Abstract
Background and Purpose
The adenosine A1 receptor is reported to mediate several excitatory effects in the airways and has inhibitory effects in the CNS. In this study, we investigated the role of peripheral and central A1 receptors in regulating cough and airway obstruction.
Experimental Approach
Drugs were administered to guinea pigs via inhalation or i.c.v. infusion. Following the administration of different drugs, cough was induced by exposing guinea pigs to aerosolized 0.4 M citric acid. An automated analyser recorded both cough and airway obstruction simultaneously using whole‐body plethysmography.
Key Results
The A1 receptor agonist, cyclopentyladenosine (CPA, administered by inhalation), dose‐dependently inhibited cough and also inhibited airway obstruction. Similarly, CPA, administered i.c.v., inhibited both the citric acid‐induced cough and airway obstruction; this was prevented by pretreatment with the A1 receptor antagonist DPCPX (i.c.v.). Treatment with DPCPX alone dose‐dependently enhanced the citric acid‐induced cough and airway obstruction. This effect was reversed following treatment with either the glutamate GluN1 receptor antagonist D‐AP5 or the neurokinin NK1 receptor antagonist FK‐888.
Conclusions and Implications
These findings suggest that activation of either peripheral or central adenosine A1 receptors inhibits citric acid‐induced cough and airway obstruction. The data also suggest that tonic activation of central adenosine A1 receptors serves as a negative regulator of cough and airway obstruction, secondary to inhibition of excitatory glutamatergic and tachykininergic neurotransmission.
Abbreviations
- ACSF
artificial CSF
- AMPA
α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid
- AP
anteroposterior
- AVPN
airway‐related vagal preganglionic neuron
- CHS
cough hypersensitivity syndrome
- CPA
cyclopentyladenosine
- d‐AP5
dl‐2‐amino‐5‐phosphonovaleric acid
- DPCPX
cyclopentyl‐1,3‐dipropylxanthine
- DV
dorsoventral
- GluN1
glutamate ionotropic receptor NMDA type subunit 1
- ML
mediolateral
- NK1
neurokinin 1
- nTS
nucleus tractus solictarus
- PE‐20
polyethylene tubing
- Penh
enhanced pause
Introduction
Cough has been classically viewed as a simple brainstem defensive reflex in response to increased airway sensory input resulting from inhalation of foreign particles, mucus, aspiration of gastric content or due to sensitization of sensory airway nerves (Ohi et al., 2005; Canning and Mori, 2010; Mazzone et al., 2015). However, whilst the brainstem plays a critical role in regulating the reflex cough (Bonham et al., 2006; Mutolo et al., 2008; Canning and Mori, 2011; El‐Hashim et al., 2013), recent evidence shows that higher brain regions may also play an important role (Ando et al., 2016; Eccles, 2006). For example, the urge to cough can be consciously suppressed in humans (Hegland et al., 2011; Mazzone et al., 2011). Also, a placebo effect has been demonstrated to be responsible for a significant component of the action of anti‐tussive drugs (Eccles, 2002; Leech et al., 2012).
Chronic cough accounts for a large proportion of respiratory outpatients seen, but treatment options are not only limited but are also ineffective (Irwin et al., 1981; Schroeder and Fahey, 2002). One reason for the lack of efficacy of these drugs is that they are not thought to affect neuronal sensitization – an important determinant of chronic cough. Indeed, the term ‘cough hypersensitivity syndrome’ (CHS) has been recently coined and reflects both the mechanisms underlying chronic cough and the nature of its triggers (Morice et al., 2014b; Keller et al., 2017). These mechanisms include neuronal activation, sensitization and/or dysfunction, and the triggers are usually low threshold thermal, mechanical or chemical exposures (El‐Hashim and Jaffal, 2017; Morice et al., 2014a). Whilst much attention has been given to the sensitization of airway nerves, CHS is also thought to be driven by enhanced sensitization of certain regions in the CNS involved in the regulation of cough (Lee et al., 2003; El‐Hashim and Jaffal, 2017; Morice et al., 2014b).
Glutamatergic and substance P‐dependent excitatory signalling pathways have been identified as being important in the CNS processing of cough‐inducing stimuli (Mutolo et al., 2007; Smith et al., 2012). Despite these pathways being demonstrated to have this role in preclinical animal models (Canning, 2009; El‐Hashim et al., 2004b), antagonism of these pathways has failed to show any significant benefit in clinical studies (Fahy et al., 1995; Dicpinigaitis et al., 2015). In addition to possible pharmacokinetic issues, it may be that both excitatory pathways need to be simultaneously blocked in order to achieve significant inhibition of cough. Alternatively, the mechanisms underlying CHS may be associated with a dysfunction in the inhibitory inputs, rather than increased excitatory inputs, in the neural circuits of the cough centre. Recent evidence shows that a dysfunction of the opioidergic and GABAergic systems, both of which are known to be important inhibitory pathways for cough (Bolser et al., 1993; Barnabe et al., 1995; Dicpinigaitis et al., 1998; Poliacek et al., 2010), may contribute to CHS (Ando et al., 2016). This may explain why blockade of the excitatory systems has not been very successful in effectively treating cough.
Increased levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2844 have long been known to mediate pulmonary effects associated with lung diseases such as inflammation, airway hyperresponsiveness and airway obstruction (Polosa and Holgate, 2006; Burnstock et al., 2012), possibly through activation of airway sensory C‐fibres and cholinergic nerves (Hong et al., 1998; Chuaychoo et al., 2006; Reynolds et al., 2008). Adenosine activates the GPCRs, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=18, A2A, A2B and A3 receptors (also known as P1 receptors; Burnstock et al., 2012). In the CNS, adenosine has been reported to exert both inhibitory and stimulatory effects via adenosine A1 and A2A receptors respectively (Ralevic and Burnstock, 1998; Burnstock and Ralevic, 2014). Activation of the A1 receptors in the nucleus tractus solictarus (nTS), the putative cough centre, inhibits regional sympathetic responses evoked by activation of cardiopulmonary chemoreflex (Ichinose et al., 2012). In mice, an enhanced expiration response with some similarities to cough has been shown to be inhibited following activation of central adenosine A1 receptors (Kamei et al., 1994).
In this study, we investigated (i) whether airway and/or central adenosine A1 receptors play a role in regulating cough and airway obstruction in response to inhaled citric acid and (ii) the mechanisms by which CNS adenosine A1 receptors may modulate cough and airway obstruction in response to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2478.
Methods
Animals
In‐house‐bred Dunkin–Hartley guinea pigs (400–600 g) of either sex were maintained under temperature‐controlled conditions with a 12 h light/dark cycle with free access to standard chow and water ad libitum. Animals were arbitrarily assigned to control and experimental groups. All experimental protocols were approved by the Animal Welfare and Use of Laboratory Animals Committee in the Health Sciences Centre, Kuwait University, and complied with the BJP Guidelines and were carried out in accordance with the EU Directive 2010/63/EU for animal experiments and the National Institutes of Health guide for the care and use of laboratory animals (National Institutes of Health Publications No. 8023, revised 1978). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Measurement of cough response and enhanced pause
Cough and enhanced pause (Penh; a correlate of airway obstruction) were measured simultaneously in conscious unrestrained guinea pigs using whole‐body plethysmography (Buxco, Troy, NY, USA). In brief, coughs and Penh were recorded using the Buxco system analyser that differentiates coughs from other events like sneezes and has a >99.0% correlation with manual counting. Penh is a dimensionless value that reflects changes in the waveform of the box pressure signal and combines a timing comparison factor of early versus late expiration (Pause). Penh has been reported to be an index of airway function, and increases in Penh correlate with lower airway obstruction in a number of animal models including mice, guinea pigs and dogs (Hamelmann et al., 1997; Chong et al., 1998; Lewis et al., 2007; Agrawal et al., 2008; Hirt et al., 2008; El‐Hashim et al., 2011).
Implantation of chronic i.c.v. cannula
A cannula was implanted in the cerebroventricles as described previously (El‐Hashim et al., 2013). Guinea pigs were anaesthetized with a combination of ketamine (50 mg·kg−1; i.m.) and xylazine (5 mg·kg−1; i.m.). The depth of anesthesia was assessed by checking the toe‐pinch reflex. Supplemental anesthesia was administered in case of any reflex response. After 20 min, each animal was injected with the antibiotic enrofloxacin (0.25 mg·kg−1; s.c.). The fur above the skull was shaved, and the animal was placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A 2 cm midline incision was made in the skin above the skull with a sharp surgical blade – no. 10 (World Precision Instruments, Sarasota, FL, USA) – and the skull was cleaned with 3% hydrogen peroxide. A 20‐gauge stainless steel guide cannula and its suitable dummy cannula, HTX‐20T and HTX‐25R stainless steel hypo tubes (Small Parts, Inc., Logansport, IN, USA), were placed in the lowering arm of the stereotaxic apparatus. The tip of the guide cannula was placed directly above the bregma, and this was taken as the zero coordinate of the animal. The cannula was then moved in three dimensions: anteroposterior (AP), mediolateral (ML) and dorsoventral (DV). The coordinates used in our protocol (2.0 mm AP, 1.8 mm ML and 4.8 mm DV relative to bregma), which corresponded to the lateral ventricles, were based on previously published reports (Mazzone et al., 2005; El‐Hashim et al., 2011). The calculated coordinates were determined, and a small burr hole was drilled in the skull. Two additional holes were drilled for two anchor screws. The guide cannula (with the dummy cannula inside) was lowered gradually into the brain using the stereotaxic lowering arm according to the predetermined coordinates, and the cannula was cemented to the skull with screws. Surgical suturing was performed using stainless steel surgical needles with 36 mm cutting edge (1/2 circle) and silk non‐absorbable surgical sutures. Animals were treated with tramadol (1 mg·kg−1; i.m.) and enrofloxacin (0.25 mg·kg−1; s.c.) once a day for three consecutive days.
