Abstract
Focal segmental glomerulosclerosis (FSGS) is a histologically defined form of kidney injury typically mediated by podocyte dysfunction. Podocytes rely on their intricate actin-based cytoskeleton to maintain the glomerular filtration barrier in the face of mechanical challenges resulting from pulsatile blood flow and filtration of this blood flow. This review summarizes the mechanical challenges faced by podocytes in the form of stretch and shear stress, both of which may play a role in the progression of podocyte dysfunction and detachment. It also reviews how podocytes respond to these mechanical challenges in dynamic fashion through rearranging their cytoskeleton, triggering various biochemical pathways, and, in some disease states, altering their morphology in the form of foot process effacement. Furthermore, this review highlights the growing body of evidence identifying several mutations of important cytoskeleton proteins as causes of FSGS. Lastly, it synthesizes the above evidence to show that a better understanding of how these mutations leave podocytes vulnerable to the mechanical challenges they face is essential to better understanding the mechanisms by which they lead to disease. The review concludes with future research directions to fill this gap and some novel techniques with which to pursue these directions.
Keywords: alpha-actinin-4, cytoskeleton protein, FSGS, mechanical challenges, podocyte
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is a histologically defined form of kidney injury, with its cardinal clinical feature being progressive glomerular scarring associated with significant leakage of protein into the urine and decline in renal function (9). It is known that FSGS, whether primary or secondary, is characterized by podocyte dysfunction whereby podocyte effacement and detachment are the initial events leading to the development of overt kidney disease (6, 18, 27, 32–34). Podocytes are essential cells that maintain the glomerular filtration barrier. To carry out their function, they rely on their intricate actin-based cytoskeleton to uphold their structural integrity and generate contractile forces in the face of mechanical challenges resulting from pulsatile blood flow and glomerular filtration (40, 41, 50). It is thought that these mechanical challenges play a role in contributing to podocyte dysfunction and detachment in glomerular kidney disease. Moreover, any alteration that leaves the cytoskeleton compromised likely in turn leaves the podocyte more vulnerable to these mechanical challenges (33). The purpose of this review is to briefly summarize 1) the mechanical challenges podocytes face, 2) the studied responses of podocytes to these mechanical challenges, and 3) the known genetic defects to the actin cytoskeleton that may impair podocytes’ ability to withstand these challenges. This overview highlights the need to study the interaction between mechanical challenges and cytoskeleton impairments in mapping out the mechanisms of podocyte-mediated kidney disease.
MECHANICAL CHALLENGES EXPERIENCED BY PODOCYTES
The main mechanical challenges that podocytes face in vivo include stretch and shear stress (11, 33). In the literature, it is understood that stretch primarily results from chronic expansion of the glomerular basement membrane, most often due to the pathologic states of glomerular hyperfiltration and hypertension (30, 31, 33). Glomerular hyperfiltration and hypertension are pathologic processes contributing to glomerular damage commonly seen in diabetes mellitus and hypertension, which are not only the most prevalent causes of chronic kidney disease but are also forms of secondary FSGS (2, 29). This resultant expansion of the glomerular basement membrane in turn may lead to stretching of adhered podocyte foot processes and cell bodies (11, 40). For example, Ferrell et al. (17) found in the 5/6 nephrectomy rat model, long used to study the effect of hyperfiltration secondary to a reduction in functioning nephrons, the diameter of glomeruli capillary vessels increased by 15%. Additionally, Brähler et al. (4) provided support that podocytes experience cyclic stretch in demonstrating that podocytes oscillate at the rate of the heartbeat in mice. There is further evidence to suggest that podocytes experience cyclic stretch corresponding to the heartbeat-to-beat fluctuations in glomerular volume, which is amplified under states of hyperfiltration (7, 8). Shear stress is thought to challenge podocytes both at the slit diaphragm as filtrate flows across podocyte foot processes from the capillary into the Bowman’s space, and within the Bowman’s space as the filtrate flows across podocyte cell bodies toward the proximal tubules (11, 12, 20). This shear stress has been estimated and used as reference for in vitro study (20). Similar to stretch, shear stress may be enhanced in the context of glomerular hyperfiltration. Srivastava et al. (48) found in a uninephrectomy rat model that shear stress is increased over the apical portions of podocyte cell bodies. Altogether, there is a growing body of evidence supporting stretch and shear stress as mechanical challenges continuously faced by podocytes. It has been hypothesized that these mechanical challenges constitute a principal factor contributing to podocyte detachment when they are exacerbated in systemic diseases (e.g., hypertension and diabetes leading to glomerular hypertension and hyperfiltration) (33).
