Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors enhance urinary glucose, Na+ and fluid excretion, and lower hyperglycemia in diabetes by targeting Na+ and glucose reabsorption along the proximal convoluted tubule. A goal of this study was to predict the effects of SGLT2 inhibitors in diabetic and nondiabetic patients with chronic kidney disease. To that end, we employed computational rat kidney models to explore how SGLT2 inhibition affects renal solute transport and metabolism when nephron populations are normal or reduced. Model simulations suggested that in a nondiabetic rat, acute and chronic SGLT2 inhibition induces glucosuria, diuresis, natriuresis, and kaliuresis. Those effects were stronger with chronic SGLT2 inhibition (due to SGLT1 downregulation) and tempered by nephron loss. In a diabetic rat with normal nephron number, acute SGLT2 inhibition similarly elevated urine fluid, Na+, and K+ excretion, whereas the urinary excretory effects of chronic SGLT2 inhibition were attenuated in proportion to its plasma glucose level lowering effect. Nephron loss in a diabetic kidney was predicted to lower the glucosuric and blood glucose-reducing effect of chronic SGLT2 inhibition, but due to the high luminal glucose delivery in the remaining hyperfiltering nephrons, nephron loss enhanced proximal tubular paracellular Na+ secretion, thereby augmenting the natriuretic, diuretic, and kaliuretic effects. A proposed shift in oxygen-consuming active transport to the outer medulla, which may simulate systemic hypoxia and enhance erythropoiesis, was also preserved with nephron loss. These effects may contribute to the protective effects of SGLT2 inhibitors on blood pressure and heart failure observed in diabetic patients with chronic kidney diseases.
Keywords: diabetes, epithelial transport, glucose, hypoxia, oxygen consumption, remnant kidney, sodium-glucose cotransporter, SGLT2 inhibitor
INTRODUCTION
Diabetes is one of the most common causes of chronic kidney disease (9). Despite intense research, the pathways via which diabetes leads to the development or progression of chronic kidney diseases remain incompletely understood. One key mechanism is thought to be hypoxia in the outer medulla, where blood flow is substantially lower than in the renal cortex, but tubular oxygen consumption is substantial, given the brisk active Na+ reabsorption in the proximal straight tubules (S3) and medullary thick ascending limbs. The balance between low oxygen supply and high oxygen demand results in an outer medullary oxygen tension that is typically ~20 mmHg, compared with ~50 mmHg in the cortex (3, 28). Both early diabetes (36, 44) and the loss of functional nephrons in kidney disease (11, 13) are accompanied by glomerular hyperfiltration and tubular hypertrophy. These pathophysiological responses increase transport work and the oxygen demand on the level of the single tubules, which when not met by a compensatory increase in oxygen supply, can induce hypoxic injury. A goal of this study was to apply computational models to assess the impact of diabetes on the metabolic demand of the kidney, and how that metabolic demand changes when functional nephrons are lost as observed in chronic kidney disease and as modeled in a nephrectomized rat.
A new therapeutic approach for lowering blood glucose levels in diabetes is to enhance glucose excretion by inhibiting glucose reabsorption via Na+-glucose cotransporter 2 (SGLT2) in the early proximal tubule. Although SGLT2 inhibitors can reduce the risk of kidney and heart failure in patients with Type 2 diabetes mellitus (27, 46, 49), many aspects remain poorly understood. One concern is that inhibiting SGLT2 lowers the proximal tubule uptake of not only glucose but Na+ as well, thereby shifting glucose and Na+ reabsorption to downstream nephron segments, including the S3 segment and the medullary thick ascending limb. The downstream shift of Na+ transport may increase overall kidney oxygen demand, because the efficiency of Na+ transport (namely, the number of Na+ moles reabsorbed per O2 mole consumed) changes along the nephron, with the early proximal tubule being more efficient than the distal segments (15). This can be counteracted as long as SGLT2 inhibitors reduce glomerular filtration rate (GFR) and thereby tubular transport load (24). SGLT2 inhibitors have also been approved in diabetic patients with moderately impaired kidney function. Thus a second goal of this study is to better understand the nuances and long-term effects of SGLT2 inhibitors by determining the extent to which diabetes and SGLT2 inhibition can affect transport activity and oxygen consumption when kidney function is impaired.
Most importantly and in addition to lowering blood sugar levels, SGLT2 inhibitors have also been reported to reduce blood pressure and protect from heart failure, even in patients with chronic kidney diseases and reduced GFR [estimated GFR (eGFR) ≥ 30 ml·min−1·1.73 m2] (21a, 30, 47). Moreover, new clinical trials have been initiated to explore the renocardiovascular benefits of SGLT2 inhibitors in nondiabetic patients (25). Hence another goal of this study is to determine if a persistent diuretic and natriuretic effect of SGLT2 inhibitors can be predicted when the nephron number and GFR are getting reduced in diabetes, and to which extent such beneficial effects may also extend to the nondiabetic setting.
MODELING METHODOLOGY
Model simulations were performed using our published models of epithelial transport along rat nephrons in a kidney with normal nephron number (i.e., a sham kidney), an uninephrectomized rat (UNX, 50% of nephrons remaining), and a 5/6-nephrectomized rat (5/6-NX, 1/6 of nephrons remaining) (20). The model accounts for 15 solutes: Na+, K+, Cl−, , H2CO3, CO2, NH3, , HPO, H+, , H2CO2, urea, and glucose. The model is formulated for steady state and predicts luminal fluid flow, hydrostatic pressure, luminal fluid solute concentrations, and, with the exception of the descending limb segment, cytosolic solute concentrations, membrane potential, and transcellular and paracellular fluxes. Model parameters under baseline conditions can be found in Ref. 20 and the references therein.
Sodium-glucose cotransport in the proximal tubule.
Under physiological conditions, the filtered load of glucose is fully reabsorbed in the proximal tubule via SGLTs on the apical side and glucose transport facilitators (GLUT) on the basolateral side. The proximal convoluted tubule expresses the high-capacity, low-affinity transporter SGLT2 together with basolateral GLUT2, whereas the S3 segment expresses the lower capacity, higher affinity transporter SGLT1 and basolateral GLUT1. The modeling of glucose and Na+ fluxes across SGLT2 and SGLT1 cotransporters, and glucose fluxes across GLUT1 and GLUT2 have been described in our previous studies (21, 22, 23, 24).
Oxygen consumption along the nephron.
The rate of oxygen () consumption can be divided into two parts: the active component () provides the energy needed to actively reabsorb Na+, and the basal component () supplies the energy for other transport processes and intracellular biochemical reactions. is calculated based on the ATP consumption of basolateral Na/K-ATPase pumps, given by
| (1) |
where is the rate of Na+ transport across Na/K-ATPase pumps, and RTQ indicates the number of moles of Na+ transported per mole of O2 consumed. Since 1 mole of ATP is required to pump out 3 moles of Na+ via the pump, and oxidative metabolism yields ~5 moles of ATP per mole of O2 consumed (37); in a nondiabetic kidney, RTQ is taken to be 15.
As in our previous study (24), we assume that in a given segment, is fixed and equal to 25% of (total) QO2 under baseline conditions.
Simulating a nephrectomized kidney.
When renal mass is reduced, the remaining nephrons can adapt with appropriately elevated urinary excretion per nephron to maintain total excretion rates. Such compensatory response involves adaptive changes in GFR, in tubular growth, and in transepithelial transport. Following our previous approach to this setting (20), we assumed in UNX that single-nephron glomerular filtration rate (SNGFR) was increased 50 and 25% in the superficial and juxtamedullary nephrons, respectively, based on measurements in Ref. 13. And we assumed in 5/6-NX that SNGFR was increased by 110 and 50% in the superficial and juxtamedullary nephrons, respectively, (14). Additional model parameters for simulating a UNX kidney and a 5/6-NX kidney can be found in Ref. 20.
Simulating a diabetic rat kidney.
Diabetes induces renal hypertrophy, hyperfiltration, and alterations in transporter expression. As in our previous model (24), we simulated diabetic conditions by simultaneously raising plasma glucose, SNGFR, tubular diameter and length (see Table 1), and the expression levels of SGLT2 and other selected Na+ transporters, and by decreasing SGLT1 expression. Details on changes in transporter expression can be found in Ref. 24. The same diabetes-induced fractional changes in transporter expression were assumed in sham and nephrectomized kidneys. Plasma glucose concentration was taken to be 25 mM in sham and slightly higher at 30 mM in UNX and 5/6-NX kidneys (29).
Table 1.
Changes in SNGFR, tubular dimensions, and plasma glucose concentration in diabetic sham, nondiabetic UNX and 5/6-NX, and diabetic UNX and 5/6-NX kidneys
| SNGFR |
Diameter |
Length |
|||||
|---|---|---|---|---|---|---|---|
| SF | JM | PT | distal | PT | distal | Plasma [glucose] | |
| Diabetic sham | +50% (44) | +17% | +28% (36) | +42% (36) | +28% (36) | +7% (36) | 25 mM (29) |
| Nondiabetic UNX | +54% (13) | +27% (13) | +17% (11) | +12% (11) | +35% (11) | +17% (11) | 5 mM |
| Diabetic UNX | +95.6% (13, 29) | +38.4% | +49.8% | +59.0% | +72.8% | +25.2% | 30 mM (29) |
| Nondiabetic 5/6-NX | +100% (13) | +45% (13) | +32% (11) | +48% (11) | +68% (11) | +45% (11) | 5 mM |
| Diabetic 5/6-NX | +154% | +58.1% | +69.0% | +87.4% | +115% | +55.2% | 30 mM (29) |
Percentage changes given relative to nondiabetic sham. Changes to parameters for diabetic uninephrectomized (UNX) and 5/6-nephrectomized (5/6-NX) kidneys were obtained by multiplying percentage changes in diabetic sham and nondiabetic UNX and 5/6-NX. SNGFR, single-nephron glomerular filtration rate; SF, superficial; JM, juxtamedullary; PT, proximal tubule.
Diabetes also decreases the ratio in the proximal tubule by 20% as a result of increased fatty acid metabolism (1); we assumed here that in diabetic rats, RTQ equals 12 (vs. 15 in control animals) along the entire nephron. To account for the higher basal O2 consumption in diabetic rats (16, 32, 33), as in our previous study (24), was taken to increase (relative to nondiabetic nephrons) by 40% in all cortical segments and by 160% each in the S3 segment and medullary collecting duct, and to remain unchanged in the medullary thick ascending limb.
