Abstract
Autosomal dominant mutations in cullin-3 (Cul3) cause the most severe form of familial hyperkalemic hypertension (FHHt). Cul3 mutations cause skipping of exon 9, which results in an internal deletion of 57 amino acids from the CUL3 protein (CUL3-∆9). The precise mechanism by which this altered form of CUL3 causes FHHt is controversial. CUL3 is a member of the cullin-RING ubiquitin ligase family that mediates ubiquitination and thus degradation of cellular proteins, including with-no-lysine [K] kinases (WNKs). In CUL3-∆9-mediated FHHt, proteasomal degradation of WNKs is abrogated, leading to overactivation of the WNK targets sterile 20/SPS-1 related proline/alanine-rich kinase and oxidative stress-response kinase-1, which directly phosphorylate and activate the thiazide-sensitive Na+-Cl− cotransporter. Several groups have suggested different mechanisms by which CUL3-∆9 causes FHHt. The majority of these are derived from in vitro data, but recently the Kurz group (Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T. EMBO Mol Med 7: 1285–1306, 2015) described the first mouse model of CUL3-∆9-mediated FHHt. Analysis of this model suggested that CUL3-∆9 is degraded in vivo, and thus Cul3 mutations cause FHHt by inducing haploinsufficiency. We recently directly tested this model but found that other dominant effects of CUL3-∆9 must contribute to the development of FHHt. In this review, we focus on our current knowledge of CUL3-∆9 action gained from in vitro and in vivo models that may help unravel this complex problem.
Keywords: cullin-3, kinases, distal nephron, familial hyperkalemic hypertension, potassium, sodium transport
INTRODUCTION
In the kidney, phosphorylation and hence activation of the Na+-Cl− cotransporter (NCC) by the kinases sterile 20/SPS-1 related proline/alanine-rich kinase (SPAK) and oxidative stress-response kinase-1 (OSR1), which lie downstream of with-no-lysine [K] kinases (WNKs) (Fig. 1A), plays a critical role in ion homeostasis and blood pressure regulation (8, 11, 15, 25). The major advances in the delineation of this pathway have come from the investigation of the human disease familial hyperkalemic hypertension (FHHt, also known as Gordon syndrome or pseudohypoaldosteronism II) (9, 27). A better understanding of the mechanisms by which this pathway regulates renal ion handling may be of more broad clinical importance. For example, in non-FHHt patients, polymorphisms in both WNK1 (23, 24, 29, 34) and WNK4 (13) have been associated with increased risk of hypertension. Furthermore, polymorphisms in WNK1 have also been associated with variation in urinary potassium excretion, which may be related to predisposition to hyperkalemia (23).