PODOCYTE RESPONSE TO MECHANICAL CHALLENGES
There are an increasing number of studies that have investigated how podocytes respond to stretch and shear stress in vitro (11–13). Several studies showed that in response to cyclic stretch podocytes demonstrated actin reorganization, with more radial stress fibers connected to an actin-rich center (14, 19, 44). Friedrich et al. and Srivastava et al. (20, 49) both showed that in response to shear stress, podocytes demonstrated reduction of stress fibers. Some authors have conjectured that this actin reorganization might be an adaptive response by podocytes to mechanical challenges (15, 43, 44), although how this reorganization helps podocytes adapt is unclear. Several biochemical responses have also been observed in podocytes in association with mechanical challenges, such as increased intracellular calcium concentrations (19) and angiotensin II secretion (36, 38). Similar to actin reorganization, the implications of these biochemical responses have yet to be fully elucidated. Many of these studies compared the responses of podocytes subjected to stretch or shear stress with podocytes that were not subjected to these mechanical challenges, making it difficult to infer whether a given response is physiological or pathological since in vivo podocytes are always experiencing some degree of stretch and shear stress.
In vivo, Kriz and Lemley (32–34) hypothesized that the effacement of podocytes seen in FSGS might actually be an adaptive response of podocytes to acute mechanical challenges, aiming to reduce increased shear stresses across their foot processes. This process of effacement is associated with formation of a basal cytoskeletal mat consisting of α-actinin closely opposed to the glomerular basement membrane (45, 47) and formation of new occluding junctions (35, 46). Together with the in vitro evidence summarized above, these in vivo observations suggest that podocytes actively respond to the mechanical challenges they face both in terms of their structure and biochemistry. Further investigation is needed into whether these responses have a purpose to protect the podocytes or are themselves pathologic representations of injury.
CYTOSKELETON ABNORMALITIES THAT MAY LEAVE PODOCYTES VULNERABLE TO MECHANICAL CHALLENGES
Podocytes depend on their actin-based cytoskeleton to preserve their structure and carry out their function while facing stretch and shear stress in the glomerulus. There are several actin-based proteins localized to podocytes in which mutations have been identified to cause FSGS. These mutations include ACTN4 (28) INF2 (5), MYO1E (37), MYH9 (24), and ANLN (21), as well as other actin regulatory proteins such as ARHGAP24 (1), ARHGDIA (23), and KANK1,2,4 (22). Each mutation may compromise the podocyte cytoskeleton in different ways, largely based on the function of the affected cytoskeletal protein. For example, ACTN4 is a cross-linker protein that bundles actin filaments (13). Human disease-causing mutations in ACTN4 have been found to lead to brittleness of reconstituted actin protein network in vitro (45), impaired podocyte motility, and abnormal cellular contraction (10). Several of the other mutations listed above have been shown to alter podocyte motility (1, 21, 23, 37), and knockout MYH9 podocytes also demonstrated changes in contraction (3). A more exhaustive table listing currently known disease-causing mutations and the cytoskeleton proteins they affect can be found in a separate review by Schell and Hubera (42). Given that a normal, healthy cytoskeleton is essential for podocytes to maintain their integrity within glomerulus, one can infer that any cytoskeletal abnormality may leave the podocyte vulnerable to stretch and shear stress.
