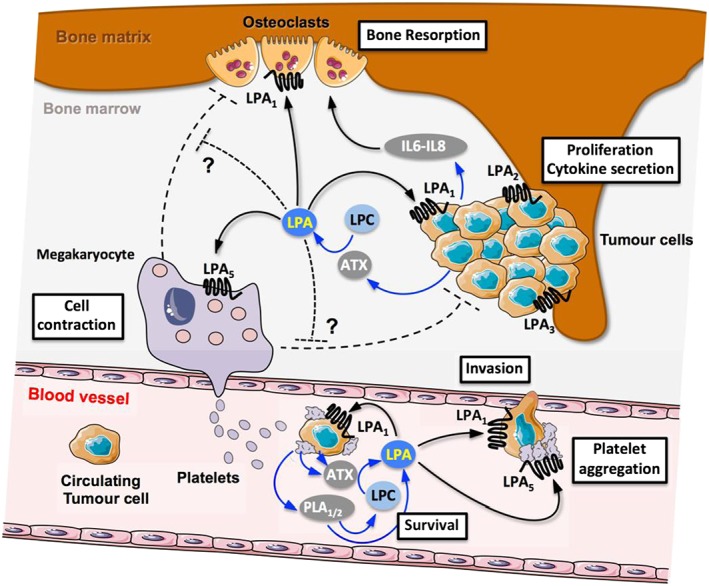
Summary of coupling actions between blood platelets, ATX, LPA and LPA receptors in bone metastasis. Interaction of circulating tumour cells with platelets induces platelet aggregation and release of LPA through mechanisms involving phospholipases A1 and A2 (PLA1/2) generating LPA directly or indirectly through synthesis of LPA precursors including LPC that was eventually degraded by ATX mobilized from the blood circulation or secreted by platelets. In the blood circulation, LPA will act on tumoural LPA1 receptors promoting survival and invasion and potentially on platelet LPA5 receptors promoting platelet aggregation. After colonizing the bone marrow, LPA will act on tumoural LPA1 receptors promoting cell proliferation and pro‐osteoclastic cytokine (IL‐6, IL‐8) secretion promoting bone resorption indirectly or directly by acting on osteoclast LPA1 receptors. LPA might act on megakaryocyte LPA5 receptors to induce cell contraction. It is proposed that the pro‐metastatic action of LPA in bone metastasis might involve the action of LPA on megakaryocytes counteracting their negative action on osteoclast and tumour cells, blunting their protective action against bone metastasis progression. ?, requires experimental validation.
Bone metastases: paramount sites involving LPA, platelets and megakaryocytes
Pharmacologically‐ or genetically‐induced thrombocytopenia dramatically affects the capacity of cancer cells to seed to distant sites (Gasic et al., 1968; Boucharaba et al., 2004; Camerer et al., 2004). Functional blocking of platelet activity also alters metastasis; combined treatments of mice with ATP102, a soluble ADPase, and aspirin were shown to significantly reduce melanoma and breast cancer bone metastasis (Uluckan et al., 2008). We showed that thrombocytopenia induced in mice treated with integrelin leads to reduced levels of circulating LPA and to a remarkable decrease in LPA‐dependent growth of skeletal tumours. Thus, at the bone site, platelet activity could generate bioactive LPA that may act on cancer cells promoting tumour progression and metastasis (Boucharaba et al., 2004) (Figure 2).
Metastatic cancer cells have the ability to interact with platelets and inducing platelet aggregation through mechanisms involving β3 integrins and P‐selectin among others (Borsig, 2008). Human MDA‐MB‐231 and MDA‐B02 breast cells display these characteristics; but beyond physical interaction with platelets, tumour cell‐induced platelet aggregation (TCIPA) mediates the production of multiple factors including LPA (Boucharaba et al., 2004). Remarkably, these cancer cell lines do not express ATX and are incapable of synthesizing LPA, indicating that LPA produced during TCIPA is derived directly from aggregated platelets. Then, LPA can act as a paracrine factor on tumour cells via the LPA1 receptor, promoting cell proliferation, migration and secretion of pro‐inflammatory and pro‐osteoclastic cytokines and growth factors [http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=821, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4942 (GM‐CSF), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=771 (CCL2)]. These tumour cell‐derived factors are major contributors to the effects of platelets on the progression of osteolytic bone metastases (Boucharaba et al., 2004; Boucharaba et al., 2006) (Figure 2).