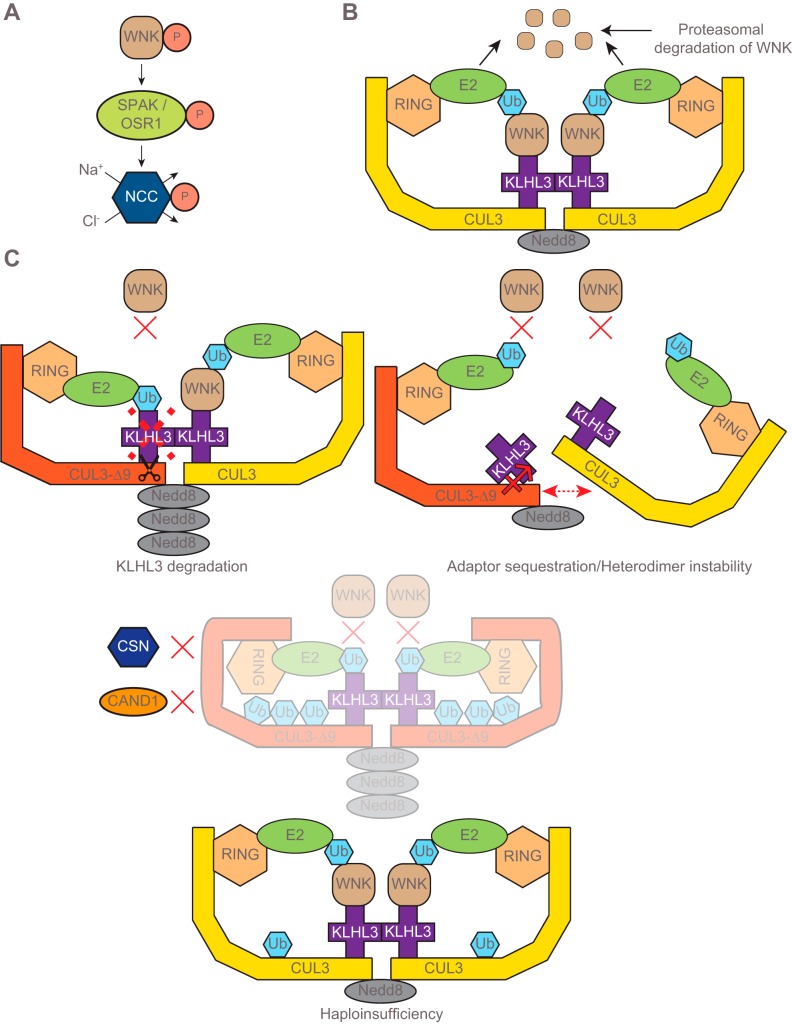
Fig. 1.
Regulation of with-no-lysine [K] kinase (WNK) abundance by the cullin-RING ligase (CRL) and proposed mechanisms by which the mutant cullin-3 protein (CUL3-∆9) dysregulates CRL activity. A: activated WNK (phosphorylated WNK) phosphorylates and activates sterile 20 (STE20)/SPS-1-related proline/alanine-rich kinase/oxidative stress-response kinase-1 (SPAK/OSR1), and upon activation SPAK/OSR1 activate Na+-Cl− cotransporter (NCC) by phosphorylating NCC at several amino-terminal residues. B: the cullin-RING ubiquitin Ligase (CRL) complex consists of the protein scaffold cullin-3 (CUL3), a substrate-binding adaptor kelch-like 3 (KLHL3), and the ubiquitin ligase really interesting new gene (RING) (5). The CRL modulates WNK abundance by regulating its proteasomal degradation. The active CRL complex functions as a dimer that may consist of a neural precursor cell expressed developmentally downregulated protein 8 (Nedd8)-modified CUL3 and an unmodified CUL3 (5). Polyubiquitinated WNK undergoes proteasomal degradation. C: there are three proposed mechanisms by which CUL3-∆9 causes familial hyperkalemic hypertension (FHHt). All of these would decrease the level of active CRL, leading to WNK accumulation and thus inappropriate NCC activation. Top left, KLHL3 degradation model; wild-type (WT) CUL3 can induce ubiquitination and degradation of the substrate adaptor KLHL3, but CUL3-∆9 has a greatly enhanced ability to do so (19). Top right, sequestration model; CUL3-∆9 displays enhanced binding to KLHL3, thereby preventing it from interacting with functional WT-containing CRLs. Note that in the vasculature, RhoBTB1 and Bacurd1, rather than KLHL3, are likely to be the sequestered adaptors (17). CUL3-∆9 and WT CUL3 may also form unstable CRL heterodimers (17). Bottom, Cul3 haploinsufficiency model; CUL3-∆9 displays autoubiquitination in vitro (32), and, consistent with this, CUL3-∆9 abundance is extremely low in mouse models of CUL3-∆9-mediated FHHt (1, 10, 32).