Intracerebroventricular drug administration
As described previously (El‐Hashim et al., 2011), prior to cough assessment, the dummy cannula was removed from the guide cannula. The infusion cannula, connected via polyethylene tubing (PE‐20) (Small Parts, Inc.) to a Hamilton syringe pump model (Harvard Apparatus, Holliston, MA, USA), was inserted into the guide cannula. The animals were infused with 15 μL of any drug over 50 min. The cannula was kept in place for an additional 15 min to prevent backflow leakage and for the drug to equilibrate. The accuracy of the placement was checked by injecting methylene blue through the guide cannula of randomly chosen guinea pigs at the end of the experiment.
At the end of the experiment, the guinea pigs were killed by CO2 asphyxiation. CO2 flow rate was adjusted to 5 L·min−1 and continued until breathing had completely stopped. After that, cervical dislocation was performed to ensure death. The total number of guinea pigs used in the study was 263.
Exposure to drug via inhalation
Aerosols were generated using a DeVilbiss aerogen ultrasonic nebulizer (DeVilbiss, Somerset, PA, USA), which had an aerodynamic mass median diameter range of 1–5 μm (manufacturer's indication). Animals were allowed to acclimatize for a period of 5 min in the whole‐body plethysmograph, and baseline airway function (Penh) was recorded for 2 min prior to aerosol administration. All animal groups were exposed to citric acid (0.4 M) aerosol for 10 min during which both cough and Penh were recorded and for a 5 min period thereafter (total 15 min). In one study, animals in four groups were exposed to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=380; 0.3, 0.6 and 1 mg·mL−1) or aerosolized distilled water (vehicle).
Preparation of buffers and drugs
A stock solution of CPA for aerosol delivery was prepared by dissolving it in distilled water with subsequent dilutions of 0.3, 0.6 and 1 mg·mL−1 made in distilled water. CPA for i.c.v. administration (1.8 and 3 nmol·mL−1) was made by initially dissolving CPA in distilled water and then diluting with artificial CSF (ACSF) to yield final dilutions of drug in 99% ACSF. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=386 was made by initially dissolving in ethanol with subsequent dilutions (0.6, 1, 3.3 and 33 nmol·mL−1) made in ACSF to yield final dilutions of drug in 5% ethanol. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2114 (0.1 and 1 nmol·mL−1) was made by initially dissolving in ethanol and diluting with distilled water to yield a final dilution of drug in 5 and 13% ethanol respectively. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4168 (15 nmol·mL−1) was made by initially dissolving in distilled water and diluting with ACSF to yield a final dilution of drug in 99% ACSF. Lastly, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1088 (6 nmol·mL−1) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1638 (2 pmol·mL−1) were made by initially dissolving in distilled water and diluting with ACSF to yield a final dilution in 99% ACSF. Citric acid (0.4 M) was dissolved in PBS. All drugs were freshly prepared for each experiment.
Experimental protocol and design (in accordance with BJP guidelines)
Effect of inhaled A1 receptor agonist CPA on citric acid‐induced cough and airway obstruction
Animals were arbitrarily divided into four groups. Group 1 (n = 10) was treated with the vehicle of CPA (99% ACSF). Groups 2 (n = 6), 3 (n = 6) and 4 (n = 10) were treated with 0.3, 0.6 and 1 mg·mL−1 CPA respectively. The drug was nebulized over a 10 min period followed by exposure to aerosolized citric acid (0.4 M) for 10 min. Cough and airway obstruction were assessed during the citric acid challenge and for 5 min thereafter.
Effect of the A1 receptor agonist CPA (i.c.v.) on citric acid‐induced cough and airway obstruction
Animals were arbitrarily divided into four groups. Group 1 (n = 14) was treated with the vehicle of CPA (99% ACSF). Groups 2 (n = 12) and 3 (n = 8) were treated with 1.8 and 3 nmol·mL−1 CPA respectively. The fourth group was pretreated with the adenosine A1 receptor antagonist, DPCPX (33 nmol·mL−1, n = 12), and 15 min after the infusion of DPCPX, animals were treated with CPA (3 nmol·mL−1). Fifteen minutes after infusion of CPA, all animals were exposed to aerosolized citric acid (0.4 M) for 10 min. Cough and airway obstruction were assessed during the citric acid challenge and 5 min thereafter.
Effect of the adenosine A1 receptor antagonist DPCPX (i.c.v.) on citric acid‐induced cough and airway obstruction
Animals were arbitrarily divided into five groups. Group 1 (n = 20) was pretreated with the vehicle of DPCPX (5% ethanol in ACSF). Group 2 (n = 9), group 3 (n = 12), group 4 (n = 15) and group 5 (n = 17) were treated with 0.6, 1, 3.3 and 33 nmol·mL−1 of DPCPX respectively. After 15 min, all treatment groups were exposed to aerosolized citric acid (0.4 M) for 10 min. Cough and airway obstruction were assessed during the citric acid challenge and 5 min thereafter.
Effect of blockade of the GluN1 receptor on DPCPX (i.c.v.)‐enhanced citric acid‐induced cough and airway obstruction
Animals were arbitrarily divided into four groups. Group 1 (n = 8) was pretreated with the vehicle of DPCPX. Fifteen minutes after the infusion of vehicle of DPCPX, animals were treated with the vehicle of d‐AP5 (5% water in ACSF). Group 2 (n = 11) was pretreated with the vehicle of DPCPX. Fifteen minutes after the infusion of the vehicle, animals were treated with d‐AP5 (15 nmol·mL−1). Group 3 (n = 9) was pretreated with 3.3 nmol·mL−1 of DPCPX. Fifteen minutes after the infusion of DPCPX, animals were treated with vehicle of d‐AP5. Group 4 (n = 12) was pretreated with 3.3 nmol·mL−1 of DPCPX, and 15 min later, animals were treated with the glutamate http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=455 antagonist d‐AP5 (15 nmol·mL−1). Fifteen minutes after i.c.v. infusion of d‐AP5 or its vehicle, all treatment groups were exposed to aerosolized citric acid (0.4 M) for 10 min. Cough and airway obstruction were assessed during the citric acid challenge and 5 min thereafter.
Effect of blockade of the NK1 receptor on DPCPX (i.c.v.)‐enhanced citric acid‐induced cough and airway obstruction
Animals were arbitrarily divided into five groups. Group 1 (n = 13) was treated with the vehicle of DPCPX (5% ethanol in ACSF). Fifteen minutes after infusion with the vehicle of DPCPX, animals were treated with the vehicle of FK‐888 (5% ethanol in ACSF). Group 2 (n = 4) was pretreated with the vehicle of DPCPX. Fifteen minutes after infusion with the vehicle of DPCPX, animals were treated with the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=360 antagonist FK‐888 (1 nmol·mL−1). Group 3 (n = 14) was pretreated with DPCPX (3.3 nmol·mL−1), and 15 min later, they were treated with the vehicle of FK‐888 (13% ethanol in ACSF). Group 4 (n = 9) was pretreated with 3.3 nmol·mL−1 of DPCPX and then treated 15 min later with FK‐888 (0.1 nmol·mL−1). Group 5 (n = 5) was pretreated with 3.3 nmol·mL−1 of DPCPX and then treated 15 min later with FK‐888 (1 nmol·mL−1). Fifteen minutes after i.c.v. infusion of FK‐888 or its vehicle, all treatment groups were exposed to aerosolized citric acid (0.4 M) for 10 min. Cough and airway obstruction were assessed during the citric acid challenge and 5 min thereafter.
Effect of blockade of GABAB and opioid μ receptors on citric acid‐induced cough and airway obstruction
Animals were arbitrarily divided into three groups. Group 1 (n = 10) was pretreated with the vehicle of CGP 55845 and the opioid http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319 antagonist naloxone hydrochloride (99% ACSF). Group 2 (n = 8) and group 3 (n = 9) were treated with CGP 55845 (6 nmol−1; http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=242 antagonist) and naloxone (2 pmol−1; μ‐opioid receptor antagonist) respectively. Fifteen minutes after i.c.v. infusion of drugs or their vehicle, all treatment groups were exposed to aerosolized citric acid (0.4 M) for 10 min. Cough and airway obstruction were assessed during the citric acid challenge and 5 min thereafter.
Statistical analyses
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). For cough experiments, data are expressed as mean number of coughs during a 15 min period ± SEM. Airway obstruction is presented as an absolute change in Penh and expressed as mean ± SEM. Differences in the degree of airway obstruction between different groups were determined as the mean AUC for the Penh values for 15 min for each animal. Data analysis was performed blind. All treatment groups were initially tested for normality and the appropriate statistical test applied depending on the distribution of the data. Normally distributed data were analysed using an ANOVA test followed by Bonferroni post hoc test, and non‐normally distributed data were analysed using Kruskal–Wallis test. All statistical analyses were performed using the Sigmaplot, version 12.3, Systat Software, Inc. SigmaPlot for Windows. In all cases, differences were considered significant at P < 0.05. The difference in group sizes was due to two factors: (i) inconsistency in supply of our in‐house bred guinea pigs and (ii) death of animals at various times after anaesthesia or even removal of the cannula. However, all groups were always time matched.