CONCLUSIONS AND FUTURE PERSPECTIVES
In summary, FSGS is a podocyte-mediated disease. While maintaining the glomerular filtration barrier, podocytes constantly face the mechanical challenges of stretch and shear stress, both of which may be enhanced in the pathologic states of glomerular hypertension and hyperfiltration. There is a growing amount of evidence demonstrating that podocytes respond to these mechanical challenges in dynamic fashion through rearranging their cytoskeleton, triggering various biochemical pathways, and altering their morphology in the form of foot process effacement. Given how vital the podocyte cytoskeleton is to podocyte structure and function, it is not surprising that numerous FSGS-causing mutations affect important cytoskeleton proteins.
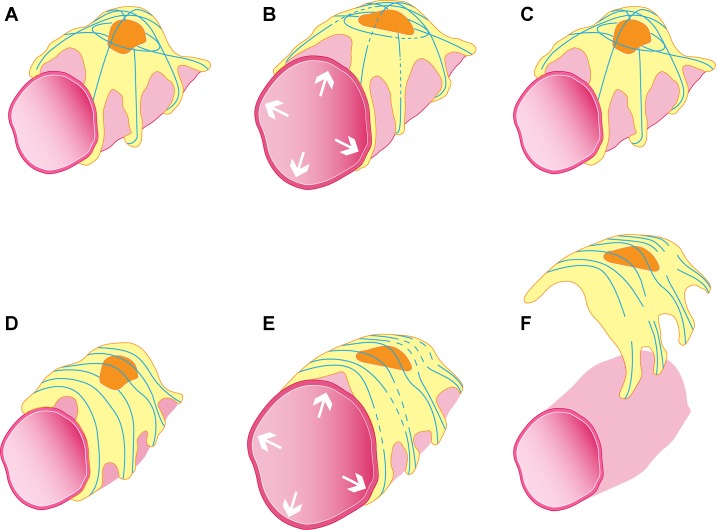
Despite the discovery of FSGS-associated cytoskeletal mutations, there is a lack of targeted, individualized therapies for these rare forms of FSGS. There remains an incomplete understanding of the mechanisms by which the alterations to the podocyte cytoskeleton conferred by these mutations impair the ability of podocytes to respond to the mechanical challenges that they constantly face. Future studies can investigate this impairment by comparing the responses of podocytes harboring mutant cytoskeletons to stretch and shear stress with the responses of podocytes with wild-type cytoskeletons. The findings of such investigations will not only shed light on what constitutes an abnormal podocyte response to mechanical challenges for a given disease-causing mutation, but through this comparison how a wild-type podocyte can properly adapt. Building on the example of ACTN4, although human disease-causing mutations in ACTN4 have been found to lead to brittleness of reconstituted actin protein networks, how these brittle networks impact the in vivo podocyte’s response to stretch and shear stress is in question. To attempt to address this question, our recent studies used primary podocytes isolated from wild-type and FSGS-causing point-mutant Actn4 knock-in mice (ACTN4: human gene or protein; Actn4: mouse gene or protein) and subjected these podocytes to periodic stretch in vitro (16). Whereas wild-type podocytes are able to consistently recover their actin structure and contractile forces after each stretch, mutant Actn4 podocytes develop irrecoverable reductions in their contraction and irreparable disruptions in their actin cytoskeletons. We also found that mutant Actn4 podocytes demonstrated a higher rate of detachment after stretch. These findings could represent the process by which mutant ACTN4 leaves the podocyte brittle and vulnerable to failure in terms of its structure and function when faced with mechanical challenges. Figure 1 illustrates our working hypothesis regarding how mutant ACTN4 impairs the podocyte’s response to stretch in vivo.
Fig. 1.
Schematic illustration hypothesizing the in vivo interaction between glomerular capillary expansion-induced stretch in wild-type (A–C) and FSGS-causing point-mutant ACTN4 podocytes (E–F). In response to a transient stretch, wild-type podocytes develop small areas of actin breakages (represented by blue lines; see B) that repair (C), allowing the podocyte to maintain its contraction and remain adhered to the glomerular basement membrane. In contrast, mutant ACTN4 podocytes show breakages in their actin structure after stretch (E) that fail to repair (F). Hence, the podocyte’s contraction fails in association with its detachment.