Mutations in WNKs (WNK1 and WNK4), which like SPAK and OSR1 are serine-threonine kinases, were the first identified as causing FHHt by the Lifton group (37). More recently, mutations in cullin-3 (Cul3) and kelch-like 3 (KLHL3) were identified as causing FHHt (6, 18). The proteins encoded by these genes, CUL3 and KLHL3, together with really interesting new gene (RING) form an E3 ubiquitin ligase complex [cullin-RING ligase (CRL)] that mediates ubiquitination and thus proteasomal degradation of cellular proteins (9). In the CRL complex CUL3 acts as a protein scaffold for many possible substrate-binding adaptors that confer cell-type and substrate specificity, including KLHL3 (5). The CRL complex was proposed to modulate NCC activity by regulating proteasomal degradation of WNK1 and WNK4 (Fig. 1B) (5, 9). Subsequent in vitro studies confirmed this and showed that mutations in Cul3, KLHL3, or WNK4 impair this process (19, 31, 40). WNK1 mutations reported to date are intronic and increase WNK1 protein abundance independent of the CRL. The resulting increase in WNK abundance leads to inappropriate phosphorylation and activation of SPAK/OSR1 and thus aberrant phosphorylation and activation of NCC along the distal convoluted tubule (DCT) (9, 14, 16, 20, 36). Overactive NCC increases Na+ reabsorption, leading to high blood pressure. It also diminishes Na+ delivery to the epithelial sodium channel (ENaC) along the connecting tubule (CNT) and cortical collecting duct, which reduces the electrogenic drive for K+ secretion through the renal outer medullary potassium channel (ROMK) (7, 26, 28, 30, 33). The development of hyperkalemia may also involve direct effects of increased WNK abundance on ENaC and ROMK surface expression (14), and decreased Na+ delivery may also cause CNT remodeling that decreases K+ secretion (12). Mutations in Cul3 are autosomal dominant and cause the most severe FHHt phenotype (6), which may reflect effects on both the kidney and the vasculature (see “CUL3 HAPLOINSUFFICIENCY”). FHHt-causing mutations can occur at intron 8 splicing donor, acceptor or enhancer sites, or at the branch point, and all lead to the generation of CUL3 mRNA lacking exon 9. This results in an internal deletion of 57 amino acids (403–459) in the translated protein (6, 18). The exact role of this altered form of CUL3 protein (CUL3-∆9) in the pathogenesis of FHHt is still unclear (9), although several mechanisms have been proposed. While these mechanisms differ from one another, they all agree that impaired function of the CRL complex abrogates WNK degradation. Here, we will describe the various models in the chronological order in which they were proposed and then discuss controversies and future directions that may resolve them.
CUL3-∆9-MEDIATED DEGRADATION OF KLHL3
We and others have reported that KLHL3 is expressed at extremely high levels along the DCT, the site of NCC expression (18, 19). Consistent with this, KLHL3 mutations also cause FHHt, which is known to be primarily a disease of NCC dysfunction because thiazide diuretics normalize the blood pressure and electrolyte abnormalities. This led us to propose that CUL3-∆9 may cause FHHt by exerting a specific effect on KLHL3. Immunoprecipitation studies using transfected HEK 293 cells revealed that CUL3-∆9 exhibited a greater affinity for KLHL3 than wild-type (WT) CUL3 (19). CUL3-∆9 also displayed a greater ability to promote ubiquitination and degradation of KLHL3 (19). These data suggest that CUL3-∆9 prevents WNK4 degradation by depleting KLHL3. These findings were subsequently confirmed by other groups (17, 32) and are reminiscent of a normal physiological process in which CUL3 triggers ubiquitination and degradation of the adaptor Keap1 in response to oxidative stress (42, 43). This permits accumulation of the CUL3/Keap1 substrate, nuclear factor-erythroid 2-related factor 2 (Nrf2), a transcription factor that induces expression of genes that protect against oxidative stress. The CRL containing CUL3-∆9 may also display altered activity. CRL activity is modulated by covalent linkage of the small (8-kDa) ubiquitin-like protein neural precursor cell expressed developmentally downregulated protein 8 (Nedd8) close to the carboxy-terminus of CUL3 (at lysine-712). Addition of Nedd8 (“neddylation”) is associated with CRL activation, whereas its removal “deneddylation” is associated with reduced CRL activity (38). Cycling of neddylation and deneddylation plays a role in maintaining CRL function. We found that CUL3-∆9 is a highly neddylated form of CUL3 with enhanced activity (19).