SGLT2 inhibition in a nondiabetic kidney.
When SGLT2 blockade is simulated, the change in SNGFR is determined by the tubuloglomerular feedback (TGF) response, which adjusts afferent arteriolar smooth muscle tone and hence SNGFR (4). Based on the affinities of the NKCC2 isoform in the macula densa cells for Na+, K+, and Cl− (35), the luminal (Cl−) was considered rate limiting for the TGF response. Thus the TGF signal was based on the luminal fluid (Cl−) near the macula densa, which corresponds to the model’s cortical thick ascending limb outflow (Cl−) (denoted CMD). Specifically, the model assumes that
| (2) |
For each nephron, the reference SNGFR0 is initially taken to be its baseline SNGFR, which in sham is ~30 and 45 nl/min for the superficial and juxtamedullary nephrons, respectively; baseline SNGFR0 values for UNX and 5/6-NX are shown in Table 1. For each nephron, the operating point Cop is taken to be its baseline CMD. The TGF gain γ is taken to be 3.1 for a nondiabetic kidney. This yields fractional changes in macula densa (Cl−) (~30% increase) and SNGFR (~10% decrease) in nondiabetic rats following acute intratubular application of the SGLT1/2 inhibitor phlorizin as reported by Vallon et al. (44).
To simulate acute SGLT2 inhibition, we reduced SGLT2 expression level by 75% to yield results comparable to the micropuncture study (44) in which phlorizin was administered into the proximal tubule rather than systemically. Note that we are matching model-predicted macula densa (Cl−) under SGLT2 inhibition with an experimentally determined value following phlorizin administration, which inhibits both SGLT2 and SGLT1. That discrepancy is justified because SGLT1 inhibition in the model had little effect on macula densa (Cl−).
We assumed that in chronic SGLT2 inhibition, systemic application of the inhibitor reaches the entire proximal tubule from Bowman space downward. Thus we abolished transport across SGLT2 by 100%. Furthermore, in wild-type mice, chronic inhibition of SGLT2 by empagliflozin was found to upregulate SGLT2 protein expression by 47%, while reducing that of SGLT1 by 35% (42). To simulate the chronic blockade of SGLT2, we also accounted for these changes in transporter density (which were seen in nondiabetic animals).
SGLT2 inhibition in a diabetic kidney.
Chronic SGLT2 inhibition was found to lower blood glucose levels by 60% in diabetic Akita mice, from ~25 to 10 mM, without altering the renal membrane expression of SGLT1 nor that of SGLT2 (i.e., the diabetes-induced changes were maintained) (42). Thus, in simulating chronic SGLT2 blockade in diabetic sham rats, we reduced the plasma concentration of glucose to 10 mM, in addition to decreasing SNGFR from diabetic levels. Because filtered glucose load was lower in nephrectomized rats, glucouria and thus the glucosuric efficacy of SGLT2 inhibitors were reduced as well. Thus we assumed that chronic SGLT2 inhibition in diabetic UNX and 5/6-NX lowers plasma glucose concentration to 15 and 20 mM, respectively. Plasma glucose concentration was kept constant (at 25 mM for sham and at 30 mM for UNX or 5/6-NX) during acute blockade.
We assumed that SNGFR is determined by the TGF response (Eq. 2), with γ taken to be 2.5 in diabetic rats, that is, modestly reduced compared with nondiabetic rats (41). This yielded similar SNGFR reduction (~30%) in sham following acute SGLT2 inhibition, as reported by Thomson et al. (40). Chronic SGLT2 inhibition is known to reduce or prevent diabetic-induced hyperfiltration in rodents (40, 42, 45) and humans (7) via its effects on tubular reabsorption (a TGF and tubular hydrostatic pressure effect) and possibly also by lowering effects of high blood glucose levels on vascular tone [a non-TGF effect (5)]. Thus, when simulating the effects of chronic SGLT2 inhibition in diabetic rats, we lowered SNGFR halfway to its value in nondiabetic sham and nephrectomized rats; that is, if SNGFRcontrol and SNGFRdiabetes denote SNGFR in nondiabetic and diabetic rats, respectively, then under chronic SGLT2 inhibition, SNGFR is set to 0.5 × (SNGFRcontrol + SNGFRdiabetes).
As in the nondiabetes case, we reduced SGLT2 expression level by 75% to yield results comparable to simulate acute SGLT2 inhibition so that model results are comparable with Ref. 44. Functional SGLT2 expression was entirely abolished in chronic SGLT2 inhibition (see rationale above).
SIMULATION RESULTS
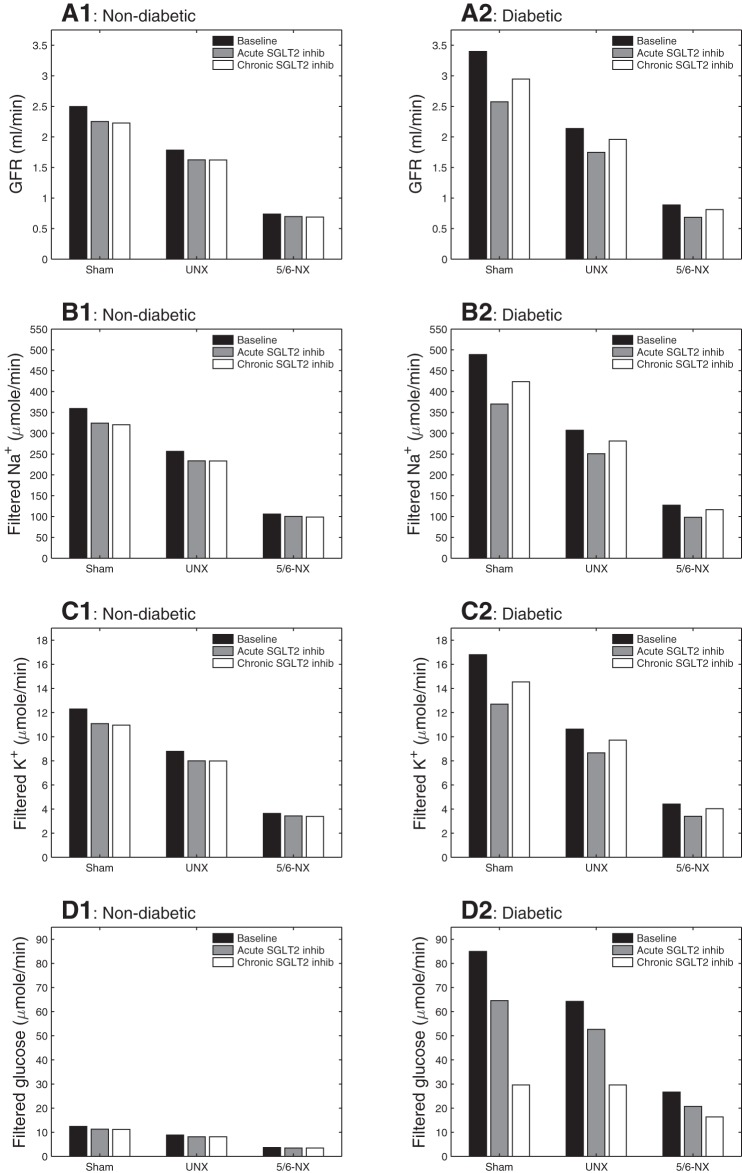
We considered six types of rat kidneys: nondiabetic sham, diabetic sham, nondiabetic UNX, diabetic UNX, nondiabetic 5/6-NX, and diabetic 5/6-NX. For each type of kidney, we simulated baseline conditions, as well as acute and chronic SGLT2 inhibition. Model parameters for each scenario are described in modeling methodology. In particular, diabetes induces hyperfiltration, whereas acute and chronic SGLT2 inhibition lowers GFR via TGF; see Fig. 1, A1 and A2. These effects on GFR are similarly reflected on filtered Na+ and K+ loads (Fig. 1, B1, B2, C1, and C2). Chronic SGLT2 inhibition in a diabetic kidney lowers plasma glucose concentration from 25 to 10 mM in sham, from 30 to 15 mM in UNX, and from 30 to 20 mM in 5/6-NX; see filtered glucose load in Fig. 1D2.
Fig. 1.
Glomerular filtration rate (GFR) (A), filtered Na+ load (B), K+ load (C), and glucose load (D), given per animal. Column at left (A1–D1): nondiabetic sham, uninephrectomized (UNX), and 5/6-nephrectomized (5/6-NX) kidneys; column at right (A2–D2): diabetic kidneys. A reduction in nephron number lowers the total filtered rates of fluid (GFR), Na+, K+, and glucose and reduces the impact of diabetes on these parameters.
Because this study contains a large number of simulations, the description of the results is inevitably lengthy. For a cursory review of these results, the reader may focus on 1) the nine figures, each supplied with a self-contained caption, and 2) the summary paragraph found at the end of each subsection in simulation results.
Effects of SGLT2 inhibition on solute transport in a nondiabetic sham kidney.
In the nondiabetic sham kidney, SGLT2 inhibition yielded glucouria (Fig. 2D1). This is a result of the much inhibited glucose reabsorption via SGLT2 along the proximal convoluted tubule, which is responsible for reabsorbing almost all the filtered glucose under baseline conditions. With acute SGLT2 inhibition, in which functional SGLT2 expression was reduced by 75%, glucose reabsorption along this segment decreased by 70%; with chronic SGLT2 inhibition, in which SGLT2 transport was completely eliminated, the corresponding glucose reabsorption decreased by 95% (Fig. 3A). The drastically elevated glucose delivery to the S3 segment increased glucose reabsorption along this segment via SGLT1 from 3 to 41 and 34% of filtered load, for acute and chronic SGLT2 inhibition, respectively. Note that a substantial amount of glucose was reabsorbed via SGLT1 under chronic SGLT2 inhibition even though SGLT1 expression was reduced by 35%. Glucose transport downstream of the proximal tubule remained negligible. SGLT2 inhibition led to glucose excretion at 3.0 and 6.8 μmol/min per animal (Fig. 2D1), for acute and chronic administration, respectively, compared with an almost negligible rate of 0.01 μmol/min under baseline conditions. These glucose excretion rates correspond to 27 and 61% of filtered load, respectively. Glucose excretion was predicted to be modestly higher with chronic vs. acute inhibition because of the downregulation of SGLT1.