Materials
Chemicals used were PBS tablets, citric acid (Sigma‐Aldrich, St. Louis, MO, USA), 8‐cyclopentyl‐1,3‐dipropylxanthine (DPCPX), N 6‐cyclopentyladenosine (CPA) and dl‐2 aminuteso‐5‐phosphonopentanoic acid (d‐AP5) (Sigma‐Aldrich), FK‐888, CGP 55845, naloxone hydrochloride (Tocris; Cookson Ltd., Langford, UK), tramadol (Grünenthal, Aachen, Germany), enrofloxacin (Baytril®; Bayer AG, Leverkusen, Germany), KCl (BDH Laboratory, Poole, UK), NaH2PO4 (Sigma‐Aldrich), NaCl (Merck, Darmstadt, Germany), D(+)‐glucose anhydrous, NaHCO3 (Merck), MgCl2 (Fluka; BioChemika, Messerschmitt, Switzerland), CaCl2 (Surechem, Suffolk, UK), ketaminutese (Hikma Pharmaceuticals, Amman, Jordan), xylazine (Sigma‐Aldrich), stainless steel surgical needles with 36 mm cutting edge (1/2 circle) (Mani, Inc., Tochigi, Japan), silk non‐absorbable surgical sutures (Look Surgical Specialties Corporation, Wyomissing, PA, USA), surgical blades (Feather Safety Razar Co., Osaka, Japan), dual syringe pump‐model 11 Plus (Harvard Apparatus), polyethylene tubes (PE‐20) and HTX‐20T and HTX‐25R stainless steel hypo tubes (Small Parts, Inc.). HTX‐20T and HTX‐25R hypo tubes were cut to form 20‐gauge guides and infusion cannulae respectively.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to the corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017,b).
Results
Effect of inhaled A1 receptor agonist CPA on citric acid‐induced cough and airway obstruction
Inhalation of CPA dose‐dependently inhibited the citric acid‐induced cough response (Figure 1A). Although inhalation of CPA did not cause any immediately noticeable changes in Penh (data not shown), it dose‐dependently inhibited the citric acid‐induced airway obstruction (Figure 1B, C).
Figure 1.
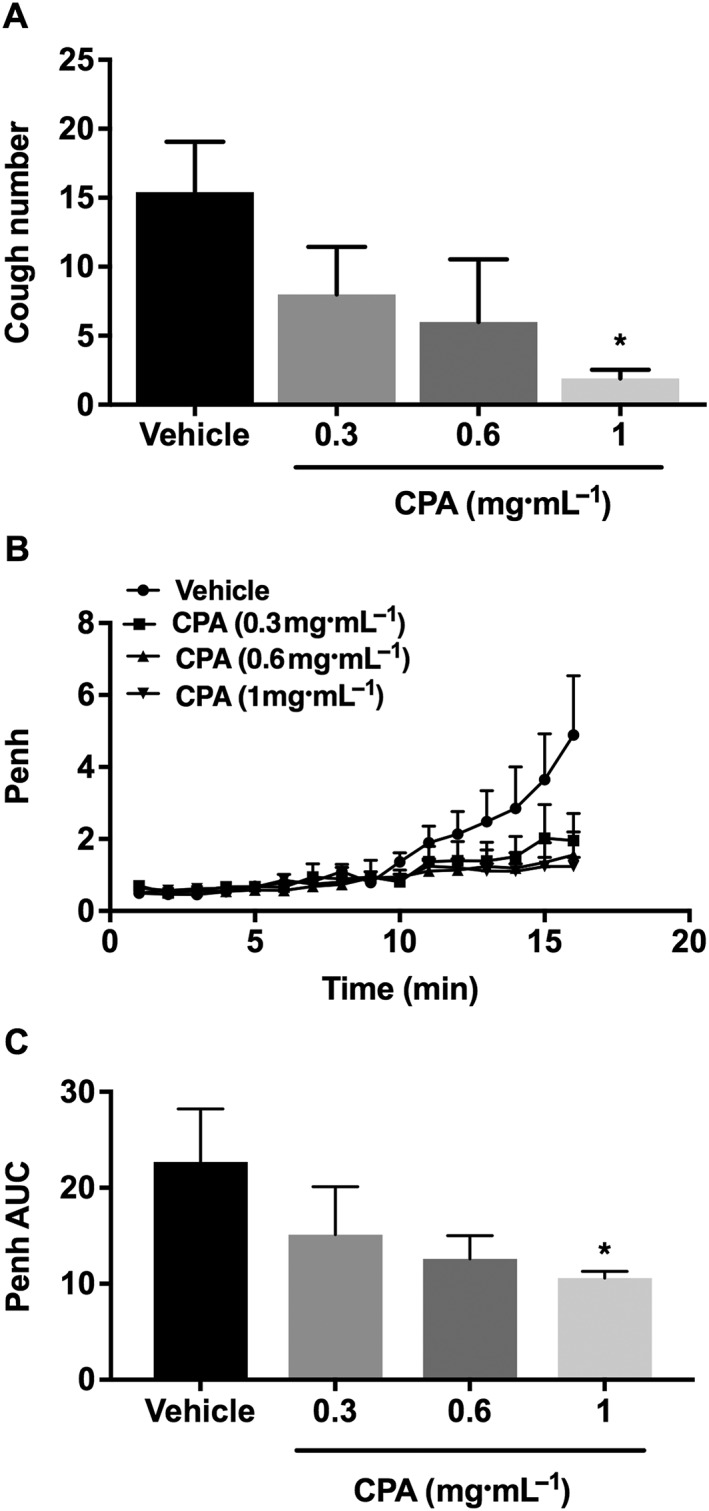
Effect of inhaled A1 receptor agonist, CPA, 0.3, 0.6 and 1 mg·mL−1 (n = 6–10) on citric acid‐induced cough (A), changes in Penh (B) and Penh AUC (C). Values represent means + SEM; *P<0.05, compared to vehicle‐treated animals.
Effect of CPA (i.c.v.) on citric acid‐induced cough and airway obstruction
CPA, at 3 but not at 1.8 nmol·mL−1, significantly reduced the citric acid‐induced cough by more than 60% compared with the vehicle group (Figure 2A). This effect was reversed in animals pretreated with DPCPX and was comparable with the vehicle‐treated group (Figure 2A). CPA, at both 1.8 and 3 nmol·mL−1, significantly reduced the citric acid‐induced airway obstruction compared with the vehicle‐treated group (Figure 2B, C). Again, treatment with DPCPX completely reversed the CPA (at 3 nmol·mL−1)‐induced reduction of the citric acid‐induced airway obstruction compared with vehicle (Figure 2B, C).
Figure 2.
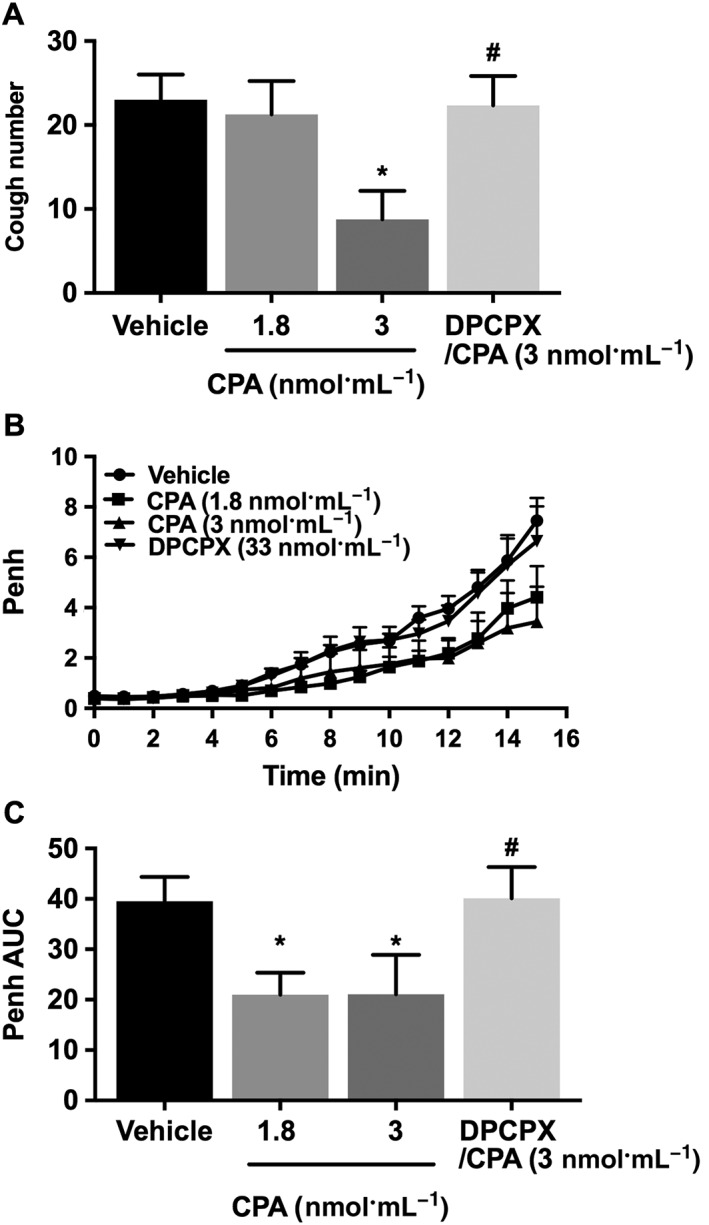
Effect of treatment with CPA (i.c.v.) 1.8 nmol·mL−1 (n = 12) and 3 nmol·mL−1 (n = 8) and pretreatment with DPCPX (33 nmol·mL−1; n = 12) on citric acid‐induced cough (A), changes in Penh (B) and Penh AUC (C). Values represent means + SEM. * P < 0.05, significant difference compared with vehicle‐treated animals. # P < 0.05, significant difference compared with CPA (3 nmol·mL−1)‐treated animals.
Effect of the adenosine A1 receptor antagonist DPCPX (i.c.v.) on citric acid‐induced cough and airway obstruction
During the DPCPX infusion, no cough was induced, and the average Penh values, immediately after the infusion, were not significantly different between the groups (data not shown). However, treatment of animal with DPCPX (0.6, 1, 3.3 and 33 nmol·mL−1) dose‐dependently enhanced the citric acid‐induced cough response compared with control animals (Figure 3A). This was significant at doses of 3.3 and 33 nmol·mL−1. Similarly, treatment with DPCPX (0.6, 1, 3.3 and 33 nmol·mL−1) dose‐dependently enhanced the citric acid‐induced airway obstruction compared with vehicle treatment (Figure 3B, C). In subsequent experiments, we used DPCPX at 3.3 mol·mL−1 as this dose resulted in a maximum enhancement of the parameters measured (mean cough number increased by 116.0%, and mean Penh AUC increased by 95.2%).