UNSTABLE CUL3-∆9 DIMERS AND ADAPTOR SEQUESTRATION
To explore interactions between CUL3-∆9 and WT CUL3, the Sigmund group performed immunoprecipitation studies in HEK 293 cells. CUL3-∆9/CUL3-WT heterodimers were formed only in the presence of proteasomal inhibitors or a neddylation inhibitor, suggesting that these might be less stable than homodimers of CUL3-WT, and not simply inactive complexes (17). Similar to our observations for KLHL3 (and two other adaptors BTB1 and KCTD6; see Ref. 19), CUL3-∆9 also displayed enhanced interaction with the adaptors RhoBTB1 and Bacurd1 (17). However, rather than promoting degradation of these adaptors, as we observed for KLHL3, CUL3-∆9 increased RhoBTB1 abundance and had no effect on Bacurd1 abundance. This led to the hypothesis that CUL3-∆9 may decrease the availability of substrate adaptors for formation of active CRL complexes. Instability of the complex containing CUL3-∆9 and CUL3-WT and substrate-binding adaptor sequestration may thus contribute to reduced WNK4 degradation (Fig. 1C).
CUL3 HAPLOINSUFFICIENCY
The Kurz group focused on CUL3-∆9 dysfunction (32). Using ubiquitin-release assays, they demonstrated that CUL3-∆9 not only maintained but also appeared to have an enhanced ability to hydrolyze E2 enzyme-ubiquitin conjugates, consistent with our proposal that CUL3-∆9 is a highly active form of CUL3 (19). Consistent with this, they reported that CUL3-∆9 is highly neddylated with aberrant neddylation of lysine residues in addition to lysine-712. CUL3-∆9 also displayed reduced interaction with the constitutive photomorphogenesis 9 (COP9) signalosome, a large protein complex that mediates deneddylation, and with CAND1, a substrate adaptor exchange factor that only binds deneddylated CUL3. These data suggest that CUL3-∆9 is a highly dysregulated form of CUL3. CUL3-∆9 also displayed defective autoubiquitination, which can induce degradation of the E3 ligase. In vitro ubiquitin assays using a methylated form of ubiquitin unable to form chains revealed that, while WT CUL3 was monoubiquitinated at a single residue, CUL3-∆9 was ubiquitinated at multiple lysines. To explore the molecular mechanism of CUL3-∆9 dysfunction, the Kurz group performed structural analyses, using prior work with CUL1 as a guide. The deleted region in CUL3-∆9 corresponds with a region of CUL1 that displays decreased substrate ubiquitination when its flexibility is increased (44). It was proposed that greater flexibility of CUL3-∆9 may play a role in enhanced autoubiquitination and may also lead to decreased WNK ubiquitination by forming nonproductive conformations. These somewhat diffuse in vitro studies (summarized in Fig. 1C) did not establish a definitive mechanism by which CUL3-∆9 causes FHHt but raised many possibilities. However, generation of a knock-in mouse with deletion of exon 9 at a single Cul3 allele (CUL3WT/∆9) provided strong evidence that CUL3-∆9 autoubiquitination plays a central role. CUL3WT/∆9 mice displayed the salient features of FHHt, with increased abundances of WNK4 and phosphorylated NCC, and elevated blood pressure and plasma potassium concentration. CUL3WT/∆9 mice also displayed thickening of the aorta and a greater constrictor response to phenylephrine and angiotensin II (32), suggesting that, in addition to inappropriate NCC activation, vascular dysfunction may contribute to the more severe hypertension seen in patients with Cul3 mutations. This is consistent with a more recent report by the Sigmund group that selective expression of CUL3-∆9 in vascular smooth muscle cells phenocopied higher blood pressure and caused vascular stiffness, which involves effects on RhoA, a key regulator of vascular smooth muscle tone (1). Critically, although abundance of WT CUL3 was decreased by 50% in the kidney, as expected with a single WT allele, CUL3-∆9 was almost undetectable. The Kurz group also reported that KLHL3 abundance was not lower, providing evidence against our KLHL3 degradation model. Thus, they proposed that CUL3-∆9 causes FHHt by inducing haploinsufficiency, which decreases the amount of functional CRL, and Cul3 heterozygosity should phenocopy the disease.