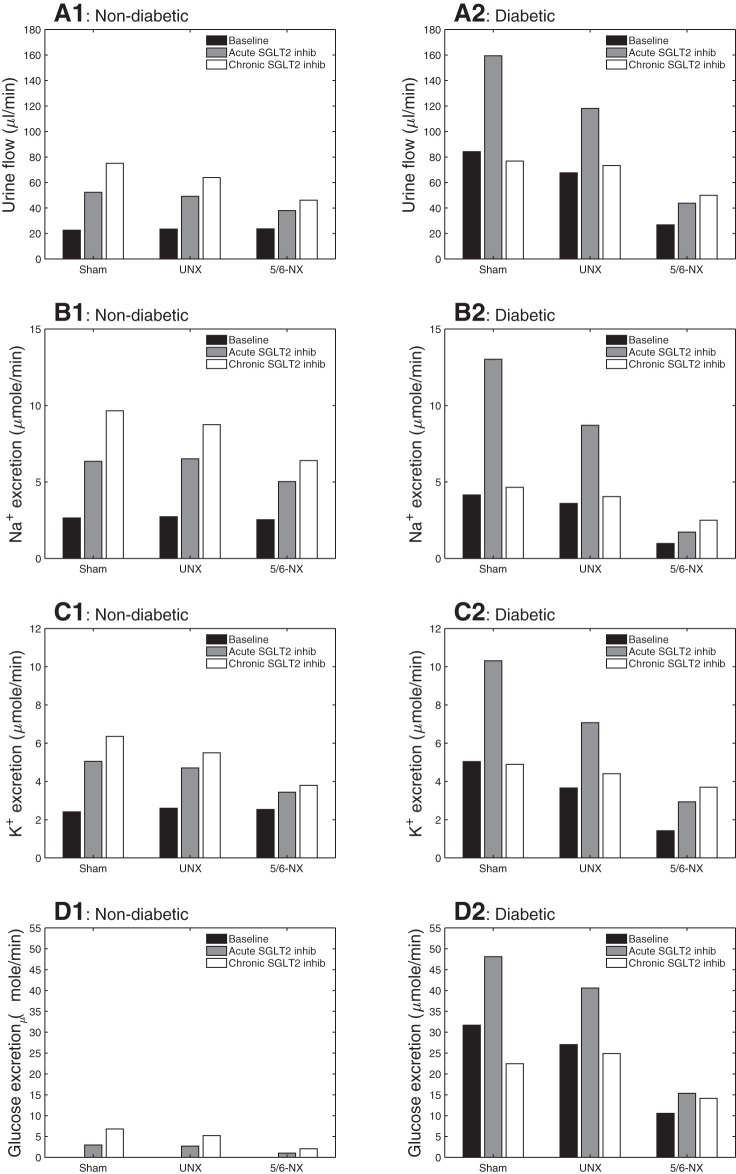
Fig. 2.
Predicted urinary flow (A), Na+ excretion (B), K+ excretion (C), and glucose excretion (D), given per animal. Column at left (A1–D1): nondiabetic sham, UNX, and 5/6-NX kidneys; column at right (A2–D2): diabetic kidneys. In nondiabetic rat, urine outputs are increased by acute Na+-glucose cotransporter 2 (SGLT2) inhibition, and even more so by chronic SGLT2 inhibition, which reduces SGLT1 expression and its compensation. This is attenuated because of lower GFR when nephrons are lost in UNX and 5/6-NX. In diabetic rat, the increase in urine output seen with acute SGLT2 inhibition is abolished with chronic SGLT2 inhibition in sham and UNX. This is due to the blood glucose-lowering effect of chronic SGLT2 inhibition. In a diabetic 5/6-NX rat, chronic inhibition of SGLT2 enhances total urinary excretion rates of fluid, Na+, K+ and glucose. This is because in 5/6-NX the reduction in proximal tubular Na+ reabsorption is not matched by a sufficient reduction in GFR and filtered Na+ and K+.
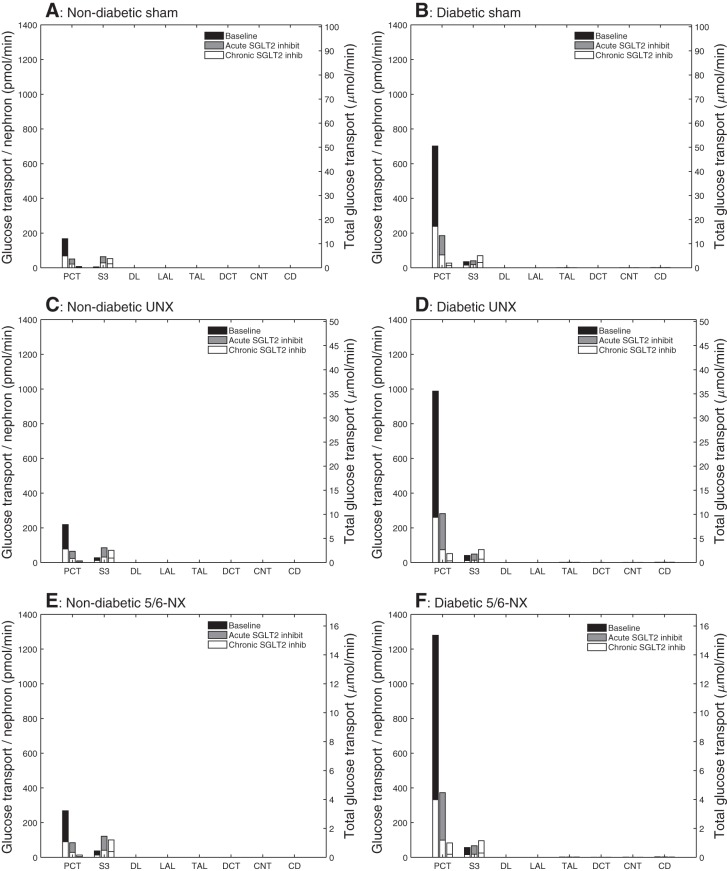
Fig. 3.
Comparison of glucose transport obtained for nondiabetic sham (A), diabetic sham (B), nondiabetic UNX (C), diabetic UNX (D), nondiabetic 5/6-NX (E), and diabetic 5/6-NX (F). Diabetes increases, and SGLT2 inhibition reduces glucose reabsorption in PCT. Diabetes and SGLT2 inhibition increase glucose transport along the S3 segment. Glucose uptake in individual S3 segments is increased to similar absolute levels by chronic SGLT2 inhibition in the diabetic and nondiabetic setting and largely independent of nephron number. PCT, proximal convoluted tubule; DL, descending limb; LAL, long ascending limb; DCT, distal convoluted tubule; CNT, connecting duct; CD, collecting duct.
Model simulations indicated that acute or chronic SGLT2 inhibition under normoglycemic conditions decreased both transcellular and paracellular Na+ transport relative to baseline conditions. SGLT2 inhibition reduced SNGFR by ~10% via TGF. The lower luminal flow suppressed active transcellular transport, via reduction of torque-induced luminal membrane transporter expression (8, 48). Also, SGLT2 inhibition elicited osmotic diuresis in the proximal tubule (see below). The higher water content diluted luminal Na+ and reduced passive Na+ reabsorption via the paracellular route along the proximal convoluted tubule. Together, these factors resulted in 18 and 22% reduction in total Na+ reabsorption along the proximal convoluted tubule for acute and chronic administration, respectively (Fig. 4A). Along the S3 segment, SGLT2 inhibition increased transcellular Na+ absorption but lowered paracellular transport. The two competing factors yielded ~20% reduction in total Na+ transport. The increased Na+ delivery downstream raised Na+ reabsorption along some segments, for example, by 14% along the thick ascending limbs under chronic SGLT2 inhibition. Nevertheless, urinary Na+ excretion increased by 2.4- and 3.6-fold under acute and chronic inhibition, respectively. These excretion rates correspond to an increase in baseline fractional Na+ excretion of 0.7 to 1.6 and 2.3%, respectively, with acute and chronic SGLT2 inhibition.
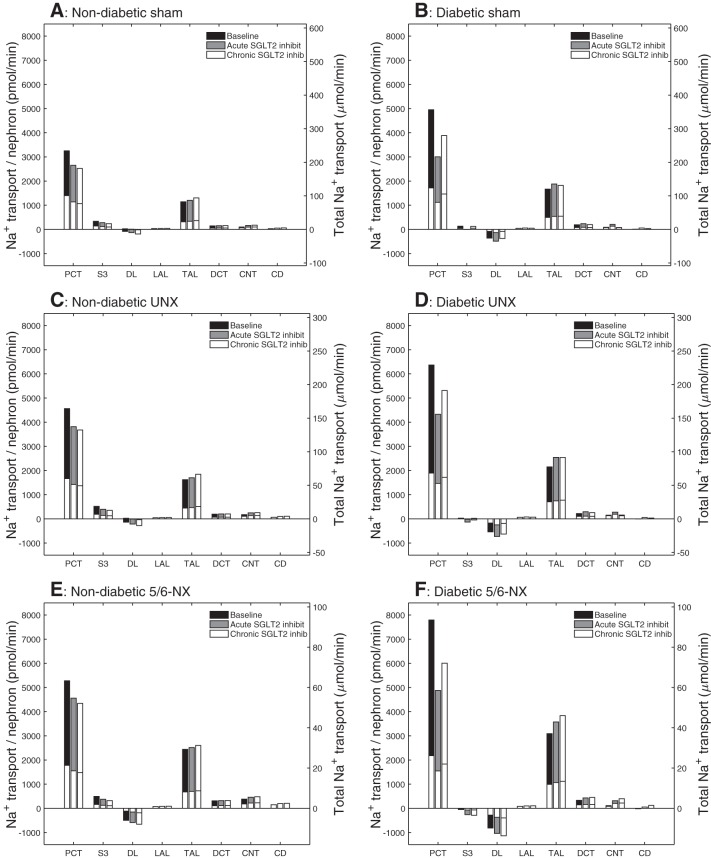
Fig. 4.