Figure 3.
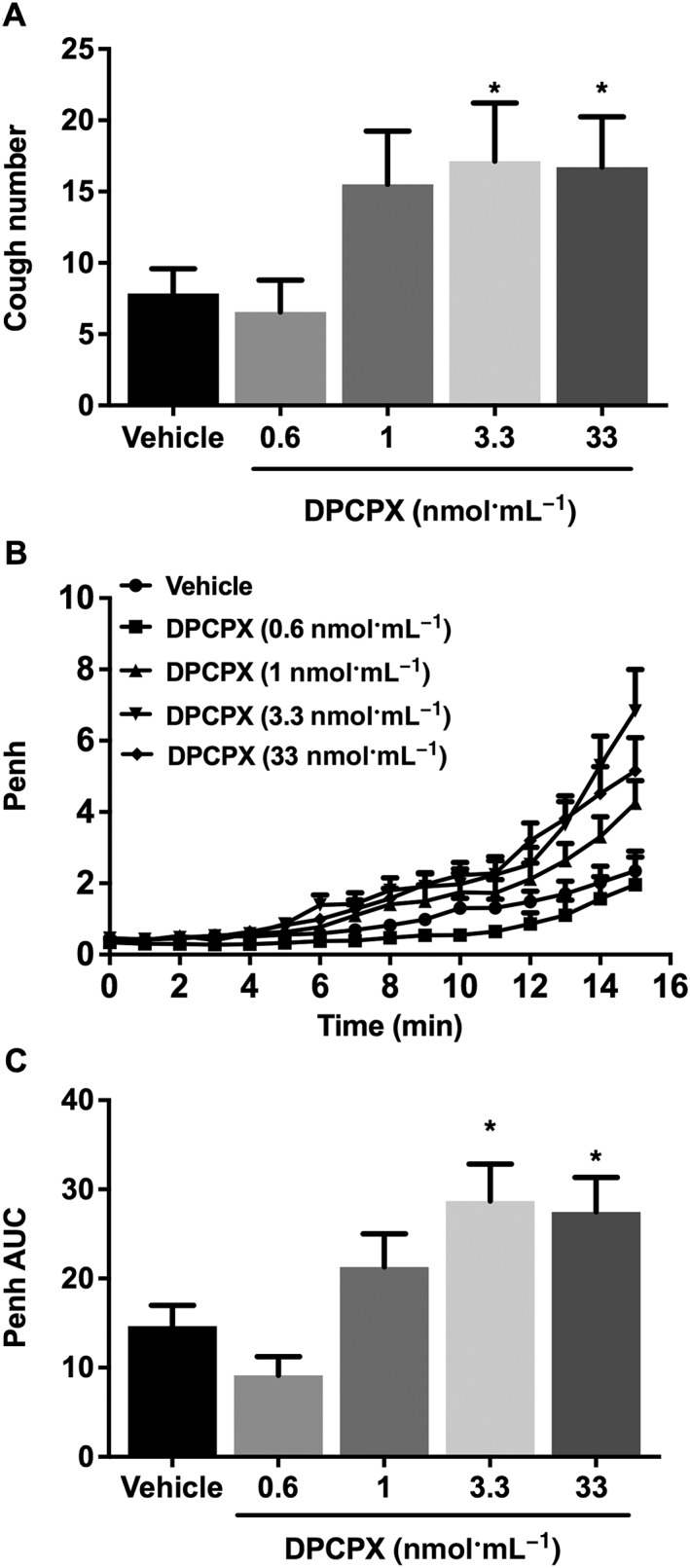
Effect of DPCPX (0.6, 1, 3.3 and 33 nmol·mL−1; i.c.v., n = 9–20) treatment on citric acid‐induced cough (A), changes in Penh (B) and Penh AUC (C). Values represent means + SEM. * P < 0.05, significant difference compared with vehicle‐treated animals.
Effect of blockade of the GluN1 receptor on DPCPX (i.c.v.)‐enhanced citric acid‐induced cough and airway obstruction
Treatment with http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=455 antagonist d‐AP5 (15 nmol·mL−1) significantly inhibited the DPCPX (3.3 nmol·mL−1)‐enhanced citric acid‐induced cough compared with vehicle pretreated animals (Figure 4A) and also significantly reduced the DPCPX‐enhanced citric acid‐induced airway obstruction compared with vehicle treatment (Figure 4B, C). Treatment with d‐AP5, alone, did not have any significant effects on either baseline cough or Penh compared with vehicle‐treated guinea pigs (P > 0.05; Figure 4A–C).
Figure 4.
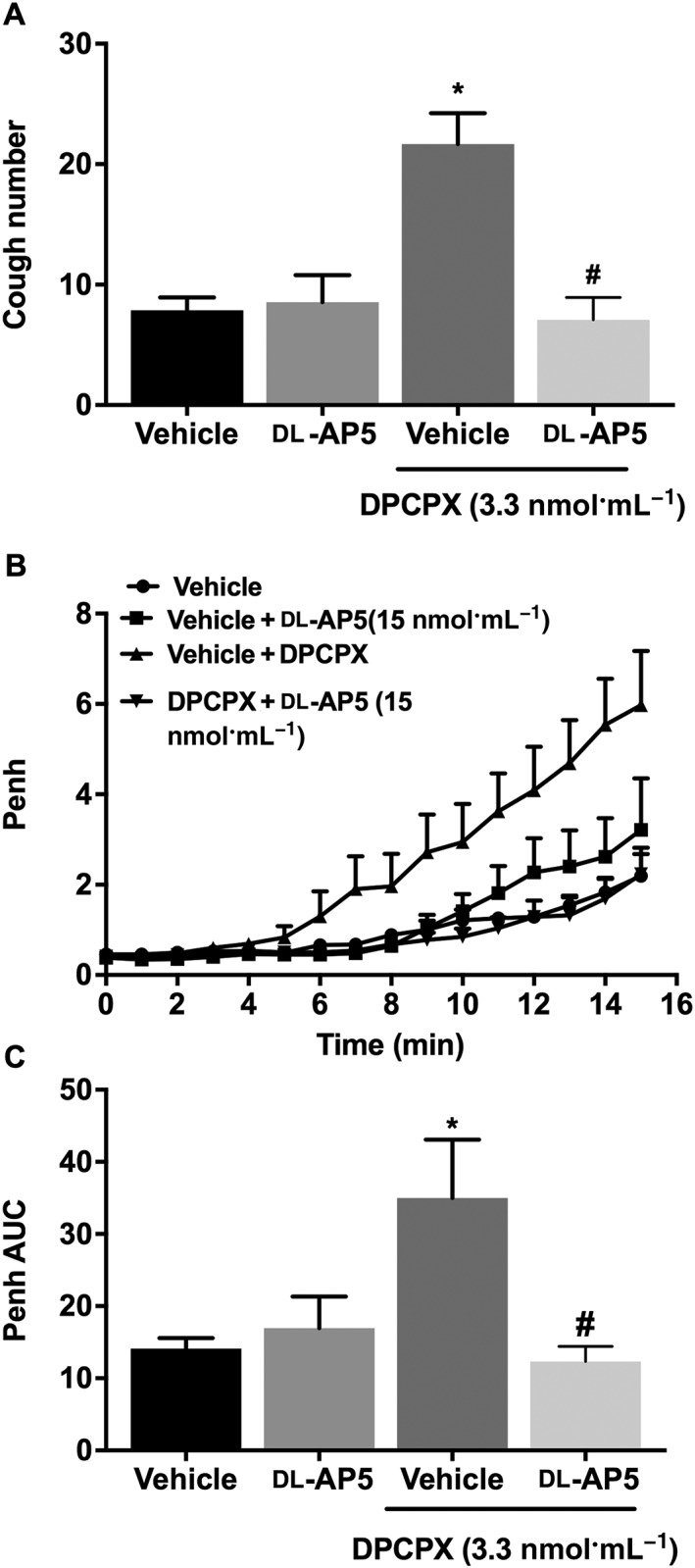
Effect of pretreatment with d‐AP5 (15 nmol·mL−1; i.c.v., n = 12) on DPCPX‐enhanced citric acid‐induced cough (A), changes in Penh (B) and Penh AUC (C). Values represent means + SEM. * P < 0.05, significant difference compared with vehicle alone (n = 8) and d‐AP5 alone (n = 11)‐treated animals. # P < 0.05, significant difference compared with DPCPX (3.3 nmol·mL−1)‐treated animals.
Effect of blockade of the NK1 receptor on DPCPX (i.c.v.)‐enhanced citric acid‐induced cough and airway obstruction
Treatment with the NK1 receptor antagonist FK‐888 (at 0.1 and 1 nmol·mL−1) dose‐dependently reduced the DPCPX (3.3 nmol·mL−1) enhancement of citric acid‐induced cough compared with vehicle; this was a significant 72% reduction at the 1 nmol·mL−1 dose (Figure 5A). Similarly, treatment with FK‐888 dose‐dependently reduced the DPCPX enhancement of citric acid‐induced airway obstruction; this was a significant 74.2% (Figure 5B) reduction at the 1 nmol·mL−1 dose. Treatment with FK‐888 (1 nmol·mL−1) did not have any significant effect on either baseline cough or Penh compared with vehicle‐treated guinea pigs (P > 0.05; Figure 5A–C).
Figure 5.