DOMINANT EFFECT OF CUL3-∆9 IN THE PATHOGENESIS OF FHHT
Rather than resulting in expression of CUL3-∆9, an attempt by the Uchida group to generate CUL3WT/∆9 knockin mice by mutating exon 9 splice sites only resulted in decreased abundance of WT CUL3, and these mice did not phenocopy FHHt. However, these mice may express additional truncated forms of CUL3 (22). Therefore, to directly test whether heterozygosity for Cul3 leads to FHHt, we generated mice heterozygous for Cul3, with or without simultaneous CUL3-∆9 expression (10) from a Cre-induced transgene (1). Our approach took advantage of an inducible renal epithelia-specific Cre recombinase system (Pax8-rtTA/LC1) in which recombination at loxP sites is induced when doxycyline is administered in drinking water (35). As expected, abundance of CUL3 protein was reduced by ~50% in Cul3 heterozygotes lacking CUL3-∆9 (CUL3-Het) compared with uninduced controls. Consistent with the observation of the Kurz group that CUL3-∆9 triggers its own degradation, CUL3-∆9 protein was not detectable in kidney lysates from induced heterozygous mice carrying the CUL3-∆9 transgene (CUL3-Het/∆9 mice). However, it was detected in cultured primary epithelial cells. CUL3-Het/Δ9 mice phenocopied FHHt, but CUL3-Het mice did not, suggesting that haploinsufficiency is not sufficient to cause the disease and dominant effects of CUL3-∆9 are required. Further support for this conclusion came from mice in which the CUL3-∆9 transgene was expressed on a background of two WT Cul3 alleles (CUL3-WT/∆9 mice) in which WT CUL3 abundance did not differ following doxycycline induction (10). CUL3-WT/∆9 mice had higher abundances of WNK4 and phosphorylated NCC, the molecular hallmarks of FHHt. Additionally, in collaboration with the Sigmund group, we recently reported that mice heterozygous for Cul3 specifically in vascular smooth muscle do not have elevated blood pressure (2).
CONTROVERSIES, FUTURE DIRECTIONS, AND CONCLUSIONS
The precise functional consequences of deletion of residues 403–459 on CUL3-∆9 function are unclear. The Kurz group proposed that it increases CUL3-∆9 flexibility, leading to its aberrant actions, but this is based on CUL1 structural data, with no CUL3 adaptors incorporated into the model. Defective neddylation may play a central role in the mechanism of CUL3-∆9-mediated FHHt as we and the Kurz group independently reported that CUL3-∆9 displays increased neddylation. However, the Sigmund group did not observe increased neddylation in HEK 293 cells in which endogenous CUL3 was disrupted by CRISPR-Cas9 (17). Perhaps interactions with WT CUL3 are required for the neddylation defect to manifest, or other changes in the CUL3 regulatory machinery occur in response to its disruption. The mechanism by which defective CUL3-∆9 activity causes FHHt is also uncertain. In vitro studies have shown that it has gain-of-function properties that ultimately prevent proteasomal degradation of substrates. CUL3-∆9 clearly induces its own degradation in vivo, but our data suggest that the resulting haploinsufficiency of WT CUL3 is not sufficient to cause FHHt (10). Our earlier work suggested that CUL3-∆9 decreases KLHL3 abundance. This seemed reasonable since Klhl3 mutations also cause the disease, and KLHL3 is expressed at high levels in the NCC-expressing DCT. However, the Kurz group provided evidence against this mechanism, since KLHL3 abundance was not shown to be lower in their FHHt model. One limitation of their findings is that the antibody used may also detect KLHL2. The Uchida group recently validated a commercially available KLHL3 antibody in KLHL3 knockout mice (31), and in a recently developed CUL3-∆9-expressing mouse model found that KLHL3 abundance was 75% lower than in WT mice (41). We have thus far been unable to replicate this finding using the same antibody. Although a 50% decrease in KLHL3 abundance does not phenocopy FHHt (31), it is possible that a 75% decrease in abundance can, or perhaps a combination of haploinsufficiency and KLHL3 degradation is required. Another intriguing possibility is that CUL3-∆9 specifically dysregulates WT CUL3 along the DCT, leading to KLHL3 degradation. Using an antibody validated in CUL3 knockout mice, we observed a decrease in nuclear CUL3 in NCC-expressing segments of the nephron (10). We previously reported that, in transfected cells, CUL3-∆9 mislocalizes to the cytoplasm (19), and CUL3-∆9 could cause relocalization of WT CUL3 specifically along the DCT, where it inappropriately degrades KLHL3.