However, the murine models used in these studies are not relevant for addressing LPA‐mediated crosstalk between platelets and cancer cells. As demonstrated previously, in contrast to those of humans, murine platelets do not respond to LPA. In humans, LPA produced during TCIPA could potentially act as an autocrine factor on platelets through LPA5 receptors, which could further enhance platelet aggregation and increase tumour metastasis (Figure 2). As a consequence, the LPA5 receptor appears to be an attractive target for the development of new therapies against the metastatic effects of platelets. However, pharmacological investigations into this approach would require animal subjects with competent platelets that respond to LPA stimulation, such as those from dogs or guinea pigs.
Transgenic mice overexpressing http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5063 (TPO) exhibit an increased number of megakaryocytes in the bone marrow and develop a high bone mass phenotype (Yan et al., 1996). The increased expansion of megakaryocytes in mice in response to a 5‐day TPO administration prior to intracardiac injection of PC3 prostate cancer cells remarkably decreases the extent of skeletal lesions and tumour burden (Li et al., 2011). TPO was shown to inhibit osteoclast differentiation and their resorption activity in vitro (Wakikawa et al., 1997). Blocking osteoclast function with anti‐resorptive agents is the current care of patients with hypercalcaemia and bone metastases. Thus, TPO might prevent osteolysis directly and indirectly through increased production of megakaryocyte‐derived osteoclast inhibitors (Wakikawa et al., 1997). In addition, murine primary megakaryocytes inhibit the proliferation and increase the apoptosis of prostate cancer cells in co‐culture systems, and this may contribute to the inhibition of skeletal tumour growth (Figure 2). These results support recent clinical findings that increased levels of circulating megakaryocytes tend to correlate with good prognosis in patients with prostate cancer and that a combination of circulating tumour cell levels and megakaryocyte count may predict survival in advanced case of this disease (Xu et al., 2017).
Megakaryocytes are located adjacent to bone marrow blood vessels allowing plasma membrane expansion through the endothelium and production of platelets (Kaushansky, 2008) (Figure 2). Megakaryocytes express a large series of growth factors including http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5041 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4924, which promote osteoblast differentiation and may help to maintain a high bone mass (Kacena et al., 2004). Megakaryocytes also express functionally active anti‐angiogenic factors such as thrombospondins 1 and 2 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2368 and TSP2), as seen from Matrigel plugs loaded with double KO‐TSP1/TSP2 megakaryocytes that had reduced sprouting vessels compared to that with wild‐type megakaryocytes (Kopp et al., 2006). These findings suggest that megakaryocytes potentially control osteoblastic and haematopoietic vascular niches that are functionally important anatomical structures for the successful establishment of bone metastasis (Psaila and Lyden, 2009). Until recently, opposite theories were proposed for the impact of megakaryocytes on these niches in promoting or inhibiting metastasis (Zaslavsky et al., 2006; Li et al., 2011; Psaila et al., 2012). However, in 2017, using preclinical models of breast cancer metastasis, Jackson and colleagues (2017) demonstrated that an increase in the number of megakaryocytes occurs in response to metastatic cells entering the bone marrow. The molecular mechanisms involved in this process have not yet been elucidated. However, compared with wild‐type mice, TPO−/− animals injected orthotopically with 4T1.2 metastatic murine carcinoma cells displayed more aggressive metastasis and a decreased survival. In this context, the presence of megakaryocytes may protect against skeletal metastasis (Figure 2). Both PC3 prostate cancer cells and 4T1 breast carcinoma cells express ATX and produce LPA autonomously, whereas megakaryocytes are incapable of this (Leblanc et al., 2014). The contribution of the different‐shaped megakaryocytes induced by LPA, through the LPA5 receptor, to a host's reaction to metastasis is totally elusive but would deserve further investigation (Figure 2).