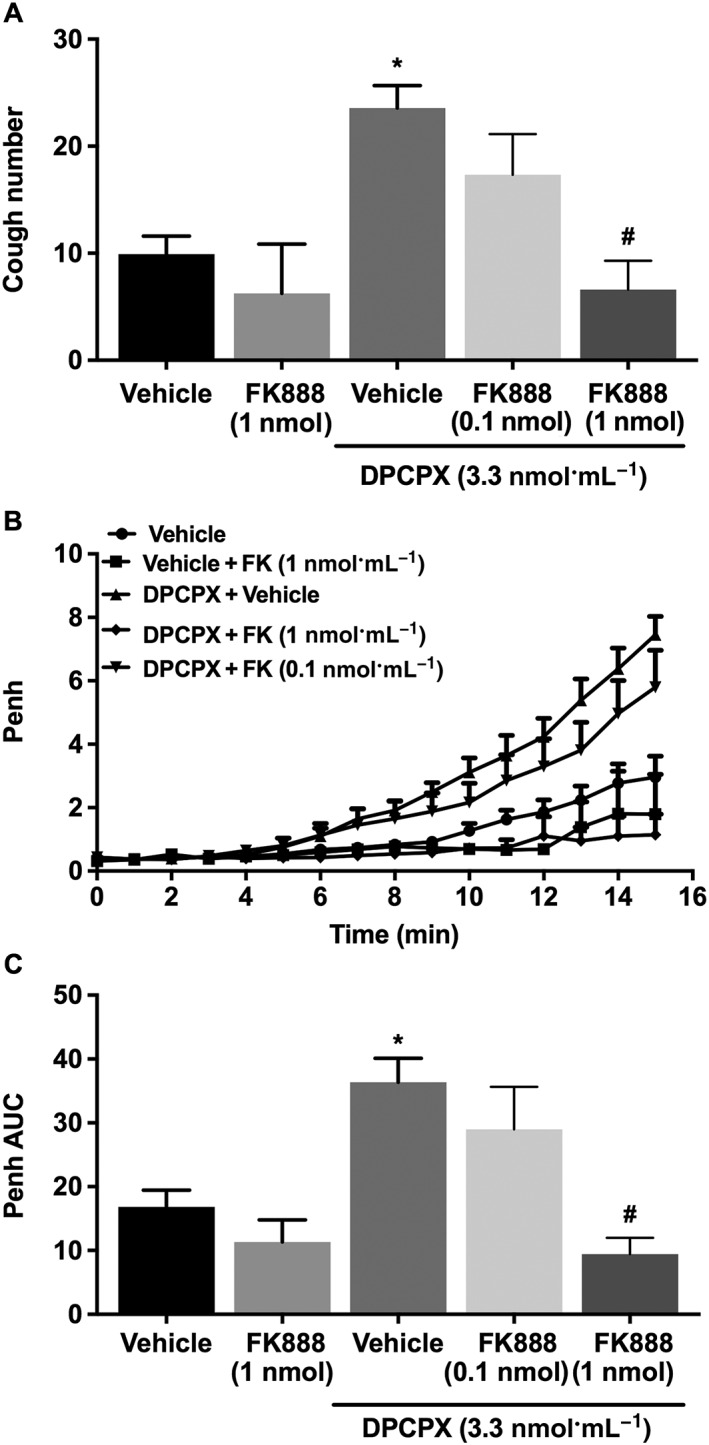
Effect of pretreatment with FK‐888 (0.1 nmol·mL−1; i.c.v., n = 9 and 1.0 nmol·mL−1, n = 5) on DPCPX‐enhanced citric acid‐induced cough (A), changes in Penh (B) and Penh AUC (C). Values represent means + SEM. * P < 0.05, significant difference compared with vehicle alone (n = 13) and FK‐888 alone (n = 4)‐treated animals. # P < 0.05, significant difference compared with DPCPX (3.3 nmol·mL−1)‐treated animals.
Effect of blockade of the GABAB and opioid μ receptors on citric acid‐induced cough and airway obstruction
Treatment of guinea pigs with the GABAB receptor antagonist, CGP 55845 (6 nmol·mL−1, i.c.v.), or the μ receptor antagonist, naloxone (2 pmol·mL−1, i.c.v.), did not affect the citric acid‐induced cough (Figure 6A) nor the citric acid‐induced airway obstruction (Figure 6B, C).
Figure 6.
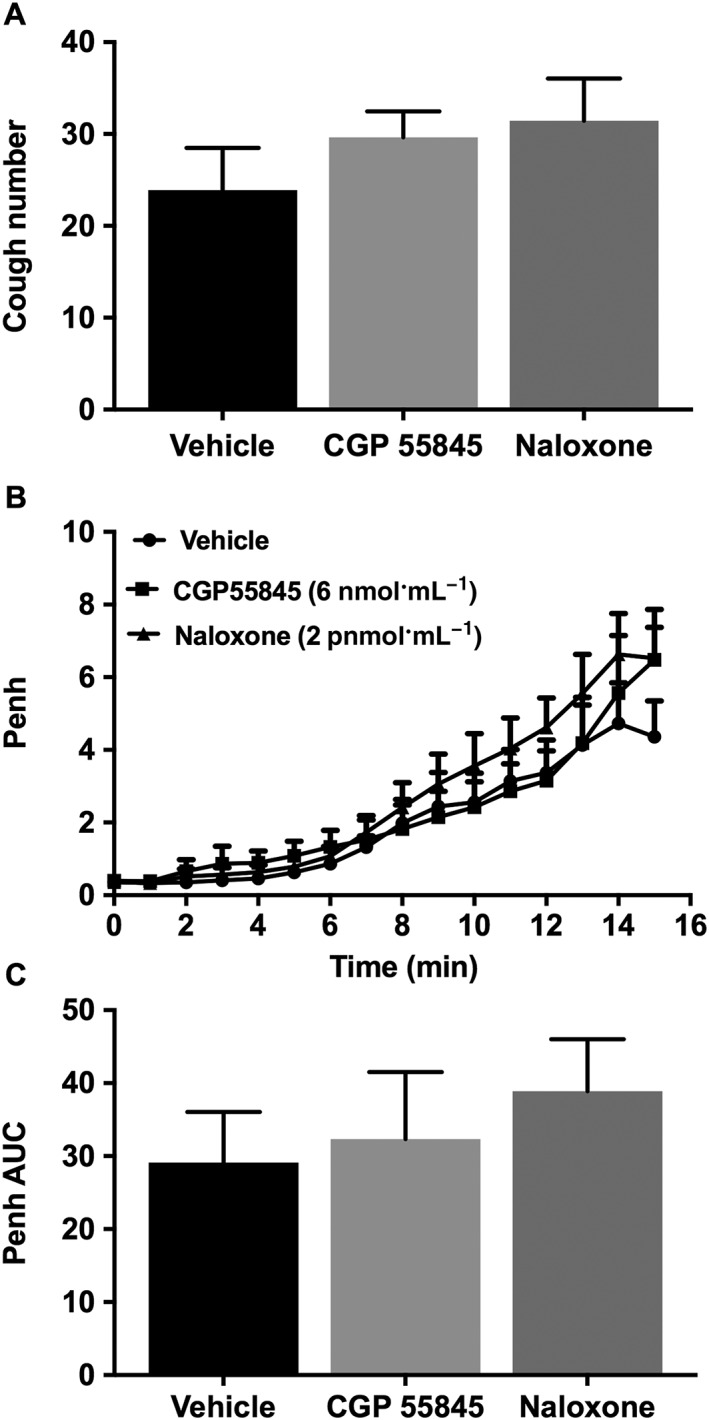
Effect of CGP 55845 (6 nmol·mL−1; i.c.v., n = 8) and naloxone (2 pmol·mL−1; i.c.v., n = 9) on citric acid‐induced cough (A), changes in Penh (B) and Penh AUC (C). Values represent means + SEM.
Discussion
The classical inhibitory pathways that are thought to regulate the cough reflex are the opioidergic and GABAergic, in addition to the more recently described cannabinergic neurotransmission (Dicpinigaitis and Dobkin, 1997; Kotzer et al., 2000; McLeod et al., 2002; Patel et al., 2003). In this study we showed, for the first time, that activation of the adenosine A1 receptor, both in the airways and centrally, inhibits cough and airway obstruction. We also showed that tonic activation of the central adenosine A1 receptors acts as ‘a braking mechanism’ to limit cough and airway obstruction via inhibition of excitatory glutamatergic and tachykininergic transmission.
Ex vivo and in vivo studies have shown that adenosine, via the A1 receptor, can activate airway C‐fibre terminals, specifically those arising from the nodose ganglia but not those of jugular origin (Hong et al., 1998; Chuaychoo et al., 2006). Stimulation of airway A1 receptors in allergic animals and patients with respiratory diseases such as asthma also results in bronchoconstriction, whereas adenosine has no effects in naïve animals or normal individuals (el‐Hashim et al., 1996; Polosa and Holgate, 2006; Chou et al., 2008). On the other hand, adenosine has also been reported to induce relaxation of precontracted tracheal spirals (Darmani and Broadley, 1986). Therefore, whether adenosine constricts or relaxes airways is not entirely clear. Our data show that CPA, an A1 receptor agonist, administered by aerosol, dose‐dependently inhibited the citric acid‐induced cough. This is in line with findings from a very recent study showing that adenosine inhibited coughing evoked by different stimuli in both anaesthetized and conscious models of cough (Chou et al., 2017). Our results are also consistent with data from a clinical study showing that exposure to AMP (adenosine precursor) decreased the cough sensitivity to capsaicin (Basoglu et al., 2017).