If KLHL3 degradation is indeed part of the mechanism, one major question is why does CUL3-∆9 specifically target this substrate-binding adaptor? CUL3 interacts with many adaptors, some of which cause very specific diseases when mutated. For example, mutations in the gene encoding gigaxonin (Gan) lead to a severe neurodegenerative disorder diagnosed early in infancy. Gan mutations decrease gigaxonin abundance in transfected cells by an average of 86% (4). This is similar to the decrease in KLHL3 observed by the Uchida group in their CUL3-∆9 mouse model, but these mice display FHHt and not neurodegeneration, suggesting CUL3-∆9 does not affect gigaxonin abundance. This is supported by the finding that CUL3-∆9 degrades some substrate-binding adaptors but not others (17). Future structural studies examining CUL3-∆9-adaptor interactions may shed light on this puzzle.
Finally, the molecular mechanisms by which CUL3-∆9 contributes to hypertension may differ in the vasculature. The Sigmund group showed that, although CUL3-∆9 displays higher affinity for KLHL3, Bacurd1, and RhoBTB1, it increased RhoBTB1 abundance, decreased that of KLHL3, but had no effect on Bacurd1 abundance (17). Because the Bacurd1 target RhoA modulates vascular tone, and RhoBTB1 may normally exert antihypertensive effects through an as-yet-unidentified pathway (21), sequestration of these adaptors by CUL3-∆9 in the vasculature may therefore be mechanistically important in the development of hypertension in FHHt. It is also possible that dysregulation of the WNK-SPAK/OSR1 pathway, which can modulate vascular tone through effects on NKCC1 (3, 39), plays a role in the vascular defects reported. The relative contributions of vascular vs. renal defects to the hypertensive phenotype in mice expressing CUL3-∆9 have also not been determined. Finally, in transgenic mice expressing CUL3-∆9 specifically in vascular smooth muscle on a background of two WT Cul3 alleles, abundance of WT CUL3 in aorta was decreased by ~50%. Direct effects of CUL3-∆9 on abundance were not observed in kidney (10, 32), further suggesting tissue-specific effects of CUL3-∆9.
Additional studies are clearly required to determine the mechanisms by which CUL3-∆9 causes FHHt. Haploinsufficiency alone does not appear to cause the disease but may contribute in combination with tissue-specific effects of CUL3-∆9. These are likely to include effects on specific substrate-binding adaptors such as degradation (e.g., KLHL3) and sequestration of adaptors (RhoBTB1) that may be determined by their unique physical interactions.
GRANTS
Funding was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-098141 to J. A. McCormick and American Heart Association Postdoctoral Fellowship 17POST33670206 to M. Z. Ferdaus.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Z.F. drafted manuscript; M.Z.F. and J.A.M. edited and revised manuscript; M.Z.F. and J.A.M. approved final version of manuscript.