Comparison of Na+ transport obtained for nondiabetic sham (A), diabetic sham (B), nondiabetic UNX (C), diabetic UNX (D), nondiabetic 5/6-NX (E), and diabetic 5/6-NX (F). Transport is taken positive for reabsorption. Top and bottom bars denote superficial and juxtamedullary nephron values, respectively. Transport values are scaled with the corresponding nephron population fraction (i.e., 2/3 and 1/3 for superficial and juxtamedullary nephron, respectively). Left axis shows per nephron values. Height of top bars denotes whole-kidney values, right axis. Note different scales for sham, UNX, and 5/6-NX. Diabetes and the reduction in nephron number enhance Na+ reabsorption/nephron in PCT and thick ascending limb. Diabetes reduces Na+ reabsorption in S3. In a nondiabetic or diabetic kidney, both acute and chronic SGLT2 inhibition reduces Na+ reabsorption along the PCT and the S3 segment, increases Na+ secretion along the descending limb, and increases Na+ reabsorption along the thick ascending limb. In a diabetic kidney, acute SGLT2 inhibition yields a larger decrease in Na+ transport in the PCT than with chronic SGLT2 inhibition because of lower blood glucose levels in the latter in sham, UNX, and 5/6-NX. Notations are analogous to Fig. 3.
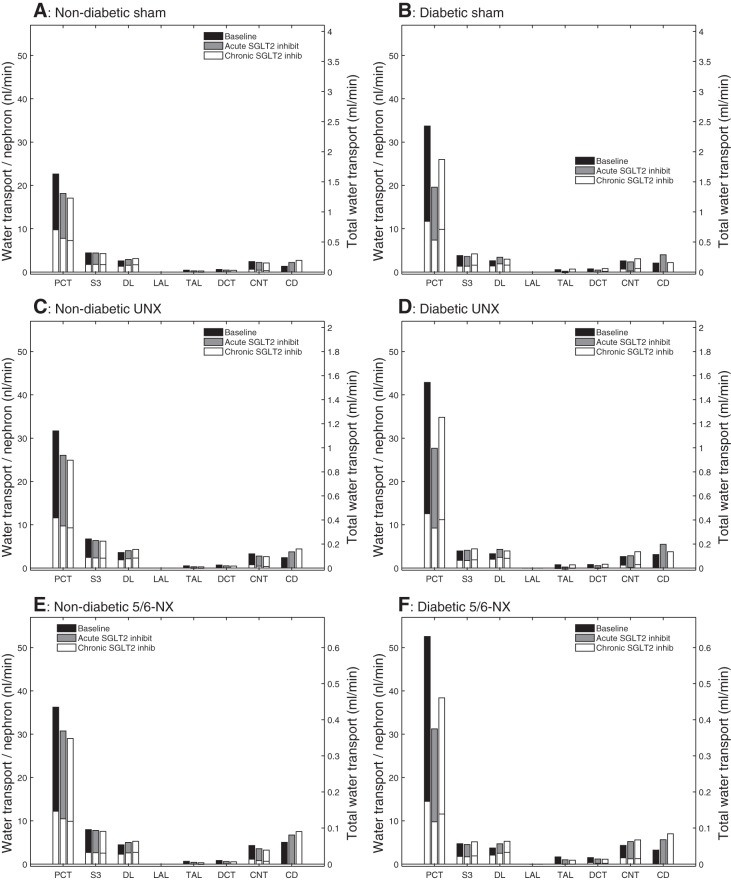
The retention of Na+ and glucose in the tubular fluid increased tubular fluid osmolality and limited water reabsorption (Fig. 5A). In particular, water reabsorption along the proximal convoluted tubule decreased by 20 and 25% for acute and chronic administration of the SGLT2 inhibitor, respectively (Fig. 5A). As a result, the model predicted that acute and chronic SGLT2 inhibition resulted in diuresis, with urine flow increased by 2.3- and 3.3-fold over baseline, respectively (Fig. 2A1), despite the ~10% reduction in SNGFR.
Fig. 5.
Comparison of water transport obtained for nondiabetic sham (A), diabetic sham (B), nondiabetic UNX (C), diabetic UNX (D), nondiabetic 5/6-NX (E), and diabetic 5/6-NX (F). Notations are analogous to Fig. 3. SGLT2 inhibition reduces water reabsorption along the PCT but not in S3 segments. This is observed in sham, UNX, and 5/6-NX in the presence and absence of diabetes and largely independent of nephron number.
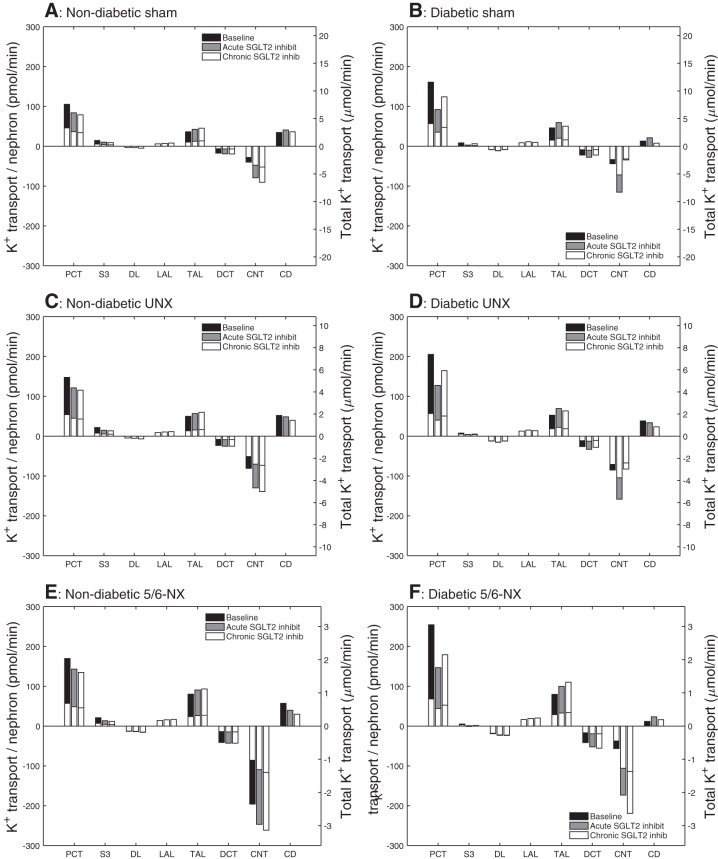
The elevated water content diluted K+ and reduced its reabsorption along some of the nephron segments, for example, the proximal convoluted tubule. But more importantly, the increased Na+ delivery to the connecting tubules enhanced K+ secretion by 17 and 24% under acute and chronic SGLT2 inhibition, respectively (Fig. 6A). Consequently, K+ excretion increased by 2.1- and 2.6-fold, respectively, with acute and chronic SGLT2 inhibition (Fig. 2C). The effect of chronic SGLT2 inhibition is masked by activation of neurohumoral and potentially morphological compensation mechanisms that match Na+, K+, and fluid excretion to intake. In accordance, studies in nondiabetic SGLT2 knockout mice showed an increase in plasma renin activity to enhance renal Na+ retention, whereas plasma aldosterone was reduced to limit urinary K+ loss (43).
Fig. 6.
Comparison of K+ transport obtained for nondiabetic sham (A), diabetic sham (B), nondiabetic UNX (C), diabetic UNX (D), nondiabetic 5/6-NX (E), and diabetic 5/6-NX (F). Notations are analogous to Fig. 3. In a nondiabetic kidney, both acute and chronic SGLT2 inhibition reduces K+ reabsorption along the PCT and increases K+ secretion along the CNT. In a diabetic kidney, only acute inhibition increases K+ secretion along the CNT, unless the nephron number is strongly reduced as in 5/6-NX, in which case chronic SGLT2 inhibition induces a further increase in K+ secretion.
Effects of SGLT2 inhibition on solute transport in the kidney of a nondiabetic nephrectomized rat.
How does nephron loss affect the effects of SGLT2 inhibition? Total filtered glucose load in UNX and 5/6-NX is 29 and 70%, respectively, less than sham. However, nephron loss induces hyperfiltration in remaining nephrons (13); thus single-nephron-filtered glucose load is 43 and 77% higher in UNX and 5/6-NX, respectively, than sham. With intact SGLT2 and SGLT1, the filtered glucose load per nephron remains below the single-nephron transport maximum, with the contribution of SGLT1-mediated glucose reabsorption increasing from ~4% in sham to 11 and 14% in UNX and 5/6-NX, respectively, and no glucose is excreted into the urine.
Overall, the effects of SGLT2 inhibition in a nondiabetic UNX or 5/6-NX kidney are qualitatively similar to those for the sham kidney. Under acute and chronic SGLT2 inhibition, 34 and 28%, respectively, of filtered glucose was reabsorbed via SGLT1 along the S3 segment in UNX (46 and 38% in 5/6-NX). Urinary glucose excretion was predicted to be 2.7 and 5.2 μmol/min, respectively, in UNX (33 and 64% of filtered glucose) and 1.0 and 2.1 μmol/min, respectively, in 5/6-NX (29 and 60% of filtered glucose); see Fig. 2D1. The effect of SGLT2 inhibition on Na+ in UNX and 5/6-NX is slightly smaller than in sham, especially for 5/6-NX; compare Fig. 4, A, C, and E. Urinary Na+ excretion increased by ~1.4- and 2.2-fold above baseline in UNX under acute and chronic SGLT2 inhibition, respectively, and by ~1- and 1.5-fold, respectively, in 5/6-NX (compared with 2.4- and 3.6-fold in sham).
Absolute urine flow and urinary K+ excretion also increased substantially, albeit by a smaller magnitude compared with sham, especially for 5/6-NX; see Fig. 2. Specifically, acute and chronic SGLT2 inhibition in UNX increased urine flow by 1.1- and 1.7-fold, respectively, above baseline, and urinary K+ excretion by 81 and 112%, respectively, above baseline. Smaller increases were seen in 5/6-NX: acute and chronic SGLT2 inhibition increased urine flow by 60 and 95%, respectively, above baseline, and urinary K+ excretion by 36 and 50%, respectively, above baseline. Overall, a reduction in nephron number lowered the glucosuric, diuretic, natriuretic and kaliuretic effect of SGLT2 inhibition.
Taken together, SGLT2 inhibition in a nondiabetic kidney results in glucosuria and increased urinary output. The latter is significantly more pronounced in sham than in UNX and 5/6-NX because of lesser total glucose filtration in the latter. A diuretic, natriuretic, and kaliuretic potential of SGLT2 inhibition was preserved in UNX and 5/6-NX kidneys, although reduced compared with sham.
Effects of SGLT2 inhibition on metabolism in the kidney of a nondiabetic sham and nephrectomized rat.