Inhaled CPA did not result in any changes in Penh. This is consistent with the data from both clinical and non‐clinical studies showing that neither naïve animals nor healthy non‐asthmatic individuals do not respond to adenosine (el‐Hashim et al., 1996; Basoglu et al., 2017). In contrast to the lack of direct effect on Penh, exposure to CPA dose‐dependently reduced the citric acid‐induced airway obstruction. This is consistent with the inhibitory effects of CPA on citric acid‐induced cough and is in agreement with findings from earlier studies showing that adenosine induces relaxation of guinea pig, isolated tracheal spirals and airway‐perfused lungs from naïve animals constricted with carbachol (Darmani and Broadley, 1986; Thorne and Broadley, 1992). Therefore, the bronchoconstrictor versus bronchodilator response to adenosine in animal models of asthma or asthmatic patients compared with that of naïve animals, respectively, could be partly due to the differences in the expression of adenosine receptor subtypes between diseased and normal airways.
Given that the inhaled adenosine A1 receptor agonist CPA suppresses cough and that adenosine has inhibitory actions in several CNS regions (caudal regions of the nTS, the hypoglossal nucleus and the ventrolateral) (Bisserbe et al., 1985; St Lambert et al., 1996), we sought to determine whether CPA, administered centrally, can also modulate the citric acid‐induced cough and airway obstruction. I.c.v. administration of CPA dose‐dependently inhibited both responses; this was prevented by pretreatment with the adenosine A1 receptor antagonist DPCPX. A previous study, using mice, showed that i.c.v. injection of the adenosine A1 receptor antagonist, N 6‐cyclohexyladenosine, reduced enhanced expiration – a reflex with some similarities to cough (Kamei et al., 1994). Although this was described as a ‘cough response’, it is generally accepted that mice and rats do not possess a cough reflex but rather an expiration reflex originating from the larynx (Belvisi and Bolser, 2002). Nonetheless, the suppressive effects on the breathing response highlight the inhibitory role of the adenosine A1 receptors in the brainstem. Our findings are also generally consistent with studies showing that inhibitory effects of adenosine A1 receptors are dominant in most central structures (Ralevic and Burnstock, 1998; Tupone et al., 2013). The nTS is considered to be an important site for termination of vagal afferent nerves and thus a key site for control of autonomic breathing and sympathetic activities, both during resting conditions and during an acute hypoxic response similar to the situation following citric acid challenge and reflexes such as cough. Our findings are also in line with other studies, which have reported inhibition of neural and cardiopulmonary chemoreflex responses following activation of adenosine A1 receptors in nTS – an effect blocked by treatment with an A1 receptor antagonist (Ginsborg and Hirst, 1972; Eldridge et al., 1985). Although the nTS is the most studied CNS region in the context of cough, the paratrigeminal nucleus (Pa5), located in the dorsalateral pole of the medullary spinal trigeminal tract, with ascending pathways that terminate broadly throughout the CNS, has been shown to receive primary afferent inputs from the airways (Driessen et al., 2015). Hence, the involvement of regions in CNS, other than the nTS, in the adenosine A1 receptor – mediated modulation of reflexes such as cough, cannot be ruled out.
Our data also show that CPA (i.c.v.) dose‐dependently inhibited citric acid‐induced airway obstruction. This suggests that activation of central A1 receptors plays a role in limiting the airway response to bronchoconstrictor stimuli such citric acid. It is well known that full expression of not only the cough reflex but also bronchoconstriction requires sensory fibres to ascend in the vagus nerve and enter the brain stem through the solitary tract, making their first synapse with the nTS second‐order neurons (Bonham and Joad, 1991; Haxhiu et al., 1997). Indeed, the anatomical convergence of visceral afferent nerve fibre subtypes in the nTS may explain why different organ reflexes such as cough, bronchospasm and bradycardia tend to occur at the same time under similar physiological conditions (Mazzone and Canning, 2002a). In the nTS, the afferent vagal terminals responsible for transmitting information from the airway sensory receptors, related to airway tone, form a distinct wiring organization with specific second‐order neurons that project to airway‐related vagal preganglionic neurons (AVPNs) (Haxhiu et al., 2005). Several studies have reported an important role for the brainstem in controlling the airway calibre and show that the net airway motor response results not only from local interaction of airway afferent and efferent nerves but also due to interaction at the brainstem of airway sensory vagal projections (Solomon, 1998; Haxhiu et al., 2000; Canning et al., 2004).
Based on the findings that adenosine A1 receptor activation suppresses both citric acid‐induced cough and airway obstruction, we hypothesized that tonic activation of the A1 receptor may ‘switch off’ a cough response. This hypothesis was tested by blocking the A1 receptor with a competitive antagonist, DPCPX. Our data showed that DPCPX dose‐dependently enhanced the citric acid‐induced cough and airway obstruction. This suggests that during activation of airway responses, such as cough and airway obstruction, adenosine release in brain regions that regulate cough, such as the nTS, may be increased thereby enhancing adenosine A1 receptor‐mediated purinergic transmission. The effect of this is to limit the frequency and intensity of cough and airway obstruction, thus acting as ‘a braking system’. These data concur with previous studies showing that blockade of the adenosine A1 receptor in the nTS significantly changes the pattern of autonomic responses in pathological conditions such as hypoxia and ischaemia (Park et al., 1988; Scislo and O'Leary, 2006). Indeed, a recent study has shown that the cardiopulmonary reflex‐mediated inhibition of renal and adrenal sympathetic activity is significantly attenuated during severe haemorrhage. This was shown to be due to increased adenosine release as the effect was reversed by blockade of adenosine A1 receptors in the caudal nTS (Minic et al., 2014).
To address the issue of how central A1 receptors could control these airway protective reflexes, we assessed whether blockade of GluN1 receptors would affect the DPCPX‐enhanced citric acid‐induced cough and airway obstruction. The potent GluN1 antagonist d‐AP5 (Jafari‐Sabet et al., 2005) completely blocked the DPCPX‐enhanced cough. This effect was not due to inhibition of baseline cough, since treatment with d‐AP5 alone had no effect. The lack of effect on baseline cough was surprising, since central GluN1 receptors were previously shown to have a role in the mediation of cough (Canning, 2009). This may have been due to an already low cough response, possibly because of the use of ethanol as a solvent/vehicle to dissolve DPCPX. Ethanol has been reported to have inhibitory effects in the CNS possibly mediated through increased adenosine release, increased GABAergic transmission or inhibition of GluN1 receptors (Peoples and Stewart, 2000; Theile et al., 2008; Ruby et al., 2010). Another possible explanation may be the medium dose of the citric acid used in this study (0.4 M), not the usual higher doses of 0.6 to 1 M, which was chosen so as to not to induce maximal cough and airway response stimulation. Whether any of these factors, singly or in combination, may have contributed to the decrease in the cough response in controls with ethanol remains to be determined.
The interaction between the A1 receptor‐mediated purinergic signalling system and glutamate/GluN1 receptors is likely taking place in the nTS (see Figure 7), where adenosine A1 receptors and glutamate/GluN1 receptors are co‐expressed (Bisserbe et al., 1985; St Lambert et al., 1996; Haxhiu et al., 2005). Interestingly, an interaction between adenosine A1 receptor‐mediated purinergic signalling system and glutmate/GluN1 receptors, in several other brain regions, has also been reported to control glutamate‐induced neuronal excitability and excitotoxicity, primarily via presynaptic A1₁ receptor‐mediated inhibition of glutamate release (Poli et al., 1991; Yang et al., 2007; Nascimento et al., 2010; Rau et al., 2014).
Figure 7.
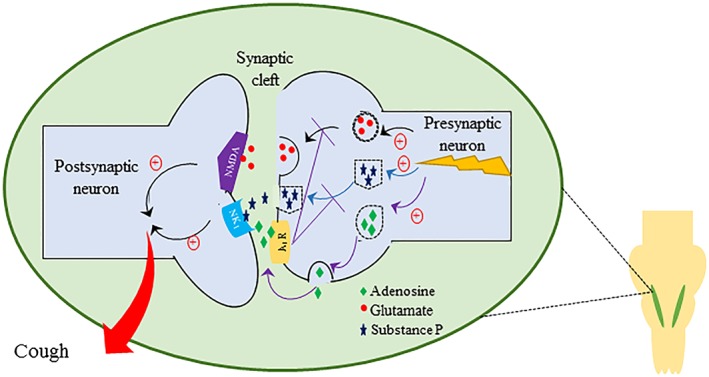
Summary of our findings and a proposed mechanism for the interaction between the adenosine/A1 receptor and glutamate/GluN1 (NMDA) and the substance P/NK1 receptor signalling pathways in controlling reflexes such as cough and airway obstruction. Blockade of the A1 receptor results in an increased cough response and airway obstruction that is reversed following antagonism of the GluN1 and NK1 receptors. This suggests that stimulation of airway nerves results in an increased central adenosine release which, via the A1 receptor, limits the cough response possibly by reducing central glutamate and substance P release. We therefore propose that in conditions such as CHS, this cough inhibitory adenosine pathway may be dysfunctional.
Our data also showed that d‐AP5 completely reverses the DPCPX‐enhanced citric acid‐induced airway obstruction. This further highlights the important role for brainstem glutamate/GluN1 receptors in mediating signals from sensory afferents to the brainstem and also underscores the inhibitory role of adenosine A1 receptors on this pathway. These findings are in line with studies showing that glutamate release in the nTS is increased following airway sensory nerve stimulation, and this also correlated with airway smooth muscle contraction (Haxhiu et al., 2000). Moreover, denervation of the airways inhibits glutamate release in the nTS (Haxhiu et al., 2005). It is thus of interest to note that glutamate‐mediated EPSPs in the AVPN, in the rostral nucleus ambiguous, results in increased ACh‐induced bronchoconstriction in the airways (Haxhiu et al., 2005; Haxhiu et al., 2000). In this regard, both GluN1 and AMPA receptors have been reported to be involved in transmitting the bronchoconstrictive inputs from the airways to the nTS to the AVPN.