REFERENCES
- 1.Agbor LN, Ibeawuchi SC, Hu C, Wu J, Davis DR, Keen HL, Quelle FW, Sigmund CD. Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight 1: e91015, 2016. doi: 10.1172/jci.insight.91015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agbor LN, Nair AR, Wu J, Davis DR, Keen HL, Quelle FW, McCormick JA, Singer JD, Sigmund CD. Smooth muscle-specific deletion of cullin-3 causes severe early onset hypertension. FASEB J 31: 1015–1017, 2017. [Google Scholar]
- 3.Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P, Henrion D, Loirand G, Achard JM, Jeunemaitre X, Hadchouel J. WNK1 regulates vasoconstriction and blood pressure response to alpha 1-adrenergic stimulation in mice. Hypertension 58: 439–445, 2011. doi: 10.1161/HYPERTENSIONAHA.111.172429. [DOI] [PubMed] [Google Scholar]
- 4.Boizot A, Talmat-Amar Y, Morrogh D, Kuntz NL, Halbert C, Chabrol B, Houlden H, Stojkovic T, Schulman BA, Rautenstrauss B, Bomont P. The instability of the BTB-KELCH protein gigaxonin causes giant axonal neuropathy and constitutes a new penetrant and specific diagnostic test. Acta Neuropathol Commun 2: 47, 2014. doi: 10.1186/2051-5960-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosu DR, Kipreos ET. Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div 3: 7, 2008. doi: 10.1186/1747-1028-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 482: 98–102, 2012. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol 27: 981–989, 2016. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferdaus MZ, Barber KW, Lopez-Cayuqueo KI, Terker AS, Argaiz ER, Gassaway BM, Chambrey R, Gamba G, Rinehart J, McCormick JA. SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J Physiol 594: 4945–4966, 2016. doi: 10.1113/JP272311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferdaus MZ, McCormick JA. The CUL3/KLHL3-WNK-SPAK/OSR1 pathway as a target for antihypertensive therapy. Am J Physiol Renal Physiol 310: F1389–F1396, 2016. doi: 10.1152/ajprenal.00132.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferdaus MZ, Miller LN, Agbor LN, Saritas T, Singer JD, Sigmund CD, McCormick JA. Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight 2017: 2, 2017. doi: 10.1172/jci.insight.96700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover M, O’Shaughnessy KM. SPAK and WNK kinases: a new target for blood pressure treatment? Curr Opin Nephrol Hypertens 20: 16–22, 2011. doi: 10.1097/MNH.0b013e32834132bc. [DOI] [PubMed] [Google Scholar]
- 12.Grimm PR, Coleman R, Delpire E, Welling PA. Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017. doi: 10.1681/ASN.2016090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo XG, Ding J, Xu H, Xuan TM, Jin WQ, Yin X, Shang YP, Zhang FR, Zhu JH, Zheng LR. Comprehensive assessment of the association of WNK4 polymorphisms with hypertension: evidence from a meta-analysis. Sci Rep 4: 6507, 2014. doi: 10.1038/srep06507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 15.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 22: 605–614, 2011. doi: 10.1681/ASN.2010080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008. doi: 10.1097/MNH.0b013e32830dd580. [DOI] [PubMed] [Google Scholar]
- 17.Ibeawuchi SR, Agbor LN, Quelle FW, Sigmund CD. Hypertension-causing mutations in Cullin3 protein impair RhoA protein ubiquitination and augment the association with substrate adaptors. J Biol Chem 290: 19208–19217, 2015. doi: 10.1074/jbc.M115.645358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X; International Consortium for Blood . KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 44: 456–460, 2012. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 19.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori Y, Wakabayashi M, Mori T, Araki Y, Sohara E, Rai T, Sasaki S, Uchida S. Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms. Biochem Biophys Res Commun 439: 30–34, 2013. doi: 10.1016/j.bbrc.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 21.Mukohda M, Ibeawuchi SC, Hu C, Nair AR, Agbor LN, Wu J, Quelle FW, Sigmund CD. RhoBTB1 is a novel gene protecting against hypertension. FASEB J 31: 1032–1034, 2017. [Google Scholar]