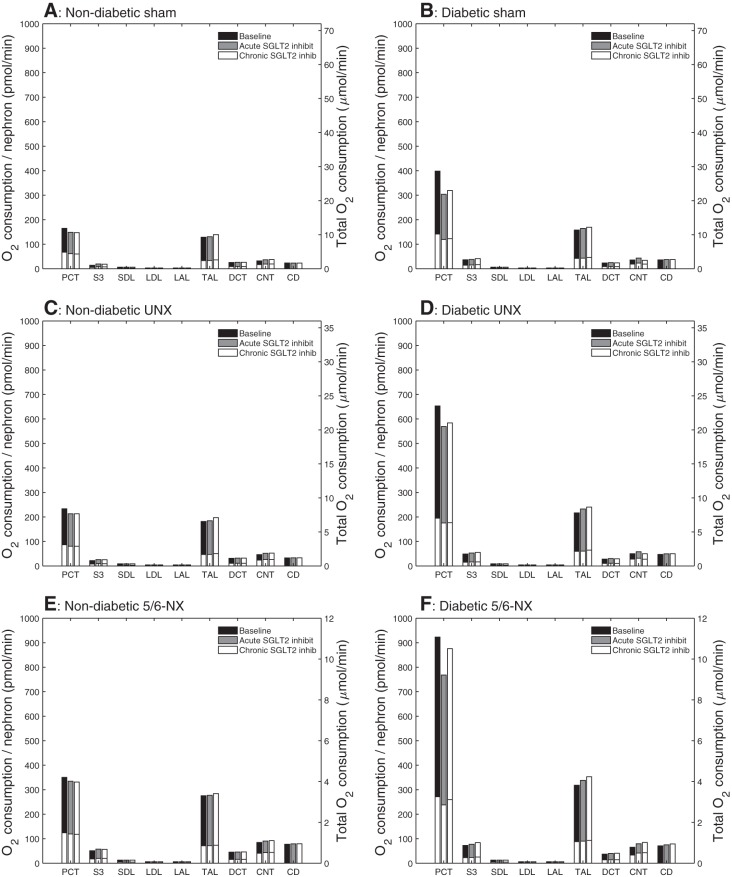
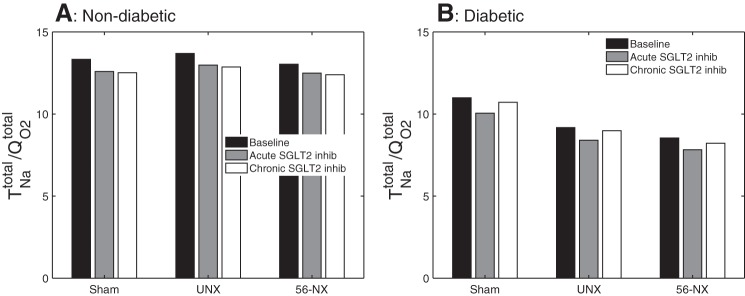
As previously noted, acute and chronic SGLT2 inhibition lowered active Na+ reabsorption along the proximal convoluted tubule. This results in an ~10% reduction in total transport O2 consumption along this segment, in sham, UNX, and 5/6-NX. In contrast, a shift to transcellular Na+ transport was observed along the S3 segment because of enhanced SGLT1-mediated transport. The absolute increase in transcellular Na+ transport and O2 consumption was similar in sham, UNX, and 5/6-NX, but given the higher baseline O2 consumption in the nephrectomized kidneys, the model predicted that total S3 segmental O2 consumption increase by 37 and 34%, respectively, with acute and chronic SGLT2 inhibition in the sham kidney, by 20 and 18%, respectively in the UNX kidney, and by 13 and 11%, respectively, in the 5/6-NX kidney. On the level of a single superficial S3 segment, total O2 consumption increased from 14, 22, and 51 pmol/min in untreated sham, UNX, and 5/6-NX rats to 19, 26, and 57 pmol/min in response to chronic SGLT2 inhibition (basal O2 consumption is taken to be 1/4 of the total O2 consumption in the untreated case). Along the distal segments, O2 consumption was predicted to increase slightly, in UNX and 5/6-NX vs. sham, on a single-nephron level (Fig. 7). Due primarily to the shift from paracellular to transcellular Na+ reabsorption along the proximal tubules under SGLT2 inhibition, Na+ transport efficiency decreased in sham from its baseline value of 13.3 to 12.6 and 12.5 for acute and chronic inhibition, in UNX from 13.7 to 13.0 and 12.9, respectively, and in 5/6-NX from 13.0 to 12.5 and 12.4, respectively (Fig. 8).
Fig. 7.
Segmental total O2 consumption (). Notations are analogous to Fig. 3. SGLT2 inhibition reduces in PCT and increases it in S3 segments and thick ascending limb in all six subgroups. In a nondiabetic kidney, the largest relative increase in in response to SGLT2 inhibition is seen in the S3 segment. A reduction in nephron number increases absolute nephron O2 consumption, particularly in PCT and thick ascending limb, and similarly in nondiabetic and diabetic settings. Diabetes primarily increases nephron O2 consumption in PCT; those increases are attenuated by SGLT2 inhibition, especially with acute administration. The reduction in PCT O2 consumption in a diabetic kidney in response to chronic SGLT2 inhibition is attenuated by the loss in nephron population, especially in 5/6-NX. This is due to the reduced blood glucose-lowering effect of chronic SGLT2 inhibition in nephrectomized rats.
Fig. 8.
Whole-animal tubular Na+ transport efficiency given by the ratio of total Na+ transport () to oxygen consumption (), obtained for nondiabetic sham, UNX, and 5/6-NX kidneys (A) and the corresponding diabetic kidneys (B). Diabetes significantly lowers as does SGLT2 inhibition and nephron loss. In a nondiabetic kidney, acute and chronic SGLT2 inhibition reduces to a similar extent. In a diabetic kidney, is partially recovered by chronic SGLT2 inhibition, in part due to its plasma glucose-lowering effect.
Taken together, SGLT2 inhibition in a nondiabetic kidney significantly elevates O2 consumption along the S3 segment, with a similar absolute increase on the single-nephron level when nephron number is reduced. SGLT2 inhibition reduces renal Na+ transport efficiency to similar extent in sham, UNX, and 5/6-NX (by <10%).
Effects of SGLT2 inhibition on solute transport in a diabetic sham kidney.
Diabetes induces hyperfiltration (44), but SGLT2 inhibition lowers GFR by ~30% with acute SGLT2 inhibition and halfway to its value in nondiabetic rat with chronic SGLT2 inhibition (see above). In a hyperfiltering diabetic sham kidney with blood glucose of 25 mM, 59% of the filtered glucose was predicted to be reabsorbed via SGLT2 along the proximal convoluted tubule under baseline conditions. That transport fraction was reduced to 21 and 7%, respectively, of filtered glucose under acute and chronic SGLT2 inhibition. (Recall that filtered glucose load in acute and chronic inhibition is 76 and 31% of baseline because of differential effects on GFR and blood glucose; see Fig. 1D2.) Unlike a nondiabetic sham kidney, a significant amount of glucose exited the proximal convoluted tubule in a diabetic sham kidney since the glucose load exceeded transport capacity. Under baseline diabetic conditions, the S3 segment reabsorbed 3% of filtered glucose via SGLT1. Under acute and chronic SGLT2 inhibition in diabetes, 5 and 19% of filtered glucose was reabsorbed along the S3 segment. In all three scenarios, a significant amount of glucose was excreted in the urine (32, 48, and 22 μmol/min per animal for baseline, acute, and chronic inhibition, respectively). These results can be contrasted with those obtained for nondiabetic kidneys above. For a nondiabetic kidney, acute and chronic SGLT2 inhibition yielded glucouria at similar levels (Fig. 2D1). In contrast, for a diabetic kidney, acute SGLT2 inhibition resulted in the highest glucose excretion, ~1.5 times of baseline and twice of chronic inhibition. Chronic SGLT2 inhibition lowered plasma glucose concentration, GFR, and filtered glucose load, resulting in the lowest glucose excretion in the new steady state.
In the diabetic sham kidney, SGLT2 inhibition raised tubular fluid glucose concentration and thus osmolality, thereby reducing water reabsorption. Because chronic SGLT2 inhibition lowered plasma glucose concentration, proximal convoluted tubule water reabsorption was lowest with acute SGLT2 inhibition. That scenario also yielded the highest urine flow at 159 μl/min, compared with 84 and 77 μl/min for baseline and chronic SGLT2 inhibition.
Acute and chronic SGLT2 inhibition under hyperglycemic and sham conditions reduced both transcellular and paracellular Na+ transport along the proximal convoluted tubule; total Na+ reabsorption along this segment decreased to 61 and 78% of baseline, respectively. Na+ reabsorption was decreased more with acute inhibition because it also yielded the largest reduction in water reabsorption; the higher water content diluted luminal Na+ concentration and decreased Na+ reabsorption, particularly via the paracellular pathway. Indeed, luminal Na+ was sufficiently low along the S3 segment that Na+ was secreted via the paracellular pathway, resulting in almost no Na+ reabsorption along this segment. Na+ delivery to the thick ascending limbs, and thus Na+ transport along these segments, was the highest with acute SGLT2 inhibition. Compared with baseline, Na+ delivery to the connecting tubules increased by ~3-fold with acute SGLT2 inhibition. As a result, connecting tubule K+ secretion increased by 163%. Na+ delivery and K+ secretion along the connecting tubule with chronic SGLT2 inhibition were similar to baseline. Taken together, in diabetic conditions, urinary Na+ excretion was predicted to more than triple with acute inhibition, and to increase by 12% with chronic inhibition; urinary K+ excretion doubled with acute SGLT2 inhibition and was essentially unchanged from baseline with chronic inhibition because of the strong blood glucose-lowering effect.
Effects of SGLT2 inhibition on solute transport in the kidney of a diabetic nephrectomized rat.
How does nephron loss affect the effects of SGLT2 inhibition in diabetes? The effects of SGLT2 inhibition in the diabetic UNX and 5/6-NX kidneys were qualitatively similar to sham. Under acute and chronic SGLT2 inhibition, 3 and 9% of that filtered glucose was reabsorbed via SGLT1 along the S3 segment in the diabetic UNX kidney (4 and 7%, respectively, in 5/6-NX). Acute SGLT2 inhibition in UNX and 5/6-NX yielded glucose excretion of 41 and 15 μmol/min, which was lower than in sham (48 μmol/min). That acute effect can be explained by the reduced GFR and thus filtered glucose load in UNX and 5/6-NX. The effect of chronic SGLT2 inhibition was more complex: whereas GFR was reduced in UNX and 5/6-NX, plasma glucose concentration was assumed to remain higher in these models (15 and 20 mM, respectively, compared with 10 mM in sham). Thus, compared with sham, the filtered glucose load was higher in UNX but lower in 5/6-NX (see Fig. 1D2). And compared with the glucose excretion of 20 μmol/min in sham, chronic SGLT2 inhibition yielded a slightly higher glucose excretion of 25 μmol/min in UNX and a lower excretion of 14 μmol/min in 5/6-NX.