In this study, we also investigated whether the substance P/NK1 receptor, another well‐established pathway in cough, is also involved in the DPCPX‐enhanced cough and airway obstruction. Our data show that there was a dose‐dependent reduction in the DPCPX‐enhanced cough following blockade of the substance P/NK1 receptor pathway. This effect was not due to interference with the baseline cough since blockade of the NK1 receptor with FK‐888 alone had no significant effect. This is in contrast to previous studies that have reported central substance P/NK1 receptor signalling is involved in the mediation of cough. The lack of effect of FK‐888 on baseline cough may be due to the already low baseline cough. Nevertheless, it is clear that treatment with FK‐888 significantly inhibited the DPCPX‐enhanced cough. This implies that activation of the adenosine A1 receptor results in reduced excitatory tachykininergic transmission and points to an interaction between the adenosine A1 receptor and the central substance P/NK1 receptor signalling pathway. There is a paucity of studies looking at this interaction in the CNS, and only few studies have looked at this interaction in the peripheral nervous system. For example, endogenous adenosine has been reported to inhibit evoked substance P release from perifused networks of myenteric ganglia (Moneta et al., 1997). Adenosine A1 receptors have also been demonstrated to inhibit tachykinin release from perifused enteric nerve endings (Christofi et al., 1990; Broad et al., 1992). Taken together, these studies indicate that adenosine receptor(s), at least on the myenteric nerve endings, are coupled negatively to tachykinin release. Thus, it is possible that activation of A1 receptors, on tachykininergic nerves in the nTs, or other cough controlling brain regions, decreases the release of excitatory substance P (see Figure 7). Furthermore, treatment with FK888 also resulted in dose‐dependent reduction in the DPCPX‐enhanced airway obstruction as evidenced by the reduction in Penh. This suggests that central substance P/NK1 receptor signalling affects the airway obstruction response. This is in agreement with reports that i.c.v. administration of substance P induces a significant degree of bronchospasm and that bradykinin‐induced hyperresponsiveness to histamine is reversed by centrally administered neurokinin receptor antagonists (Mazzone and Canning, 2002a; Mazzone and Canning, 2002b).
Since activation of the A1 receptor inhibited both the citric acid‐induced cough and airway obstruction, and its blockade potentiates both, we investigated whether blockade of the GABAergic and opioidergic pathways would also result in the enhancement of cough or airway obstruction. If this was the case, it would imply that they are also tonically activated in response to tussigenic stimulation. Our data show that blockade of the GABAB or the μ receptors, at doses shown to have effects in vivo (Takahama and Shirasaki, 2007), had no significant effect on cough or airway obstruction. These findings suggest that the role of the opioidergic and the GABAergic systems, in the cough reflex, is different from that of the adenosine A1 receptor‐mediated purinergic pathway and that the latter may play a distinct role in controlling cough.
In summary, our data show that activation of airway and central adenosine A1 receptors suppress citric acid‐induced cough and airway obstruction whilst blockade of central adenosine A1 receptors has an enhancing effect on both these responses. This is likely to be secondary to modulation of central excitatory glutamatergic and tachykininergic activity in the nTS or other brain regions that regulate cough (Figure 7). Thus, adenosine A1 receptors act as a ‘braking system’ to limit excessive airway responses. Our findings may have relevance to patients with chronic cough where their demonstrated CHS may not be due to enhanced activity of the central excitatory pathways but rather to decreased activity of the inhibitory pathways such as the A1 receptor‐mediated purinergic pathway.
Author contributions
A.Z.E.‐H. devised the experiments and wrote the manuscript. S.M. and F.A.‐S. performed the experiments and the statistical analyses.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
We are greatly indebted to our friend and mentor the late Dr Dom Spina, a great respiratory pharmacologist, for his insightful feedback on the work and constructive criticism of this manuscript. We are also grateful to Professors Samuel Kombian and Yunus Luqmani for their help with the preparation of the manuscript and to Ala Abdelali for preparing the schematic diagram. We also acknowledge the support of Ms Ola Zahran and Mr Sunny Ojoko from the Animal Resources Center of the Health Sciences Centre, Kuwait University.
El‐Hashim, A. Z. , Mathews, S. , and Al‐Shamlan, F. (2018) Central adenosine A1 receptors inhibit cough via suppression of excitatory glutamatergic and tachykininergic neurotransmission. British Journal of Pharmacology, 175: 3162–3174. https://doi.org/10.1111/bph.14360.
References
- Agrawal A, Singh SK, Singh VP, Murphy E, Parikh I (2008). Partitioning of nasal and pulmonary resistance changes during noninvasive plethysmography in mice. J Appl Physiol (1985) 105: 1975–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017). The Concise Guide To PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando A, Smallwood D, McMahon M, Irving L, Mazzone SB, Farrell MJ (2016). Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax 71: 323–329. [DOI] [PubMed] [Google Scholar]
- Barnabe R, Berni F, Clini V, Pirrelli M, Pisani Ceretti A, Robuschi M et al (1995). The efficacy and safety of moguisteine in comparison with codeine phosphate in patients with chronic cough. Monaldi Arch Chest Dis 50: 93–97. [PubMed] [Google Scholar]
- Basoglu OK, Pelleg A, Kharitonov SA, Barnes PJ (2017). Contrasting effects of ATP and adenosine on capsaicin challenge in asthmatic patients. Pulm Pharmacol Ther 45: 13–18. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Bolser DC (2002). Summary: animal models for cough. Pulm Pharmacol Ther 15: 249–250. [DOI] [PubMed] [Google Scholar]
- Bisserbe JC, Patel J, Marangos PJ (1985). Autoradiographic localization of adenosine uptake sites in rat brain using [3H]nitrobenzylthioinosine. J Neurosci 5: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DC, Aziz SM, DeGennaro FC, Kreutner W, Egan RW, Siegel MI et al (1993). Antitussive effects of GABAB agonists in the cat and guinea‐pig. Br J Pharmacol 110: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Chen CY, Sekizawa S, Joad JP (2006). Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol 101: 322–327. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Joad JP (1991). Neurones in commissural nucleus tractus solitarii required for full expression of the pulmonary C fibre reflex in rat. J Physiol 441: 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad RM, McDonald TJ, Brodin E, Cook MA (1992). Adenosine A1 receptors mediate inhibition of tachykinin release from perifused enteric nerve endings. Am J Physiol 262: G525–G531. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Brouns I, Adriaensen D, Timmermans JP (2012). Purinergic signaling in the airways. Pharmacol Rev 64: 834–868. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V (2014). Purinergic signaling and blood vessels in health and disease. Pharmacol Rev 66: 102–192. [DOI] [PubMed] [Google Scholar]
- Canning BJ (2009). Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther 22: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ (2004). Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea‐pigs. J Physiol 557: 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N (2010). An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 24: 3916–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N (2011). Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 300: R369–R377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BTY, Agrawal DK, Romero FA, Townley RG (1998). Measurement of bronchoconstriction using whole‐body plethysmograph: comparison of freely moving versus restrained guinea pigs. J Pharmacol Toxicol 39: 163–168. [DOI] [PubMed] [Google Scholar]
- Chou YL, Mori N, Canning BJ (2017). Opposing effects of bronchopulmonary C‐fiber subtypes on cough in guinea pigs. Am J Physiol Regul Integr Comp Physiol 314: R489–R498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YL, Scarupa MD, Mori N, Canning BJ (2008). Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 295: R1572–R1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofi FL, McDonald TJ, Cook MA (1990). Adenosine receptors are coupled negatively to release of tachykinin(s) from enteric nerve endings. J Pharmacol Exp Ther 253: 290–295. [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M, Pullmann R Jr, Undem BJ (2006). Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C‐fibres in guinea pig lungs. J Physiol 575: 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Broadley KJ (1986). Actions and interactions of adenosine, theophylline and enprofylline on the guinea‐pig spirally cut trachea. Eur J Pharmacol 125: 353–362. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Canning BJ, Garner R, Paterson B (2015). Effect of memantine on cough reflex sensitivity: translational studies in guinea pigs and humans. J Pharmacol Exp Ther 352: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB (1997). Antitussive effect of the GABA‐agonist baclofen. Chest 111: 996–999. [DOI] [PubMed] [Google Scholar]
- Dicpinigaitis PV, Dobkin JB, Rauf K, Aldrich TK (1998). Inhibition of capsaicin‐induced cough by the γ‐aminobutyric acid agonist baclofen. J Clin Pharmacol 38: 364–367. [DOI] [PubMed] [Google Scholar]
- Driessen AK, Farrell MJ, Mazzone SB, McGovern AE (2015). The role of the paratrigeminal nucleus in vagal afferent evoked respiratory reflexes: a neuroanatomical and functional study in guinea pigs. Front Physiol 6: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R (2002). The powerful placebo in cough studies? Pulm Pharmacol Ther 15: 303–308. [DOI] [PubMed] [Google Scholar]
- Eccles R (2006). Mechanisms of the placebo effect of sweet cough syrups. Respir Physiol Neurobiol 152: 340–348. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Kiley JP (1985). Antagonism by theophylline of respiratory inhibition induced by adenosine. J Appl Physiol 59: 1428–1433. [DOI] [PubMed] [Google Scholar]
- El‐Hashim AZ, Jaffal SM (2017). Cough reflex hypersensitivity: A role for neurotrophins. Exp Lung Res 43: 93–108. [DOI] [PubMed] [Google Scholar]
- El‐Hashim AZ, Edafiogho IO, Jaffal SM, Yousif MH, Ezeamuzie CI, Kombian SB (2011). Anti‐tussive and bronchodilator mechanisms of action for the enaminone E121. Life Sci 89: 378–387. [DOI] [PubMed] [Google Scholar]