- 22.Murthy M, Kurz T, O’Shaughnessy KM. WNK signalling pathways in blood pressure regulation. Cell Mol Life Sci 74: 1261–1280, 2017. doi: 10.1007/s00018-016-2402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newhouse S, Farrall M, Wallace C, Hoti M, Burke B, Howard P, Onipinla A, Lee K, Shaw-Hawkins S, Dobson R, Brown M, Samani NJ, Dominiczak AF, Connell JM, Lathrop GM, Kooner J, Chambers J, Elliott P, Clarke R, Collins R, Laan M, Org E, Juhanson P, Veldre G, Viigimaa M, Eyheramendy S, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Kumari M, Marmot M, Brunner E, Caulfield M, Munroe PB. Polymorphisms in the WNK1 gene are associated with blood pressure variation and urinary potassium excretion. PLoS One 4: e5003, 2009. doi: 10.1371/journal.pone.0005003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newhouse SJ, Wallace C, Dobson R, Mein C, Pembroke J, Farrall M, Clayton D, Brown M, Samani N, Dominiczak A, Connell JM, Webster J, Lathrop GM, Caulfield M, Munroe PB. Haplotypes of the WNK1 gene associate with blood pressure variation in a severely hypertensive population from the British Genetics of Hypertension study. Hum Mol Genet 14: 1805–1814, 2005. doi: 10.1093/hmg/ddi187. [DOI] [PubMed] [Google Scholar]
- 25.Pacheco-Alvarez D, Cristobal PS, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na+:Cl- cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 26.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10: 676–687, 2015. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P. A molecular update on pseudohypoaldosteronism type II. Am J Physiol Renal Physiol 305: F1513–F1520, 2013. doi: 10.1152/ajprenal.00440.2013. [DOI] [PubMed] [Google Scholar]
- 28.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putku M, Kepp K, Org E, Sober S, Comas D, Viigimaa M, Veldre G, Juhanson P, Hallast P, Tonisson N, Tonia ES, Yi H, Shaw-Hawkins S, Caulfield MJ, Khusnutdinova E, Kozich V, Munroe PB, Laan M. Novel polymorphic AluYb8 insertion in the WNK1 gene is associated with blood pressure variation in Europeans. Hum Mutat 32: 806–814, 2011. doi: 10.1002/humu.21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodan AR, Huang CL. Distal potassium handling based on flow modulation of maxi-K channel activity. Curr Opin Nephrol Hypertens 18: 350–355, 2009. doi: 10.1097/MNH.0b013e32832c75d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki E, Susa K, Mori T, Isobe K, Araki Y, Inoue Y, Yoshizaki Y, Ando F, Mori Y, Mandai S, Zeniya M, Takahashi D, Nomura N, Rai T, Uchida S, Sohara E. KLHL3 knockout mice reveal the physiological role of KLHL3 and the pathophysiology of pseudohypoaldosteronism type ii caused by mutant KLHL3. Mol Cell Biol 37: e00508-16, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med 7: 1285–1306, 2015. doi: 10.15252/emmm.201505444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobin MD, Raleigh SM, Newhouse S, Braund P, Bodycote C, Ogleby J, Cross D, Gracey J, Hayes S, Smith T, Ridge C, Caulfield M, Sheehan NA, Munroe PB, Burton PR, Samani NJ. Association of WNK1 gene polymorphisms and haplotypes with ambulatory blood pressure in the general population. Circulation 112: 3423–3429, 2005. doi: 10.1161/CIRCULATIONAHA.105.555474. [DOI] [PubMed] [Google Scholar]
- 35.Traykova-Brauch M, Schonig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Grone HJ, Koesters R. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Reports 3: 858–868, 2013. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 38.Wu JT, Lin HC, Hu YC, Chien CT. Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat Cell Biol 7: 1014–1020, 2005. doi: 10.1038/ncb1301. [DOI] [PubMed] [Google Scholar]
- 39.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida S US, Sohara E, Araki Y, Mori T, Sasaki E, Kasagi Y, Isobe K, Susa K, Inoue Y, Rai T. Decreased protein expression of KLHL3 is involved in the pathogenesis of PHAII caused by CUL3 mutation in vivo. ASN Kidney Week 2017: PO1039, 2017. [Google Scholar]
- 42.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24: 10941–10953, 2004. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang DD, Lo SC, Sun Z, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem 280: 30091–30099, 2005. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- 44.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416: 703–709, 2002. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]