Functional interaction of ATX with platelets and cancer cells
ATX is a multidomain protein that possesses two somatomedin B (SMB1,2)‐like domains: a catalytic PDE domain and a nuclease‐like domain. A previous study on the role of ATX/LPA in murine thrombosis has shown that activated but not resting platelets are able to bind recombinant ATX. This binding process can be disrupted by 7E3 antibody, demonstrating the involvement of β3 integrin (Pamuklar et al., 2009). Although ATX possesses the classical RGD motif that mediates integrin binding, the recent crystal structure reveals that ATX‐SMB2 might interact with platelet‐http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2579 integrins using a surface similar to that used by the related vitronectin SMB domains in their interactions with plasminogen activator inhibitor‐1 (PAI‐1) and a urokinase receptor (uPAR; Hausmann et al., 2011). Fulkerson et al. also investigated the integrin signalling pathways that promote ATX binding to platelets. They found that ATX increases thrombin‐stimulated LPA production by washed platelets and provided evidence that ATX‐mediated LPA production is significantly higher in CHO cells transfected to express αIIbβ3 integrin. Moreover, blocking the ATX/αIIbβ3 interaction by performing point mutations in the SMB2 domain or using 7E3 antibody leads to a decrease in LPA production (Fulkerson et al., 2011).
Although ATX expression is elevated in several types of cancers (neuroblastoma, beta cell lymphoma, melanoma, breast carcinomas, etc.) and correlated with a poor prognosis (Leblanc and Peyruchaud, 2015), it is now well accepted that stromal cells including platelets can provide ATX to the tumour for enhancing cancer progression (Figure 2). Based on immunohistochemistry analysis of murine breast carcinoma tissue, Benesch and colleagues (2014) noticed a higher ATX staining in the stroma than in the tumour cell compartment. In addition, adipose tissue that has a remarkable impact on breast cancer is also known to be a major source of ATX in the organism (Dusaulcy et al., 2011). Recently, Brindley's lab revealed that tumour‐induced inflammation in mammary adipose tissue stimulates a vicious cycle of ATX expression and breast cancer progression (Benesch et al., 2015).
By using a human breast cancer cell line (MDA‐B02) that does not express ATX, we also found that treatment of animals with an ATX inhibitor (BMP22) inhibited both the progression of pre‐established skeletal metastases and the early steps of cancer cell colonization to the bone (Leblanc et al., 2014) (Figure 2). Furthermore, we demonstrated for the first time that ATX can be stored in platelet α‐granules isolated from healthy donors and released upon TCIPA, leading to the production of LPA via the degradation of platelet‐derived LPC (Leblanc et al., 2014). We showed that the pro‐tumoural activity of ATX derived from platelets was partially dependent on the interaction of ATX with tumoural http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=760#2583. A recent report also demonstrated a cooperative action between exogenous ATX and β3 integrin in cell migration. The binding of ATX to integrin enabled the uptake and redistribution of ATX to the leading edge of migrating cells (Wu et al., 2014). Altogether, these studies suggest that β3 integrin binding might localize ATX activity to the cancer cell/platelet surface, providing a mechanism to generate LPA in the vicinity of its receptors, and then enhance cancer cell dissemination.
There is extensive experimental evidence indicating that platelets also support cancer cell extravasation to secondary metastatic sites (Labelle et al., 2011) (Figure 2). For instance, dual blocking of the platelet‐membrane P‐selectin and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2579 reduced the ability of tumour cells to degrade Matrigel, confirming their role in assisting tumour cells in the extravasation process (Pang et al., 2015). Our recent publication also suggests that by increasing the ability of the MDA‐MB‐231 cell line to cross an endothelial monolayer and to degrade a Matrigel layer, LPA and its precursor ATX may favour breast cancer cell extravasation to the bone (Leblanc et al., 2014). Kanda and colleagues (2008) have already described such a mechanism, where they showed that ATX binds to chemokine‐activated human lymphocytes in a http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2455‐dependent manner. This binding promotes LPA production and enhances the entry of lymphocytes into secondary lymphoid organs. Interestingly, Smyth's group reported that platelet β1 integrin also interacts in an activation‐dependent manner with ATX via the SMB2 domain (Fulkerson et al., 2011). Since activated platelets can produce and bind ATX, as well as participate in lymphocyte trafficking in high endothelial venules (Diacovo et al., 1996), we could imagine platelet β1 integrin cooperates with ATX in the tumour cell extravasation process.