The effect of SGLT2 inhibition on Na+ excretion in diabetic UNX and 5/6-NX is similar to sham; compare Fig. 4, B, D, and F. Acute and chronic SGLT2 inhibition lowered Na+ reabsorption along the proximal convoluted tubule by 32 and 17%, respectively, in UNX, and by 37 and 23%, respectively, in 5/6-NX (similar to 39 and 22% reduction in diabetic sham). Along the S3 segment, acute and chronic SGLT2 inhibition reversed the direction of Na+ transport, from reabsorption in sham (see above) to secretion in UNX, and further increased Na+ secretion by >5-fold (compared with the untreated case) in 5/6-NX. This was caused by the reduction in luminal Na+ concentrations due to strong water retaining effect of the very high amounts of filtered glucose that were delivered to the S3 segment in response to SGLT2 inhibition. Along the thick ascending limbs, acute and chronic SGLT2 inhibition increased Na+ transport by 18% in UNX and by 16 and 24%, respectively, in 5/6-NX (compared with 13 and 9% in sham). Acute SGLT2 inhibition increased urinary Na+ excretion by 142 and 75%, respectively, in UNX and 5/6-NX, which is lower than in sham (214%). Chronic SGLT2 inhibition yielded similar fractional increases in urinary Na+ in sham and UNX (12%) but a much higher increase in 5/6-NX of 56% (Fig. 2B2), because of the decreased Na+ reabsorption along the proximal convoluted tubule as well as to the increased Na+ secretion along the S3 segment.
Acute SGLT2 inhibition increased urine flow, albeit to a lower extent in a nephrectomized rat: urine flow increased by 89, 75, and 63% in sham, UNX, and 5/6-NX, respectively. In contrast, whereas chronic SGLT2 inhibition reduced urine flow by 9% in sham, it increased urine flow by 8 and 87% in UNX and 5/6-NX, respectively. The latter response in 5/6-NX is due to the decreased Na+ reabsorption along the proximal convoluted tubule as well as to the increased Na+ secretion along the S3 segment, both of which inhibited water reabsorption. Acute SGLT2 inhibition increased urinary K+ excretion among the three models by similar fractions: 105, 93, and 107% in sham, UNX, and 5/6-NX, respectively, whereas chronic SGLT2 inhibition decreased urinary K+ excretion slightly in sham (3%), it increased K+ excretion by 20% in UNX, and even more so by 160% in 5/6-NX.
Overall, with acute SGLT2 inhibition, a reduction in nephron number lowered the glucosuric, diuretic, natriuretic, and kaliuretic effects, whereas the corresponding effects of chronic SGLT2 inhibition were enhanced by a strong reduction in nephron number. The relatively enhanced chronic natriuretic and diuretic effects in 5/6-NX may explain in part the observed blood pressure reduction effect and beneficial effects on heart failure of SGLT2 inhibitors in diabetic patients with impaired kidney function (34).
Effects of SGLT2 inhibition on metabolism in the kidney of a diabetic sham and nephrectomized rat.
As previously noted, acute SGLT2 inhibition reduced transcellular Na+ transport and thus along the proximal convoluted tubule of a diabetic kidney, in large part because of a lower GFR. Chronic SGLT2 inhibition has a similar effect on proximal convoluted tubule transcellular Na+ reabsorption, although that effect was attenuated by a reduction in nephron number (Fig. 7, B, D, and F). This is due to the higher plasma glucose concentration, relative to sham, assumed in a nephrectomized kidney following chronic SGLT2 inhibition. SGLT2 inhibition had smaller effects on of downstream segments.
An increase in transcellular Na+ transport was observed along the S3 segment due to enhanced SGLT1-mediated transport. The model predicted that total S3 segmental O2 consumption increase by 4 and 18%, respectively, with acute and chronic SGLT2 inhibition in the diabetic sham kidney. These percentage increases are significantly smaller than the analogous increases in the nondiabetic sham kidney (37 and 34%, respectively; see above) because of the blood glucose and GFR-lowering effect in diabetes. In the diabetic UNX and 5/6-NX kidney, total S3 segmental O2 consumption increased by 8 and 14%, and by 7 and 16%, respectively, that is, similar to diabetic sham for all S3 segments combined, but the O2 consumption of the individual S3 segment increased with the loss of nephron number (Fig. 7).
In a diabetic kidney, metabolic efficiency is assumed to be reduced (see modeling methodology). Thus Na+ transport efficiency was predicted to be 11.0, 9.2, and 8.5 for sham, UNX, and 5/6-NX, respectively, compared with ~13.5 in a nondiabetic kidney (Fig. 8). With SGLT2 inhibition, the larger reduction in paracellular relative to transcellular Na+ transport along the proximal convoluted tubule lowered Na+ transport efficiency in that segment as well as in the kidney overall. The model predicted that with acute and chronic SGLT2 inhibition, Na+ transport efficiency was 10.1 and 10.7, respectively, in a diabetic sham kidney (which correspond to 8.5 and 2.5% reduction in efficiency, respectively), 8.4 and 9.0, respectively, in a diabetic UNX kidney (8.4 and 2.1% reduction), and 7.8 and 8.2, respectively, in a diabetic 5/6-NX kidney (8.4 and 3.8% reduction); see Fig. 8.
Summary.
Compared with baseline diabetic, both acute and chronic SGLT2 inhibition was associated with lower active Na+ reabsorption along the proximal tubule in sham, UNX, and 5/6-NX, mainly driven by the reduction in GFR (Fig. 4, B, D, and F). This increased Na+ load on downstream segments, where Na+ transport efficiency is lower compared with the proximal tubule. Chronic SGLT2 inhibition in a diabetic UNX kidney and even more in a 5/6-NX kidney increased paracellular secretion of Na+ along the proximal convoluted tubule and the S3 segment, thereby enhancing natriuresis relative to baseline. On the other hand, this is the setting with the highest active Na+ transport and metabolic requirement on the individual S3 and thick ascending limb segments (Fig. 7), because of the highest rates of transcellular Na+ reabsorption, and is associated with the lowest whole-kidney Na+ transport efficiency (Fig. 8).
A notable effect of chronic SGLT2 inhibition is the reduction of plasma glucose concentration. The impact of lowered plasma glucose concentration on kidney function is considered in the appendix.
DISCUSSION
The principal objective of this study was to assess the theoretical impact of diabetes and SGLT2 inhibition on solute transport, O2 consumption, and urinary excretion in kidneys with normal and reduced nephron number. Key predictions of the present study include the following:
In a nondiabetic kidney, both acute and chronic SGLT2 inhibition induces glucosuria, diuresis, natriuresis, and kaliuresis (Fig. 2), due in large part to the reduction in reabsorption along the proximal convoluted tubule. These effects are attenuated when the nephron number is reduced because of lesser filtration.
In a diabetic kidney with normal nephron number, acute SGLT2 inhibition induces diuresis, natriuresis, and kaliuresis (Fig. 2), but such an effect is attenuated with chronic inhibition, due to GFR and blood glucose lowering.
A reduction in nephron number in the diabetic kidney attenuates the acute response to SGLT2 inhibition but enhances the chronic increase in diuresis, natriuresis, and kaliuresis (Fig. 2).
To the extent that renal fluid and Na+ excretion determine blood pressure and heart failure, this may explain why the blood pressure lowering and heart failure protective effect of chronic SGLT2 inhibition is preserved in diabetic patients with chronic kidney diseases (CKD) and reduced GFR.
These results are summarized in Fig. 9. The present nephron model can be used as an essential component in integrative computational models of whole-kidney function (6, 17, 18, 19, 26), in which interstitial fluid composition would be predicted instead of assumed known a priori.
Fig. 9.
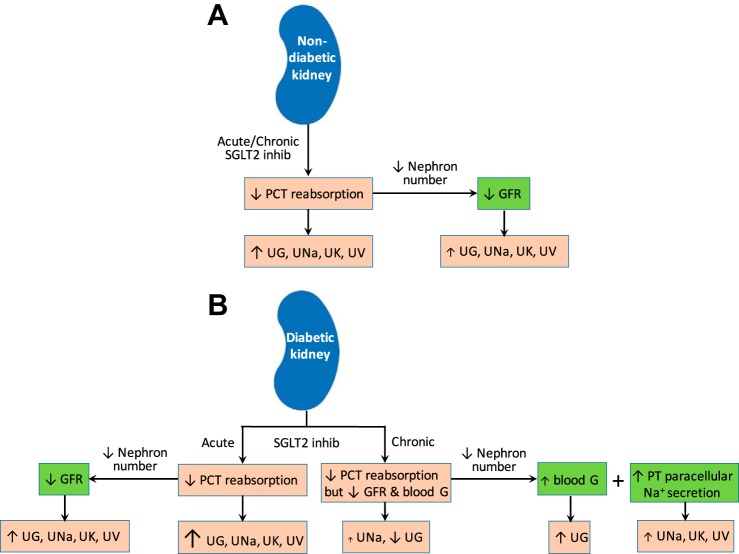
Schematic diagram summarizing predicted effects, and the underlying mechanisms, of acute and chronic SGLT2 inhibition on a nondiabetic kidney (A) and a diabetic kidney (B) in terms of urinary excretion of glucose (UG), Na+ (UNa), K+ (UK), and fluid (UV). Arrows in pink boxes indicate effects of acute/chronic SGLT2 inhibition in a nondiabetic/diabetic kidney. Arrows in green boxes indicate the effects of nephron loss in a nondiabetic/diabetic kidney treated with SGLT2 inhibition. Arrow size scales with effect. Blood G, blood glucose.
SGLT2 inhibition induces diuresis and natriuresis in nondiabetic kidneys.
Acute and chronic SGLT2 inhibition had similar effects in a nondiabetic sham kidney. Both maneuvers modestly lowered GFR and filtered Na+ load (Fig. 1) and more strongly reduced Na+ reabsorption along the proximal convoluted tubule (Fig. 4). The competing effects of reduced GFR and proximal reabsorption were predicted to yield diuresis, natriuresis, and kaliuresis in nondiabetic kidneys with normal or reduced nephron number (Fig. 2). This result suggests that SGLT2 inhibitors may offer blood pressure reduction benefit and facilitate renal K+ excretion for nondiabetic patients with or without impaired kidney function.