Indeed, the ability to test the interaction between cytoskeletal abnormalities and the mechanical challenges podocytes face is becoming increasingly more feasible, with methods such as organ-on-a-chip that allow fine control over mechanical challenges subjected to podocytes, both stretch and shear stress alone and in combination (25, 26, 39). Moreover, new techniques such as intravital microscopy may open doors into measuring the magnitude of these mechanical challenges in in vivo models, representing normal states and the pathologic states of glomerular hypertension and hyperfiltration. A better understanding of the process by which disease-causing mutations lead to podocyte dysfunction and FSGS could stem from 1) more accurately simulating the stretch and shear stress that podocytes experience in conjunction with 2) improved knowledge of how defects to the actin cytoskeleton leave podocytes vulnerable to stretch and shear stress. This understanding in turn could form the basis for the development of new therapies for FSGS.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37 DK-59588 (to M. Pollak) and T32 DK-007199 (to D. Feng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.F. and C.D. drafted manuscript; D.F., C.D., and M.R.P. edited and revised manuscript; D.F., C.D., and M.R.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jerolim Mladinov for the illustration in Fig. 1. Used with permission of the artist.
REFERENCES
- 1.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, Antignac C, Pollak M, Kopp JB, Winn MP, Shaw AS. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol 60: 18–26, 2007. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondzie PA, Chen HA, Cao MZ, Tomolonis JA, He F, Pollak MR, Henderson JM. Non-muscle myosin-IIA is critical for podocyte f-actin organization, contractility, and attenuation of cell motility. Cytoskeleton (Hoboken) 73: 377–395, 2016. doi: 10.1002/cm.21313. [DOI] [PubMed] [Google Scholar]
- 4.Brähler S, Yu H, Suleiman H, Krishnan GM, Saunders BT, Kopp JB, Miner JH, Zinselmeyer BH, Shaw AS. Intravital and kidney slice imaging of podocyte membrane dynamics. J Am Soc Nephrol 27: 3285–3290, 2016. doi: 10.1681/ASN.2015121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010. [Erratum in Nat Genet 42: 361, 2010.] doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YM, Liapis H. Focal segmental glomerulosclerosis: molecular genetics and targeted therapies. BMC Nephrol 16: 101, 2015. doi: 10.1186/s12882-015-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes P, Riser BL, Yee J, Narins RG. Mechanical strain of glomerular mesangial cells in the pathogenesis of glomerulosclerosis: clinical implications. Nephrol Dial Transplant 14: 1351–1354, 1999. doi: 10.1093/ndt/14.6.1351. [DOI] [PubMed] [Google Scholar]
- 8.Cortes P, Zhao X, Riser BL, Narins RG. Regulation of glomerular volume in normal and partially nephrectomized rats. Am J Physiol Renal Physiol 270: F356–F370, 1996. [DOI] [PubMed] [Google Scholar]
- 9.D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlicher AJ, Krishnan R, Guo M, Bidan CM, Weitz DA, Pollak MR. Alpha-actinin binding kinetics modulate cellular dynamics and force generation. Proc Natl Acad Sci USA 112: 6619–6624, 2015. doi: 10.1073/pnas.1505652112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endlich K, Kliewe F, Endlich N. Stressed podocytes-mechanical forces, sensors, signaling and response. Pflugers Arch 469: 937–949, 2017. doi: 10.1007/s00424-017-2025-8. [DOI] [PubMed] [Google Scholar]
- 12.Endlich N, Endlich K. The challenge and response of podocytes to glomerular hypertension. Semin Nephrol 32: 327–341, 2012. doi: 10.1016/j.semnephrol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Endlich N, Endlich K. Stretch, tension and adhesion—adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol 85: 229–234, 2006. doi: 10.1016/j.ejcb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Endlich N, Sunohara M, Nietfeld W, Wolski EW, Schiwek D, Kränzlin B, Gretz N, Kriz W, Eickhoff H, Endlich K. Analysis of differential gene expression in stretched podocytes: osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J 16: 1850–1852, 2002. doi: 10.1096/fj.02-0125fje. [DOI] [PubMed] [Google Scholar]