Platelets: a function beyond aggregation
Platelets are fascinating cells. They do not have a nucleus and therefore they lack gene transcription. Nevertheless, platelets benefit from the large amount of plethoric factors (proteins, lipids, nucleotides) produced by megakaryocytes that in turn are responsible for the role of platelets in haemostasis, cancer and other pathologies. Platelets also contain residual mRNAs synthesized by megakaryocytes that are efficiently used as templates for de novo protein synthesis (Rowley and Weyrich, 2013). Specific platelet transcriptional profiles and protein expressions have not yet been determined in the context of cancer, but they have been confirmed in patients with several types of diseases including cardiovascular disease, sickle cell anaemia and systemic lupus erythematosus (Rowley et al., 2012).
MicroRNAs are epigenetic factors controlling gene expression through indirect destabilization of mRNAs. They are also present and functionally active in platelets, as shown from platelet factor 4‐Cre‐mediated deletion of Dicer1 that heightened platelet reactivity (Rowley et al., 2016). In addition to the presence of factors derived from megakaryocytes, platelets have also a remarkable capacity to take up multiple factors from the surrounding environment during their short life of 9–12 days in the blood stream. This is well known for fibrinogen, albumin and immunoglobulin (Handagama et al., 1990). We recently demonstrated that this is also the case for ATX since it is not synthesized by megakaryocytes but found in platelet α‐granules and released upon TCIPA (Leblanc et al., 2014). This suggests that in the perspective of new therapeutics directed against ATX, stored protein in α‐granules is unlikely to be accessible to pharmacological drugs and would escape inactivation leading to potentially ineffective treatments.
Since platelets are circulating throughout the entire organism, they may also be able to collect release factors related to specific pathological situations appearing in different organs. In an oncological context, Best and colleagues (2015) recently demonstrated that tumour‐educated platelets (TEP) could be used as a liquid biopsy that, following up RNA‐Seq analysis, leads them to distinguish patients with localized and metastasized tumours from healthy individuals. Subsequent algorithm optimization of TEP analysis allowed this group to detect early‐ and late‐stage non‐small cell lung cancer (Best et al., 2017).
Conclusion
Beside their vital role for human health, platelets also promote cancer progression and metastasis. In this context, platelets can be used as therapeutic targets and prognostic tools. The complexity of the ATX/LPA signalling axis in cancer has recently reached a higher level since both platelets and several tumour cells were found to produce LPA, thereby promoting platelet aggregation and tumour cell proliferation and mobility. As a consequence, platelets and cancer cells participate in a vicious cycle whereby tumour cell proliferation and platelet aggregation sustain each other. In addition, ATX‐null cells that would be deficient in LPA signalling could benefit from platelet‐derived ATX for eliciting LPA‐induced programmes leading to cancer metastasis. Once established into secondary sites, metastatic cells acquire resistance to conventional treatments. Understanding how circulating tumour cells survive in flux before seeding in target organs remains crucial for the development of potent anti‐metastasis therapies.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Figures were produced using Servier Medical Art (http://www.servier.com). This work was supported by grants from INSERM (O.P.) and the Université Claude Bernard Lyon‐1 (O.P.), the Comité Départemental de la Savoie de la Ligue Contre le Cancer (O.P.), the Fondation ARC pour la Rechercher sur le Cancer (ARC, grant no. PJA20151203151) and the ANR grant LYSBONE (O.P.) (grant no. ANR‐15‐CE14‐0010‐01). R.L. is a recipient of a grant fellowship from the Fondation ARC pour la Recherche sur le Cancer.