The model predicts that in nondiabetic rats, acute and chronic SGLT2 inhibition shifts Na+ transport from the paracellular to the transcellular route along the proximal convoluted tubules. In addition, the reduced early proximal tubule transport shifts Na+ load to downstream segments (S3 and thick ascending limb), which are less efficient in transporting Na+. This enhanced O2-consuming transcellular Na+ reabsorption in the latter segments and, consequently, whole-animal Na+ transport efficiency decreased significantly (Fig. 8A). It is noteworthy that in the nondiabetic setting, nephron loss itself did not significantly reduce whole-kidney Na+ transport efficiency.
SGLT2 inhibition induced diuresis and natriuresis in diabetic kidneys.
In addition to its blood glucose-lowering effects, SGLT2 inhibitors have been shown to lower blood pressure (2, 30). Moreover, results from the recent EMPA-REG OUTCOME trial indicated that the SGLT2 inhibitor empagliflozin significantly reduced the rate of primary composite cardiovascular outcome and mortality from any cause and the rate of heart failure in patients with Type 2 diabetes at high cardiovascular risk (49). Similar effects on blood pressure and heart failure were more recently observed in Type 2 diabetic patients with canagliflozin, another SGLT2 inhibitor (27). Therefore we sought to gain mechanistic insights into any potential cardiovascular benefits of SGLT2 inhibitors for diabetic patients. We first consider the diabetic but otherwise healthy kidney, and ask whether such beneficial effects in diabetic and hypertensive patients could be attributed to a diuretic and natriuretic effect of SGLT2 inhibitors? Under diabetic conditions, acute and chronic SGLT2 inhibition decreased GFR (Fig. 1A2), with the reduction twice as large in acute compared with chronic inhibition. The lower GFR and filtered Na+ load reduces Na+ transport along the proximal convoluted tubule (Fig. 4). Despite lowering GFR, acute SGLT2 inhibition was predicted to result in strong diuresis and natriuresis in diabetic kidneys with normal or 50% reduction in nephron number with a much lesser acute effect when nephron number was further reduced (Figs. 2 and 4). This acute diuretic and natriuretic effect was mainly due to the reduction in paracellular Na+ reabsorption along the proximal tubule.
Can chronic SGLT2 inhibition induce diuresis and natriuresis when nephron number and GFR are reduced and what could be the underlying mechanism?
In response to severe nephron loss, the glucosuric and thus the plasma glucose-lowering effect of chronic SGLT2 inhibition was assumed to be impaired (from 30 to 20 mM in 5/6-NX, compared with 25 to 10 mM in sham). Taken in isolation, the higher tubular fluid osmolality would lead to osmotic diuresis (relative to the nondiabetic case). This is amplified by the increase in single-nephron GFR in the remaining nephrons and the reduction of glucose reabsorption by SGLT2 inhibition. All together this markedly decreased net proximal tubule Na+ reabsorption, in large part because of a predicted fivefold increase in paracellular Na+ secretion. A reduced but still substantial natriuretic and diuretic effect of SGLT2 inhibition was predicted in diabetic 5/6-NX when blood glucose levels were kept at diabetic sham levels. In other words, chronic SGLT2 inhibition enhances the diuretic and natriuretic effect of hyperglycemia and this effect is relatively enhanced when the nephron number is reduced (Fig. 2). A potential clinical implication of this result is that the natriuretic and thereby blood pressure lowering effects and the heart failure protection of SGLT2 inhibitors persist in diabetic patients with CKD and reduced GFR (30, 34, 46). The prediction that hyperglycemia facilitates the diuretic and natriuretic potential of SGLT2 inhibition may also explain why effects of SGLT2 inhibitors on volume status rather than HbA1C levels were the most important mediators of the reduction in risk of cardiovascular death (12). In other words, the attenuated blood glucose-lowering effect of SGLT2 inhibitors in patients with CKD may not be all detrimental. On the other hand, this preserved natriuretic and diuretic effect of chronic SGLT2 inhibition in the diabetic kidney with CKD indicated that the risk to develop volume depletion, as may happen when combined with impaired fluid intake or other mechanisms of body fluid loss, may be well preserved, especially when blood glucose levels remain very high.
To what extent does SGLT2 inhibition enhance Na+ transport of vulnerable downstream nephron segments and increase outer medullary hypoxia?
The diminished Na+ reabsorption in the proximal convoluted tubule that follows acute or chronic SGLT2 inhibition was partially compensated by enhanced downstream transport; our model predicted that this is true in a diabetic or nondiabetic kidney. In diabetic sham rats, acute SGLT2 inhibition raised active Na+ transport-induced O2 consumption by 4% along the individual S3 segment, with larger increases predicted in the diabetic UNX and 5/6-NX kidneys of 8 and 7%, respectively (see Fig. 7). Chronic SGLT2 inhibition in diabetic rats yielded higher increases in along the individual S3 segment with modestly reduced levels when the nephron number was lowered (18, 14, and 16% in sham, UNX, and 5/6-NX kidneys, respectively). Furthermore, chronic SGLT2 inhibition was predicted to increase transcellular Na+ transport and along the thick ascending limb by 3, 9, and 9% in a diabetic sham, UNX, and 5/6-NX rat, respectively. The model predicted relatively minor effects for transcellular Na+ transport along the thick ascending limb in response to SGLT2 inhibition, particularly in a sham diabetic kidney, because the luminal concentration of Na+ along the segment was sufficiently high under baseline conditions so that active Na+ transport was essentially carrier limited. Indeed, SGLT2 inhibition would have a stronger effect on medullary thick ascending limb if expression of NKCC2 were upregulated.
The S3 segment, together with the thick ascending limb, are known to be particularly vulnerable to hypoxic injury. Thus the predicted increase in S3 segment and thick ascending limb O2 consumption suggests that SGLT2 inhibitors may increase hypoxia in that segment. Given that peritubular capillary flow has been observed to decrease in diabetes (10), the risk for hypoxia may be higher in a diabetic kidney. On the other hand, the proposed SGLT2 inhibitor-induced reduction in oxygen pressure in the deep cortex and outer medulla may stimulate hypoxia-inducible factors HIF1 and HIF2 and enhance erythropoietin release from interstitial cells (38). Together with the diuretic effect, the latter may contribute to the observed modest increase in hematocrit and hemoglobin in patients in response to SGLT2 inhibition, which may improve the oxygenation of the outer medulla but also facilitate oxygen delivery to the heart and other organs. Remarkably, changes in hematocrit and hemoglobin mediated 51.8 and 48.9%, respectively, of the effect of the SGLT2 inhibitor empagliflozin vs. placebo on the risk of cardiovascular death on the basis of changes from baseline (12). In other words, SGLT2 inhibition may simulate systemic hypoxia to the sensor in deep cortex and outer medulla of the kidney, and the heart and kidney then benefit from the induced response. Moreover, our modeling approach predicted that the SGLT2 inhibition-induced changes in the oxygen signal at the renal sensor can be preserved in CKD.
Our model’s acute SGLT2 inhibition results were in general agreement with the in vivo data of O’Neill et al. (31), who administered acute dual SGLT2/SGLT1 inhibition in nondiabetic and diabetic rats by using phlorizin. Model simulations suggested that acute SGLT2 inhibition lowers tubular Na+ transport, O2 consumption, and ratio in a nondiabetic kidney, with the reduction even more pronounced in a diabetic kidney (Figs. 4, 7, and 8). These results match the observations of O’Neill et al. (31). Furthermore, our model predicts that because of the shift in Na+ transport from the proximal tubule to downstream segments, O2 consumption is increased along medullary segments (Fig. 7). That result is consistent with the significant reduction in medullary Po2 in control and diabetic rats, elicited by acute SGLT2/SGLT1 inhibition (31). Our model extends the predictions to chronic SGLT2 inhibition and relevant levels of nephron loss.
In summary, acute and chronic SGLT2 inhibition lowered O2 consumption in the individual proximal convoluted tubule. This was predicted for diabetic and nondiabetic settings as well as with normal or reduced nephron numbers. The effect was stronger in diabetic than in nondiabetic rats. Conversely, SGLT2 inhibition induced a small increase in O2 consumption in the S3 segments and thick ascending limbs in all six subgroups. In nondiabetic rats, acute SGLT2 inhibition is glucosuric, diuretic, natriuretic, and kaliuretic. This effect is further enhanced with chronic SGLT2 inhibition and attenuated by nephron loss. In diabetic rats, the diuretic, natriuretic, and kaliuretic effect observed with acute SGLT2 inhibition was blunted with chronic SGLT2 inhibition unless the nephron number was severely reduced, in which case the excretion rates increased further with chronic SGLT2 inhibition. The latter may explain the blood pressure lowering and heart failure protective effects of SGLT2 inhibitors observed in diabetic patients despite CKD and limited glucosuria. A limited antihyperglycemic effect is actually predicted to further enhance the diuretic and natriuretic effect of SGLT2 inhibition. Moreover, the preserved kaliuretic effect of chronic SGLT2 inhibition may reduce K+ retention in diabetic patients with CKD. The latter may enhance the tolerability of renin-angiotensin-aldosterone system blockade in CKD and thus allow their continued use, which is expected to be of therapeutic benefit.
GRANTS
Support for this study was provided by the Department of Veterans Affairs (to V. Vallon); by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-112042 (to V. Vallon) and R01 DK-106102 (to A. Layton and V. Vallon); and by the UAB/UCSD O’Brien Center for Acute Kidney Injury Research, National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-079337 (to V. Vallon).
DISCLOSURES
Over the past 36 months, V. Vallon has served as a consultant and received honoraria from Bayer, Boehringer Ingelheim, Intarcia Therapeutics, Astra-Zeneca, Janssen Pharmaceutical, Eli Lilly and Merck, and received grant support for investigator-initiated research from Astra-Zeneca, Bayer, Boehringer Ingelheim, Fresenius, and Janssen. No conflicts of interest, financial or otherwise, are declared by A. T. Layton.
AUTHOR CONTRIBUTIONS
A.T.L. and V.V. conceived and designed research; A.T.L. performed experiments; A.T.L. and V.V. analyzed data; A.T.L. and V.V. interpreted results of experiments; A.T.L. prepared figures; A.T.L. drafted manuscript; A.T.L. and V.V. edited and revised manuscript; A.T.L. and V.V. approved final version of manuscript.