- 16.Feng D, Notbohm J, Benjamin A, He S, Wang M, Ang LH, Bantawa M, Bouzid M, Del Gado E, Krishnan R, Pollak MR. Disease-causing mutation in alpha-actinin-4 promotes podocyte detachment through maladaptation to periodic stretch. Proc Natl Acad Sci USA 115: 1517–1522, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrell N, Sandoval RM, Bian A, Campos-Bilderback SB, Molitoris BA, Fissell WH. Shear stress is normalized in glomerular capillaries following ⅚ nephrectomy. Am J Physiol Renal Physiol 308: F588–F593, 2015. doi: 10.1152/ajprenal.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 11: 76–87, 2015. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forst AL, Olteanu VS, Mollet G, Wlodkowski T, Schaefer F, Dietrich A, Reiser J, Gudermann T, Mederos y Schnitzler M, Storch U. Podocyte purinergic P2X4 channels are mechanotransducers that mediate cytoskeletal disorganization. J Am Soc Nephrol 27: 848–862, 2016. doi: 10.1681/ASN.2014111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedrich C, Endlich N, Kriz W, Endlich K. Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006. doi: 10.1152/ajprenal.00196.2005. [DOI] [PubMed] [Google Scholar]
- 21.Gbadegesin RA, Hall G, Adeyemo A, Hanke N, Tossidou I, Burchette J, Wu G, Homstad A, Sparks MA, Gomez J, Jiang R, Alonso A, Lavin P, Conlon P, Korstanje R, Stander MC, Shamsan G, Barua M, Spurney R, Singhal PC, Kopp JB, Haller H, Howell D, Pollak MR, Shaw AS, Schiffer M, Winn MP. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J Am Soc Nephrol 25: 1991–2002, 2014. doi: 10.1681/ASN.2013090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125: 2375–2384, 2015. doi: 10.1172/JCI79504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta IR, Baldwin C, Auguste D, Ha KC, El Andalousi J, Fahiminiya S, Bitzan M, Bernard C, Akbari MR, Narod SA, Rosenblatt DS, Majewski J, Takano T. ARHGDIA: a novel gene implicated in nephrotic syndrome. J Med Genet 50: 330–338, 2013. doi: 10.1136/jmedgenet-2012-101442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heath KE, Campos-Barros A, Toren A, Rozenfeld-Granot G, Carlsson LE, Savige J, Denison JC, Gregory MC, White JG, Barker DF, Greinacher A, Epstein CJ, Glucksman MJ, Martignetti JA. Nonmuscle myosin heavy chain IIA mutations define a spectrum of autosomal dominant macrothrombocytopenias: May-Hegglin anomaly and Fechtner, Sebastian, Epstein, and Alport-like syndromes. Am J Hum Genet 69: 1033–1045, 2001. doi: 10.1086/324267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, Bahinski A, Hamilton GA, Ingber DE. Microfabrication of human organs-on-chips. Nat Protoc 8: 2135–2157, 2013. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 26.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jefferson JA, Shankland SJ. The pathogenesis of focal segmental glomerulosclerosis. Adv Chronic Kidney Dis 21: 408–416, 2014. doi: 10.1053/j.ackd.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, Han BG, Choi SO, Cha SK. Secondary focal segmental glomerulosclerosis: from podocyte injury to glomerulosclerosis. Biomed Res Int 2016: 1630365, 2016. doi: 10.1155/2016/1630365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretzler M, Koeppen-Hagemann I, Kriz W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch 425: 181–193, 1994. doi: 10.1007/BF00230355. [DOI] [PubMed] [Google Scholar]
- 31.Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M, Hähnel B. A role for podocytes to counteract capillary wall distension. Kidney Int 45: 369–376, 1994. doi: 10.1038/ki.1994.47. [DOI] [PubMed] [Google Scholar]