Leblanc, R. , Houssin, A. , and Peyruchaud, O. (2018) Platelets, autotaxin and lysophosphatidic acid signalling: win‐win factors for cancer metastasis. British Journal of Pharmacology, 175: 3100–3110. https://doi.org/10.1111/bph.14362.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T et al (2002). Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277: 48737–48744. [DOI] [PubMed] [Google Scholar]
- Barden AE, Mas E, Croft KD, Phillips M, Mori TA (2015). Specialized proresolving lipid mediators in humans with the metabolic syndrome after n‐3 fatty acids and aspirin. Am J Clin Nutr 102: 1357–1364. [DOI] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Maeda T, Ohhata A, Zhao YY, Kok BP et al (2014). Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J 28: 2655–2666. [DOI] [PubMed] [Google Scholar]
- Benesch MG, Tang X, Dewald J, Dong WF, Mackey JR, Hemmings DG et al (2015). Tumor‐induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J 29: 3990–4000. [DOI] [PubMed] [Google Scholar]
- Benesch MGK, Yang Z, Tang X, Meng G, Brindley DN (2017). Lysophosphatidate signaling: the tumor microenvironment's new nemesis. Trends Cancer 3: 748–752. [DOI] [PubMed] [Google Scholar]
- Best MG, Sol N, In 't Veld S, Vancura A, Muller M, Niemeijer AN et al (2017). Swarm intelligence‐enhanced detection of non‐small‐cell lung cancer using tumor‐educated platelets. Cancer Cell 32: 238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F et al (2015). RNA‐Seq of tumor‐educated platelets enables blood‐based pan‐cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 28: 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SR, Dadey DY, Karvas RM, Ferraro DJ, Kotipatruni RP, Jaboin JJ et al (2013). Autotaxin inhibition with PF‐8380 enhances the radiosensitivity of human and murine glioblastoma cell lines. Front Oncol 3: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L (2008). The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther 8: 1247–1255. [DOI] [PubMed] [Google Scholar]
- Boucharaba A, Serre C‐M, Gres S, Saulnier‐Blache JS, Bordet J‐C, Guglielmi J et al (2004). Platelet‐derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest 114: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucharaba A, Serre CM, Guglielmi J, Bordet JC, Clezardin P, Peyruchaud O (2006). The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc Natl Acad Sci U S A 103: 9643–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgoin SG, Zhao C (2010). Autotaxin and lysophospholipids in rheumatoid arthritis. Curr Opin Investig Drugs 11: 515–526. [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR (2004). Platelets, protease‐activated receptors, and fibrinogen in hematogenous metastasis. Blood 104: 397–401. [DOI] [PubMed] [Google Scholar]
- Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS et al (2011). Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum 63: 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Machuca‐Gayet I, Kikuta J, Ottewell P, Mima F, Leblanc R et al (2014). Lysophosphatidic acid receptor type 1 (LPA1) plays a functional role in osteoclast differentiation and bone resorption activity. J Biol Chem 289: 6551–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Ribeiro J, Descotes F, Serre CM, Barbier M, Murone M et al (2012). Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int J Oncol 40: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH (1996). Platelet‐mediated lymphocyte delivery to high endothelial venules. Science 273: 252–255. [DOI] [PubMed] [Google Scholar]
- Dusaulcy R, Rancoule C, Gres S, Wanecq E, Colom A, Guigne C et al (2011). Adipose‐specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res 52: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH (1993). The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J 291: 677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade O, Simon MF, Viode C, Rugani N, Leballe F, Ragab A et al (1995). Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 80: 919–927. [DOI] [PubMed] [Google Scholar]
- Fulkerson Z, Wu T, Sunkara M, Kooi CV, Morris AJ, Smyth SS (2011). Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem 286: 34654–34663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB, Stewart CC (1968). Antimetastatic effects associated with platelet reduction. Proc Nal Acad Sci U S A 61: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay LJ, Felding‐Habermann B (2011). Contribution of platelets to tumour metastasis. Nat Rev Cancer 11: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard JM, Kindom SE, Peterson DA, White JG (1979). Lysophosphatidic acids. II. Interaction of the effects of adenosine diphosphate and lysophosphatidic acids in dog, rabbit, and human platelets. J Pathol 97: 531–547. [PMC free article] [PubMed] [Google Scholar]