- El‐Hashim AZ, Jaffal SM, Al‐Rashidi FT, Luqmani YA, Akhtar S (2013). Nerve growth factor enhances cough via a central mechanism of action. Pharmacol Res 74: 68–77. [DOI] [PubMed] [Google Scholar]
- El‐Hashim AZ, Wyss D, Lewis C (2004b). Effect of a novel NK1 receptor selective antagonist (NKP608) on citric acid induced cough and airway obstruction. Pulm Pharmacol Ther 17: 11–18. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Wong HH, Geppetti P, Reis JM, Harris SC, Maclean DB et al (1995). Effect of an NK1 receptor antagonist (CP‐99,994) on hypertonic saline‐induced bronchoconstriction and cough in male asthmatic subjects. Am J Respir Crit Care Med 152: 879–884. [DOI] [PubMed] [Google Scholar]
- Ginsborg BL, Hirst GD (1972). The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol 224: 629–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG et al (1997). Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 156: 766–775. [DOI] [PubMed] [Google Scholar]
- el‐Hashim A, D'Agostino B, Matera MG, Page C (1996). Characterization of adenosine receptors involved in adenosine‐induced bronchoconstriction in allergic rabbits. Br J Pharmacol 119: 1262–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Erokwu B, Dreshaj IA (1997). The role of excitatory amino acids in airway reflex responses in anesthetized dogs. J Auton Nerv Syst 67: 192–199. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Kc P, Moore CT, Acquah SS, Wilson CG, Zaidi SI et al (2005). Brain stem excitatory and inhibitory signaling pathways regulating bronchoconstrictive responses. J Appl Physiol 98: 1961–1982. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Yamamoto B, Dreshaj IA, Bedol D, Ferguson DG (2000). Involvement of glutamate in transmission of afferent constrictive inputs from the airways to the nucleus tractus solitarius in ferrets. J Auton Nerv Syst 80: 22–30. [DOI] [PubMed] [Google Scholar]
- Hegland KW, Pitts T, Bolser DC, Davenport PW (2011). Urge to cough with voluntary suppression following mechanical pharyngeal stimulation. Bratisl Lek Listy 112: 109–114. [PMC free article] [PubMed] [Google Scholar]
- Hirt RA, Leinker S, Mosing M, Wiederstein I (2008). Comparison of barometric whole body plethysmography and its derived parameter enhanced pause (PENH) with conventional respiratory mechanics in healthy Beagle dogs. Vet J 176: 232–239. [DOI] [PubMed] [Google Scholar]
- Hong JL, Ho CY, Kwong K, Lee LY (1998). Activation of pulmonary C fibres by adenosine in anaesthetized rats: role of adenosine A1 receptors. J Physiol 508: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose TK, Minic Z, Li C, O'Leary DS, Scislo TJ (2012). Activation of NTS A1 adenosine receptors inhibits regional sympathetic responses evoked by activation of cardiopulmonary chemoreflex. Am J Physiol Regul Integr Comp Physiol 303: R539–R550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RS, Corrao WM, Pratter MR (1981). Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis 123: 413–417. [DOI] [PubMed] [Google Scholar]
- Jafari‐Sabet M, Zarrindast MR, Rezayat M, Rezayof A, Djahanguiri B (2005). The influence of NMDA receptor agonist and antagonist on morphine state‐dependent memory of passive avoidance in mice. Life Sci 78: 157–163. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Misawa M, Nagase H, Kasuya Y (1994). Involvement of adenosine A1 receptors in antitussive effect in mice. Life Sci 55: PL383–PL388. [DOI] [PubMed] [Google Scholar]
- Keller JA, McGovern AE, Mazzone SB (2017). Translating cough mechanisms into better cough suppressants. Chest 152: 833–841. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzer CJ, Hay DW, Dondio G, Giardina G, Petrillo P, Underwood DC (2000). The antitussive activity of δ‐opioid receptor stimulation in guinea pigs. J Pharmacol Exp Ther 292: 803–809. [PubMed] [Google Scholar]
- Lee LY, Shuei Lin Y, Gu Q, Chung E, Ho CY (2003). Functional morphology and physiological properties of bronchopulmonary C‐fiber afferents. Anat Rec A Discov Mol Cell Evol Biol 270: 17–24. [DOI] [PubMed] [Google Scholar]
- Leech J, Mazzone SB, Farrell MJ (2012). The effect of placebo conditioning on capsaicin‐evoked urge to cough. Chest 142: 951–957. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Ambrose C, Banner K, Battram C, Butler K, Giddings J et al (2007). Animal models of cough: literature review and presentation of a novel cigarette smoke‐enhanced cough model in the guinea‐pig. Pulm Pharmacol Ther 20: 325–333. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ (2002a). Central nervous system control of the airways: pharmacological implications. Curr Opin Pharmacol 2: 220–228. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ (2002b). Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 283: R86–R98. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ (2011). Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 31: 2948–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, McGovern AE, Farrell MJ (2015). Endogenous central suppressive mechanisms regulating cough as potential targets for novel antitussive therapies. Curr Opin Pharmacol 22: 1–8. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Mori N, Canning BJ (2005). Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea‐pigs. J Physiol 569: 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod RL, Bolser DC, Jia Y, Parra LE, Mutter JC, Wang X et al (2002). Antitussive effect of nociceptin/orphanin FQ in experimental cough models. Pulm Pharmacol Ther 15: 213–216. [DOI] [PubMed] [Google Scholar]
- Minic Z, Li C, O'Leary DS, Scislo TJ (2014). Severe hemorrhage attenuates cardiopulmonary chemoreflex control of regional sympathetic outputs via NTS adenosine receptors. Am J Physiol Heart Circ Physiol 307: H904–H909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneta NA, McDonald TJ, Cook MA (1997). Endogenous adenosine inhibits evoked substance P release from perifused networks of myenteric ganglia. Am J Physiol 272: G38–G45. [DOI] [PubMed] [Google Scholar]
- Morice AH, Jakes AD, Faruqi S, Birring SS, McGarvey L, Canning B et al (2014a). A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 44: 1149–1155. [DOI] [PubMed] [Google Scholar]
- Morice AH, Millqvist E, Belvisi MG, Bieksiene K, Birring SS, Chung KF et al (2014b). Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 44: 1132–1148. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T (2008). Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol 295: R243–R251. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Fontana GA, Pantaleo T (2007). The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull 74: 284–293. [DOI] [PubMed] [Google Scholar]
- Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ Jr, Lima DA et al (2010). Inosine reduces pain‐related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Ther 334: 590–598. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamazaki H, Takeda R, Haji A (2005). Functional and morphological organization of the nucleus tractus solitarius in the fictive cough reflex of guinea pigs. Neurosci Res 53: 201–209. [DOI] [PubMed] [Google Scholar]
- Park TS, Van Wylen DG, Rubio R, Berne RM (1988). Brain interstitial adenosine and sagittal sinus blood flow during systemic hypotension in piglet. J Cereb Blood Flow Metab 8: 822–828. [DOI] [PubMed] [Google Scholar]
- Patel HJ, Birrell MA, Crispino N, Hele DJ, Venkatesan P, Barnes PJ et al (2003). Inhibition of guinea‐pig and human sensory nerve activity and the cough reflex in guinea‐pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol 140: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples RW, Stewart RR (2000). Alcohols inhibit N‐methyl‐d‐aspartate receptors via a site exposed to the extracellular environment. Neuropharmacology 39: 1681–1691. [DOI] [PubMed] [Google Scholar]
- Poli A, Lucchi R, Vibio M, Barnabei O (1991). Adenosine and glutamate modulate each other's release from rat hippocampal synaptosomes. J Neurochem 57: 298–306. [DOI] [PubMed] [Google Scholar]
- Poliacek I, Wang C, Corrie LW, Rose MJ, Bolser DC (2010). Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J Appl Physiol 108: 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Holgate ST (2006). Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr Drug Targets 7: 699–706. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G (1998). Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492. [PubMed] [Google Scholar]
- Rau AR, Ariwodola OJ, Weiner JL (2014). Presynaptic adenosine A1 receptors modulate excitatory transmission in the rat basolateral amygdala. Neuropharmacology 77: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Docherty R, Robbins J, Spina D, Page CP (2008). Adenosine induces a cholinergic tracheal reflex contraction in guinea pigs in vivo via an adenosine A1 receptor‐dependent mechanism. J Appl Physiol 105: 187–196. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Adams CA, Knight EJ, Nam HW, Choi DS (2010). An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev 3: 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder K, Fahey T (2002). Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ 324: 329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS (2006). Adenosine receptors located in the NTS contribute to renal sympathoinhibition during hypotensive phase of severe hemorrhage in anesthetized rats. Am J Physiol Heart Circ Physiol 291: H2,453–H2,461. [DOI] [PubMed] [Google Scholar]
- Smith JA, Hilton EC, Saulsberry L, Canning BJ (2012). Antitussive effects of memantine in guinea pigs. Chest 141: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon IC (1998). Activation of NMDA and non‐NMDA receptors in the caudal ventrolateral medulla dilates the airways. J Auton Nerv Syst 74: 169–174. [DOI] [PubMed] [Google Scholar]
- St Lambert JH, Dashwood MR, Spyer KM (1996). Role of brainstem adenosine A1 receptors in the cardiovascular response to hypothalamic defence area stimulation in the anaesthetized rat. Br J Pharmacol 117: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama K, Shirasaki T (2007). Central and peripheral mechanisms of narcotic antitussives: codeine‐sensitive and ‐resistant coughs. Cough 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA (2008). Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res 32: 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JR, Broadley KJ (1992). Adenosine‐induced bronchoconstriction of isolated lung and trachea from sensitized guinea‐pigs. Br J Pharmacol 106: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF (2013). Highlights in basic autonomic neurosciences: central adenosine A1 receptor – the key to a hypometabolic state and therapeutic hypothermia? Auton Neurosci 176: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SC, Chiu TH, Yang HW, Min MY (2007). Presynaptic adenosine A1 receptors modulate excitatory synaptic transmission in the posterior piriform cortex in rats. Brain Res 1156: 67–79. [DOI] [PubMed] [Google Scholar]