APPENDIX
Impact of plasma glucose-lowering effect of SGLT2 inhibitors.
A notable effect of chronic SGLT2 inhibition is the reduction of plasma glucose concentration, from 25 to 10 mM in a diabetic sham kidney, from 30 to 15 mM in a diabetic UNX kidney, and from 30 to 20 mM in a diabetic 5/6-NX kidney. We conducted simulations to reveal the impact of lowered plasma glucose concentration on overall kidney function. In these simulations, we kept plasma glucose concentration at diabetic levels, but preserved all other renal effects of chronic SGLT2 inhibition. The model predicted that the higher tubular fluid glucose concentration augments osmotic diuresis, thereby reducing Na+ reabsorption along the proximal tubule. As a result, in a diabetic sham kidney, the absence of plasma glucose-lowering effect yielded much higher urine flow (a 2.2-fold increase above baseline chronic inhibition), Na+ excretion (4.5-fold increase), and glucose excretion (2.0-fold increase). Qualitatively similar results were predicted for the diabetic UNX and 5/6-NX kidneys. In other words, the effect of SGLT2 inhibition to enhance urinary excretion of fluid, glucose, Na+, and K+ was most prominent when conditions were compared with similar glycemia levels.
To assess the extent to which the higher plasma glucose level in diabetic 5/6-NX under chronic SGLT2 inhibition contributes to the higher diuretic and natriuretic effect compared with diabetic sham, we performed a set of simulations in which plasma glucose concentration of diabetic 5/6-NX was set to sham level in the untreated case and with chronic SGLT2 inhibition. That is, instead of 30 to 20 mM (in the “original case”; see above), we assumed that chronic SGLT2 inhibition in diabetic 5/6-NX lowered plasma glucose concentration from 25 to 10 mM (the “equal case”). In the “original case,” chronic SGLT2 inhibition in diabetic 5/6-NX increased urine flow and Na+ excretion by 87 and 156%, respectively; smaller but still substantial increases of 39 and 39% were predicted in the “equal case.”
Impact of GFR-lowering effect of SGLT2 inhibitors.
We also conducted simulations to assess the effects of SGLT2 inhibition without GFR effects in kidneys with nephron loss. Specifically, we simulated acute and chronic SGLT2 inhibition in diabetic and nondiabetic 5/6-NX, with GFR set to untreated diabetic/nondiabetic levels. Without the GFR-lowering effect, acute SGLT2 inhibition in the diabetic 5/6-NX kidney increased urine flow, urinary Na+, K+, and glucose excretion by 52, 85, 24, and 92%, respectively, relative to the case in which GFR was reduced. Qualitatively similar results were obtained for the nondiabetic and diabetic 5/6-NX kidney with chronic SGLT2 inhibition. One exception is the attenuated increase in urinary glucose excretion (by 10% only) obtained for the diabetic 5/6-NX diabetic, due to the glucose-lowering effect of chronic SGLT2 inhibition.
REFERENCES
- 1.Baines A, Ho P. Glucose stimulates O2 consumption, NOS, and Na/H exchange in diabetic rat proximal tubules. Am J Physiol Renal Physiol 283: F286–F293, 2002. doi: 10.1152/ajprenal.00330.2001. [DOI] [PubMed] [Google Scholar]
- 2.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 8: 262–275.e9, 2014. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332: 647–655, 1995. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 4.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 49: 251–273, 1987. doi: 10.1146/annurev.ph.49.030187.001343. [DOI] [PubMed] [Google Scholar]
- 5.Carmines PK, Ohishi K, Ikenaga H. Functional impairment of renal afferent arteriolar voltage-gated calcium channels in rats with diabetes mellitus. J Clin Invest 98: 2564–2571, 1996. doi: 10.1172/JCI119075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Fry BC, Layton AT. Modeling glucose metabolism in the kidney. Bull Math Biol 78: 1318–1336, 2016. doi: 10.1007/s11538-016-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006. doi: 10.1152/ajprenal.00255.2005. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 10.Futrakul N, Vongthavarawat V, Sirisalipotch S, Chairatanarat T, Futrakul P, Suwanwalaikorn S. Tubular dysfunction and hemodynamic alteration in normoalbuminuric type 2 diabetes. Clin Hemorheol Microcirc 32: 59–65, 2005. [PubMed] [Google Scholar]
- 11.Hayslett JP, Kashgarian M, Epstein FH. Functional correlates of compensatory renal hypertrophy. J Clin Invest 47: 774–782, 1968. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, Dagogo-Jack S, Ismail-Beigi F, Korytkowski MT, Pratley RE, Schwartz GG, Kernan WN; IRIS Trial Investigators . Response to Comment on Inzucchi et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care 2016; 39: 1684–1692. Diabetes Care 40: e47–e48, 2017. doi: 10.2337/dci16-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman JM, Siegel NJ, Hayslett JP. Functional and hemodynamic adaptation to progressive renal ablation. Circ Res 36: 286–293, 1975. doi: 10.1161/01.RES.36.2.286. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Heo NJ, Jung JY, Son M-J, Jang HR, Lee JW, Oh YK, Na KY, Joo KW, Han JS. Changes in the sodium and potassium transporters in the course of chronic renal failure. Nephron Physiol 115: p31–41, 2010. doi: 10.1159/000314542. [DOI] [PubMed] [Google Scholar]
- 15.Klahr S, Hamm LL, Hammerman MR, Mandel LJ. Renal metabolism: integrated responses. In: Handbook of Physiology, edited by Berliner RW. Oxford, UK: Oxford University Press, 1989. [Google Scholar]
- 16.Körner A, Eklöf A-C, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 43: 629–633, 1994. doi: 10.2337/diab.43.5.629. [DOI] [PubMed] [Google Scholar]
- 17.Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 300: F356–F371, 2011. doi: 10.1152/ajprenal.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. II. Functional implications of three-dimensional architecture. Am J Physiol Renal Physiol 300: F372–F384, 2011. doi: 10.1152/ajprenal.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layton AT, Dantzler WH, Pannabecker TL. Urine concentrating mechanism: impact of vascular and tubular architecture and a proposed descending limb urea-Na+ cotransporter. Am J Physiol Renal Physiol 302: F591–F605, 2012. doi: 10.1152/ajprenal.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Layton AT, Edwards A, Vallon V. Adaptive changes in GFR, tubular morphology, and transport in subtotal nephrectomized kidneys: modeling and analysis. Am J Physiol Renal Physiol 313: F199–F209, 2017. doi: 10.1152/ajprenal.00018.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layton AT, Laghmani K, Vallon V, Edwards A. Solute transport and oxygen consumption along the nephrons: effects of Na+ transport inhibitors. Am J Physiol Renal Physiol 311: F1217–F1229, 2016. doi: 10.1152/ajprenal.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Layton AT, Vallon V. Cardiovascular benefits of SGLT 2 inhibition in diabetes and chronic kidney diseases. Acta Physiol 222: e13050, 2018. doi: 10.1111/apha.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015. doi: 10.1152/ajprenal.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layton AT, Vallon V, Edwards A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am J Physiol Renal Physiol 311: F1378–F1390, 2016. doi: 10.1152/ajprenal.00293.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layton AT, Vallon V, Edwards A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am J Physiol Renal Physiol 310: F1269–F1283, 2016. doi: 10.1152/ajprenal.00543.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 136: 1643–1658, 2017. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss R, Layton AT. Dominant factors that govern pressure natriuresis in diuresis and antidiuresis: a mathematical model. Am J Physiol Renal Physiol 306: F952–F969, 2014. doi: 10.1152/ajprenal.00500.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 28.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. doi: 10.1146/annurev.physiol.67.031103.154456. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell MP, Kasiske BL, Daniels FX, Keane WF. Effects of nephron loss on glomerular hemodynamics and morphology in diabetic rats. Diabetes 35: 1011–1015, 1986. doi: 10.2337/diab.35.9.1011. [DOI] [PubMed] [Google Scholar]
- 30.Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 8: 330–339, 2014. doi: 10.1016/j.jash.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 31.O’Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 309: F227–F234, 2015. doi: 10.1152/ajprenal.00689.2014. [DOI] [PubMed] [Google Scholar]
- 32.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: 1153–1160, 2003. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 33.Palm F, Hansell P, Ronquist G, Waldenström A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia 47: 1223–1231, 2004. doi: 10.1007/s00125-004-1434-3. [DOI] [PubMed] [Google Scholar]
- 34.Petrykiv S, Sjöström CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol 12: 751–759, 2017. doi: 10.2215/CJN.10180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plata C, Meade P, Vázquez N, Hebert SC, Gamba G. Functional properties of the apical Na+-K+-2Cl− cotransporter isoforms. J Biol Chem 277: 11004–11012, 2002. doi: 10.1074/jbc.M110442200. [DOI] [PubMed] [Google Scholar]
- 36.Rasch R, Dørup J. Quantitative morphology of the rat kidney during diabetes mellitus and insulin treatment. Diabetologia 40: 802–809, 1997. doi: 10.1007/s001250050752. [DOI] [PubMed] [Google Scholar]
- 37.Rich PR. The molecular machinery of Keilin’s respiratory chain. Biochem Soc Trans 31: 1095–1105, 2003. doi: 10.1042/bst0311095. [DOI] [PubMed] [Google Scholar]
- 38.Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 8: 844–847, 2016. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas MC, Moran JL, Harjutsalo V, Thorn L, Wadén J, Saraheimo M, Tolonen N, Leiviskä J, Jula A, Forsblom C, Groop PH; FinnDiane Study Group . Hyperfiltration in type 1 diabetes: does it exist and does it matter for nephropathy? Diabetologia 55: 1505–1513, 2012. doi: 10.1007/s00125-012-2485-5. [DOI] [PubMed] [Google Scholar]
- 40.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallon V, Blantz RC, Thomson S. Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol 269: F876–F883, 1995. doi: 10.1152/ajprenal.1995.269.6.F876. [DOI] [PubMed] [Google Scholar]
- 42.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 306: F194–F204, 2014. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2011. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 304: F156–F167, 2013. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 47.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George JT, Woerle HJ, Broedl UC, von Eynatten M, Zinman B. Empagliflozin and clinical outcomes in patients with type 2 diabetes, established cardiovascular disease, and chronic kidney disease. Circulation 137: 119–129, 2018. doi: 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007. doi: 10.1152/ajprenal.00392.2006. [DOI] [PubMed] [Google Scholar]
- 49.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]