- Gierse J, Thorarensen A, Beltey K, Bradshaw‐Pierce E, Cortes‐Burgos L, Hall T et al (2010). A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation. J Pharmacol Exp Ther 334: 310–317. [DOI] [PubMed] [Google Scholar]
- Goh J, Godson C, Brady HR, Macmathuna P (2003). Lipoxins: pro‐resolution lipid mediators in intestinal inflammation. Gastroenterology 124: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS (2000). Gelsolin binding and cellular presentation of lysophosphatidic acid. J Biol Chem 275: 14573–14578. [DOI] [PubMed] [Google Scholar]
- Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N et al (2006). Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinol 147: 4883–4892. [DOI] [PubMed] [Google Scholar]
- Gupte R, Patil R, Liu J, Wang Y, Lee SC, Fujiwara Y et al (2011). Benzyl and naphthalene methylphosphonic acid inhibitors of autotaxin with anti‐invasive and anti‐metastatic activity. Chem Med Chem 6: 922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handagama P, Rappolee DA, Werb Z, Levin J, Bainton DF (1990). Platelet alpha‐granule fibrinogen, albumin, and immunoglobulin G are not synthesized by rat and mouse megakaryocytes. J Clin Invest 86: 1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann J, Kamtekar S, Christodoulou E, Day JE, Wu T, Fulkerson Z et al (2011). Structural basis of substrate discrimination and integrin binding by autotaxin. Nat Struct Mol Biol 18: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J (1996). Ventricular zone gene‐1 (vzg‐1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J Cell Biol 135: 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W 3rd, Sosnoski DM, Ohanessian SE, Chandler P, Mobley A, Meisel KD et al (2017). Role of megakaryocytes in breast cancer metastasis to bone. Cancer Res 77: 1942–1954. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Lee BH, Kim HJ, Cho HJ, Shin HC, Im KS et al (2013). Suppression of metastasis of intravenously‐inoculated B16/F10 melanoma cells by the novel ginseng‐derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol 42: 317–326. [DOI] [PubMed] [Google Scholar]
- Iyer P, Lalane R 3rd, Morris C, Challa P, Vann R, Rao PV (2012). Autotaxin‐lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure. PloS one 7: e42627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A et al (2004). Megakaryocyte‐osteoblast interaction revealed in mice deficient in transcription factors GATA‐1 and NF‐E2. J Bone Miner Res 19: 652–660. [DOI] [PubMed] [Google Scholar]
- Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD (2008). Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nature Immunol 9: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K (2008). Historical review: megakaryopoiesis and thrombopoiesis. Blood 111: 981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoga AL, Pandey D, Welsch U, Brandl R, Siess W (2011). GPR92/LPA(5) lysophosphatidate receptor mediates megakaryocytic cell shape change induced by human atherosclerotic plaques. Cardiovasc Res 90: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss GN, Lee SC, Fells JI, Liu J, Valentine WJ, Fujiwara Y et al (2013). Mitigation of radiation injury by selective stimulation of the LPA(2) receptor. Biochim Biophys Acta 1831: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlden SA, Hillman SE, Chapman TJ, Patil R, Miller DD, Tigyi G et al (2016). Novel inhibitory effect of a lysophosphatidic acid 2 agonist on allergen‐driven airway inflammation. Am J Respir Cell Mol Biol 54: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komachi M, Sato K, Tobo M, Mogi C, Yamada T, Ohta H et al (2012). Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo . Cancer Sci 103: 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Hooper AT, Broekman MJ, Avecilla ST, Petit I, Luo M et al (2006). Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest 116: 3277–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO (2011). Direct signaling between platelets and cancer cells induces an epithelial‐mesenchymal‐like transition and promotes metastasis. Cancer Cell 20: 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Lee SC, David M, Bordet JC, Norman DD, Patil R et al (2014). Interaction of platelet‐derived autotaxin with tumor integrin alphaVbeta3 controls metastasis of breast cancer cells to bone. Blood 124: 3141–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc R, Peyruchaud O (2016). Metastasis: new functional implications of platelets and megakaryocytes. Blood 128: 24–31. [DOI] [PubMed] [Google Scholar]
- Leblanc R, Peyruchaud O (2015). New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp Cell Res 333: 183–189. [DOI] [PubMed] [Google Scholar]