- 32.Kriz W, Lemley KV. Mechanical challenges to the glomerular filtration barrier: adaptations and pathway to sclerosis. Pediatr Nephrol 32: 405–417, 2017. doi: 10.1007/s00467-016-3358-9. [DOI] [PubMed] [Google Scholar]
- 33.Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 35.Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 141: 805–816, 1992. [PMC free article] [PubMed] [Google Scholar]
- 36.Liebau MC, Lang D, Böhm J, Endlich N, Bek MJ, Witherden I, Mathieson PW, Saleem MA, Pavenstädt H, Fischer KG. Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol 290: F710–F719, 2006. doi: 10.1152/ajprenal.00475.2004. [DOI] [PubMed] [Google Scholar]
- 37.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M; PodoNet Consortium . MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365: 295–306, 2011. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miceli I, Burt D, Tarabra E, Camussi G, Perin PC, Gruden G. Stretch reduces nephrin expression via an angiotensin II-AT1-dependent mechanism in human podocytes: effect of rosiglitazone. Am J Physiol Renal Physiol 298: F381–F390, 2010. doi: 10.1152/ajprenal.90423.2008. [DOI] [PubMed] [Google Scholar]
- 39.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, and Ingber DE. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng 1: 0069, 2017. doi: 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neal CR, Crook H, Bell E, Harper SJ, Bates DO. Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol 16: 1223–1235, 2005. doi: 10.1681/ASN.2004100822. [DOI] [PubMed] [Google Scholar]
- 41.Saleem MA, Zavadil J, Bailly M, McGee K, Witherden IR, Pavenstädt H, Hsu H, Sanday J, Satchell SC, Lennon R, Ni L, Bottinger EP, Mundel P, Mathieson PW. The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 295: F959–F970, 2008. doi: 10.1152/ajprenal.00559.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schell C, Huber TB. The evolving complexity of the podocyte cytoskeleton. J Am Soc Nephrol 28: 3166–3174, 2017. doi: 10.1681/ASN.2017020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schordan S, Grisk O, Schordan E, Miehe B, Rumpel E, Endlich K, Giebel J, Endlich N. OPN deficiency results in severe glomerulosclerosis in uninephrectomized mice. Am J Physiol Renal Physiol 304: F1458–F1470, 2013. doi: 10.1152/ajprenal.00615.2012. [DOI] [PubMed] [Google Scholar]
- 44.Schordan S, Schordan E, Endlich K, Endlich N. αV-Integrins mediate the mechanoprotective action of osteopontin in podocytes. Am J Physiol Renal Physiol 300: F119–F132, 2011. doi: 10.1152/ajprenal.00143.2010. [DOI] [PubMed] [Google Scholar]
- 45.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol 148: 1283–1296, 1996. [PMC free article] [PubMed] [Google Scholar]
- 46.Shono A, Tsukaguchi H, Yaoita E, Nameta M, Kurihara H, Qin XS, Yamamoto T, Doi T. Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephrol 18: 2525–2533, 2007. doi: 10.1681/ASN.2006101084. [DOI] [PubMed] [Google Scholar]
- 47.Smoyer WE, Mundel P, Gupta A, Welsh MJ. Podocyte alpha-actinin induction precedes foot process effacement in experimental nephrotic syndrome. Am J Physiol Renal Physiol 273: F150–F157, 1997. doi: 10.1152/ajprenal.1997.273.1.F150. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava T, Celsi GE, Sharma M, Dai H, McCarthy ET, Ruiz M, Cudmore PA, Alon US, Sharma R, Savin VA. Fluid flow shear stress over podocytes is increased in the solitary kidney. Nephrol Dial Transplant 29: 65–72, 2014. doi: 10.1093/ndt/gft387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava T, McCarthy ET, Sharma R, Cudmore PA, Sharma M, Johnson ML, Bonewald LF. Prostaglandin E2 is crucial in the response of podocytes to fluid flow shear stress. J Cell Commun Signal 4: 79–90, 2010. doi: 10.1007/s12079-010-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welsh GI, Saleem MA. The podocyte cytoskeleton–key to a functioning glomerulus in health and disease. Nat Rev Nephrol 8: 14–21, 2011. doi: 10.1038/nrneph.2011.151. [DOI] [PubMed] [Google Scholar]