- Li X, Koh AJ, Wang Z, Soki FN, Park SI, Pienta KJ et al (2011). Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner Res 26: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Umezu‐Goto M, Murph M, Lu Y, Liu W, Zhang F et al (2009). Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 15: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llona‐Minguez S, Ghassemian A, Helleday T (2015). Lysophosphatidic acid receptor (LPAR) modulators: the current pharmacological toolbox. Prog Lipid Res 58: 51–75. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM et al (2012). Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst 104: 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerschaert K, De Corte V, De Ville Y, Vandekerckhove J, Gettemans J (1998). Gelsolin and functionally similar actin‐binding proteins are regulated by lysophosphatidic acid. EMBO J 17: 5923–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter DG, Kopetz S, Hawk E, Sood AK, Loree JM, Gresele P et al (2017). Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis Rev 36: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GB, Moolenaar WH (2003). The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3: 582–591. [DOI] [PubMed] [Google Scholar]
- Miyabe Y, Miyabe C, Iwai Y, Takayasu A, Fukuda S, Yokoyama W et al (2013). Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheum 65: 2037–2047. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Selim S, Salous A, Smyth SS (2009). Blood relatives: dynamic regulation of bioactive lysophosphatidic acid and sphingosine‐1‐ phosphate metabolism in the circulation. Trends Cardiovasc Med 19: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Rivera R, Chun J (2012). Insights into the pharmacological relevance of lysophospholipid receptors. Br J Pharmacol 165: 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitopoulou I, Kaffe E, Sevastou I, Sirioti I, Samiotaki M, Madan D et al (2013). A metabolically‐stabilized phosphonate analog of lysophosphatidic acid attenuates collagen‐induced arthritis. PloS one 8: e70941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe L, Peeters K, Izzi B, Van Geet C, Freson K (2010). Regulators of platelet cAMP levels: clinical and therapeutic implications. Curr Med Chem 17: 2897–2905. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Herr D, Mutoh T, Chun J (2009). Lysophosphatidic acid (LPA) and its receptors. Curr Med Chem 17: 2897–2905. [DOI] [PubMed] [Google Scholar]
- Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G et al (2012). Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 47: 566–574. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Ye H, Huang L, Sigismund L, Macdonald T, Lynch KR (2003). Selective blockade of lysophosphatidic acid LPA3 receptors reduces murine renal ischemia‐reperfusion injury. Am J Physiol Renal Physiol 285: F565–F574. [DOI] [PubMed] [Google Scholar]
- Orosa B, Garcia S, Martinez P, Gonzalez A, Gomez‐Reino JJ, Conde C (2014). Lysophosphatidic acid receptor inhibition as a new multipronged treatment for rheumatoid arthritis. Ann Rheum Dis 73: 298–305. [DOI] [PubMed] [Google Scholar]
- Pamuklar Z, Federico L, Liu S, Umezu‐Goto M, Dong A, Panchatcharam M et al (2009). Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J Biol Chem 284: 7385–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamuklar Z, Lee JS, Cheng HY, Panchatcharam M, Steinhubl S, Morris AJ et al (2008). Individual heterogeneity in platelet response to lysophosphatidic acid: evidence for a novel inhibitory pathway. Arterioscler Thromb Vasc Biol 28: 555–561. [DOI] [PubMed] [Google Scholar]
- Pang JH, Coupland LA, Freeman C, Chong BH, Parish CR (2015). Activation of tumour cell ECM degradation by thrombin‐activated platelet membranes: potentially a P‐selectin and GPIIb/IIIa‐dependent process. Clin Exp Metastasis 32: 495–505. [DOI] [PubMed] [Google Scholar]
- Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P et al (2013). Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med 188: 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyruchaud O, David M, Macdonald TL, Lynch KR (2013). Lysophosphatidic acid (LPA) signaling in bone cancer In: Chun J, Hla T, Moolenaar W, Spiegel S. (eds). Lysophospholipid receptors. Signaling and Biochemistry. Wiley: Hoboken, NJ, pp. 627–640. [Google Scholar]
- Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P et al (2007). LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol 18: 3110–3118. [DOI] [PubMed] [Google Scholar]
- Psaila B, Lyden D (2009). The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D, Roberts I (2012). Megakaryocytes, malignancy and bone marrow vascular niches. J Thromb Haemost 10: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z (2012). Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379: 1591–1601. [DOI] [PubMed] [Google Scholar]
- Rowley JW, Chappaz S, Corduan A, Chong MM, Campbell R, Khoury A et al (2016). Dicer1‐mediated miRNA processing shapes the mRNA profile and function of murine platelets. Blood 127: 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA et al (2011). Genome‐wide RNA‐seq analysis of human and mouse platelet transcriptomes. Blood 118: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Schwertz H, Weyrich AS (2012). Platelet mRNA: the meaning behind the message. Curr Opin Hematol 19: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Weyrich AS (2013). Coordinate expression of transcripts and proteins in platelets. Blood 121: 5255–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher SM, Thotala DK, Linkous AG, Hu R, Leahy KM, Yazlovitskaya EM et al (2011). Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PloS one 6: e22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W, Tigyi G (2004). Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem 92: 1086–1094. [DOI] [PubMed] [Google Scholar]
- Su SC, Hu X, Kenney PA, Merrill MM, Babaian KN, Zhang XY et al (2013). Autotaxin‐lysophosphatidic acid signaling axis mediates tumorigenesis and development of acquired resistance to sunitinib in renal cell carcinoma. Clin Cancer Res 19: 6461–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G et al (2011). Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor‐selective antagonist. J Pharmacol Exp Ther 336: 693–700. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Bundey RA, Prodanovich PC et al (2010). A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol 160: 1699–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumura A, Fukuzawa K, Tsukatani H (1978). Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids 13: 572–574. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T et al (2002). Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether‐linked lysophosphatidic acids. Biochem J 365: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uluckan O, Eagleton MC, Floyd DH, Morgan EA, Hirbe AC, Kramer M et al (2008). APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J Cell Biochem 104: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP et al (2006). Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakikawa T, Shioi A, Hino M, Inaba M, Nishizawa Y, Tatsumi N et al (1997). Thrombopoietin inhibits in vitro osteoclastogenesis from murine bone marrow cells. Endocrinol 138: 4160–4166. [DOI] [PubMed] [Google Scholar]
- Watson SP, McConnell RT, Lapetina EG (1985). Decanoyl lysophosphatidic acid induces platelet aggregation through an extracellular action. Evidence against a second messenger role for lysophosphatidic acid. Biochem J 232: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W et al (2009). Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem 284: 17304–17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Kooi CV, Shah P, Charnigo R, Huang C, Smyth SS et al (2014). Integrin‐mediated cell surface recruitment of autotaxin promotes persistent directional cell migration. FASEB J 28: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Mao X, Guo T, Chan PY, Shaw G, Hines J et al (2017). The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin Cancer Res 23: 5112–5122. [DOI] [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG et al (1996). A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood 88: 402–409. [PubMed] [Google Scholar]
- Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T et al (2005). G protein‐coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the beta‐catenin pathway. Proc Natl Acad Sci U S A 102: 6027–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J et al (2008). Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst 100: 1630–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, Choi JW et al (2011). Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 3: 99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky A, Singh LS, Tan H, Ding H, Liang Z, Xu Y (2006). Homo‐ and hetero‐dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim Biophys Acta 1761: 1200–1212. [DOI] [PubMed] [Google Scholar]
- Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM (2004). Cyclooxygenases in cancer: progress and perspective. Cancer Lett 215: 1–20. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J et al (2009). Dual activity lysophosphatidic acid receptor pan‐antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo . Cancer Res 69: 5441–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]


