Abstract
Tubulointerstitial fibrosis (TIF) is a prominent factor in the progression of chronic kidney disease regardless of etiology. Avian erythroblastic leukemia viral oncogene homolog 4 (ErbB4) expression levels were inversely correlated to renal fibrosis in human fibrotic kidneys. In both unilateral ureteral obstruction (UUO) and ischemia-reperfusion injury followed by uninephrectomy (IRI/UNx) mouse models, expression levels of ErbB4 were elevated in the early stage of renal injury. Using mice with global ErbB4 deletion except for transgenic rescue in cardiac tissue (ErbB4−/−ht+), we determined that UUO induced similar injury in proximal tubules compared with wild-type mice but more severe injury in distal nephrons. TIF was apparent earlier and was more pronounced following UUO in ErbB4−/−ht+ mice. With ErbB4 deletion, UUO injury inhibited protein kinase B phosphorylation and increased the percentage of cells in G2/M arrest. There was also increased nuclear immunostaining of yes-associated protein and increased expression of phospho-Mothers against decapentaplegic homolog 3, snail1, and vimentin. These results indicate that ErbB4 deletion accelerates the development and progression of renal fibrosis in obstructive nephropathy. Similar results were found in a mouse IRI/UNx model. In conclusion, increased expression of ErbB4 in the early stages of renal injury may reflect a compensatory effect to lessen tubulointerstitial injury.
Keywords: avian erythroblastic leukemia viral oncogene homolog 4, cell cycle, epithelial dedifferentiation, tubulointerstitial fibrosis
tubulointerstitial fibrosis (TIF) is a component of chronic kidney disease (CKD) of any etiology and is the best predictor of progression toward end-stage kidney disease. Mechanisms underlying the development and progression of TIF are still incompletely understood. Previous studies by us and others have indicated that activation of the epidermal growth factor receptor (EGFR) is one such mechanism (4, 36). EGFR is a member of the avian erythroblastic leukemia viral oncogene (ErbB) superfamily of type I transmembrane tyrosine kinase-associated receptors that includes ErbB1 (EGFR), ErbB2 (Neu), ErbB3, and ErbB4. They present with similar structures, domains, and distinct but overlapping ligand-binding preferences. EGFR members form homo- or heterodimers upon ligand binding and transduce signals from the cell surface into the cytosol and nucleus to regulate multiple cellular functions. They play a central role in organ development and tissue repair as well as a pathogenic role in many diseases (6, 20, 33).
Unlike the other ErbB family members, whose overexpression or constitutive activation mediates progression of many types of cancers, ErbB4 expression in cancer is often associated with growth suppression and improved prognosis (15, 30). ErbB4 expression increases in CKD (18, 32), but its role in either mediating or inhibiting progression of tubulointerstitial injury has not been previously determined. Using both mouse unilateral ureteral obstruction (UUO) and IRI/UNx models, we have investigated the possible role of ErbB4 in development and progression of TIF. We found that ErbB4 deletion accelerated renal tubulointerstitial fibrosis and renal injury.
MATERIALS AND METHODS
Animal care, breeding, and genotyping.
Mice were housed at the Vanderbilt Medical Center Veterinary Facility. Animal care and all experimental protocols in our studies complied with the regulations of, and were approved by, Vanderbilt University’s Institutional Animal Care and Usage Committee. We used mice with ErbB4 deletion that were prevented from developing lethal cardiac defects by concomitant expression of human ErbB4 cDNA under the cardiac-specific myosin heavy chain promoter (ErbB4−/−MHC+, namely ErbB4−/−ht+), as described previously (32). These mice were backcrossed 10 generations to an FVB background. Heterozygous mice with ErbB4 deletion (ErbB4-/+ht+) were used for breeding to generate mice with homozygous ErbB4 deletion (ErbB4−/−ht+) and wild-type with or without the cardiac-specific myosin heavy chain promoter (MHC+). In our preliminary studies, no morphological and functional differences were found between wild-type mice with or without MHC+. Therefore, wild-type mice without MHC+ were used in the control group in subsequent experiments. Six to 8 mice were used in each group, and every experiment has been repeated. Genotypes were performed by PCR using primers as previously reported (27).
Antibodies and reagents.
Antibodies for GAPDH, EGFR, α-smooth muscle actin (α-SMA), and β-actin were purchased from Sigma-Aldrich (St. Louis, MO); kidney injury molecule-1 (KIM-1) and fatty acid-binding protein-3/heart fatty acid-binding protein (H-FABP) antibody were from R&D Systems (Minneapolis, MN); Ki-67 and Snail1 antibody were from Abcam (Cambridge, MA); antibodies for ErbB4 (c-18), heparin-binding epidermal growth factor-like growth factor (HB-EGF), and phospho (p)-EGFR were from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies for p-ErbB4 (Tyr1284), caspase-3, vimentin, yes-associated protein (YAP), Mothers against decapentaplegic homolog 3 (Smad3), p-protein kinase B (AKT), p-p38, and p-Erk were from Cell Signaling Technology (Beverly, MA); p-Smad3 (pS423/pS425) antibody was from Rockland Immunochemicals (Limerick, PA); antibodies for fibroblast-specific protein 1 (FSP-1) and p-Histone H3 were from EMD Millipore (Billerica, MA); purified mouse anti-E-cadherin was from BD Transduction Laboratories (San Jose, CA); anti-neuregulin 1 (NRG1) antibody was from Proteintech Group (Rosemont, IL); Tamm-Horsfall glycoprotein (THP) antibody was from Biomedical Technologies (Stoughton, MA); fluorescein-labeled Lotus tetragonolobus lectin (LTL), fluorescein-labeled dolichos biflorus agglutinin (DBA), and avidin-biotin complex (ABC) kits were from VectorLabs (Burlingame, CA); fluorochrome-labeled secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA); and the sirius red/fast green collagen staining kit was from Chondrex (Redmond, WA).
Unilateral ureteral obstruction.
UUO was performed on 8- to 10-wk-old male mice as previously described (17). Briefly, under anesthesia, the right kidney was exposed dorsally via a flank incision, and two sutures were placed on the ureter just distal to the renal pelvis. The same procedure was performed for the sham-operated mice without obstruction of the ureter. Mice were euthanized at 1, 3, 6, 10, or 14 days after surgery.
Ischemia-reperfusion injury followed by contralateral nephrectomy.
Ischemia-reperfusion injury followed by contralateral nephrectomy (IRI/UNx) was performed as previously described (24). Surgeries were performed on a water bath-heated platform at 38°C on 10- to 12-wk-old male mice. To induce IRI, mice underwent left renal pedicle clamping for 31 min, and delayed contralateral nephrectomy was performed after 8 days. Functional recovery was assessed on days 9 and 22 after initial injury, mice were killed, and kidneys were collected for pathological analysis (Fig. 3D).
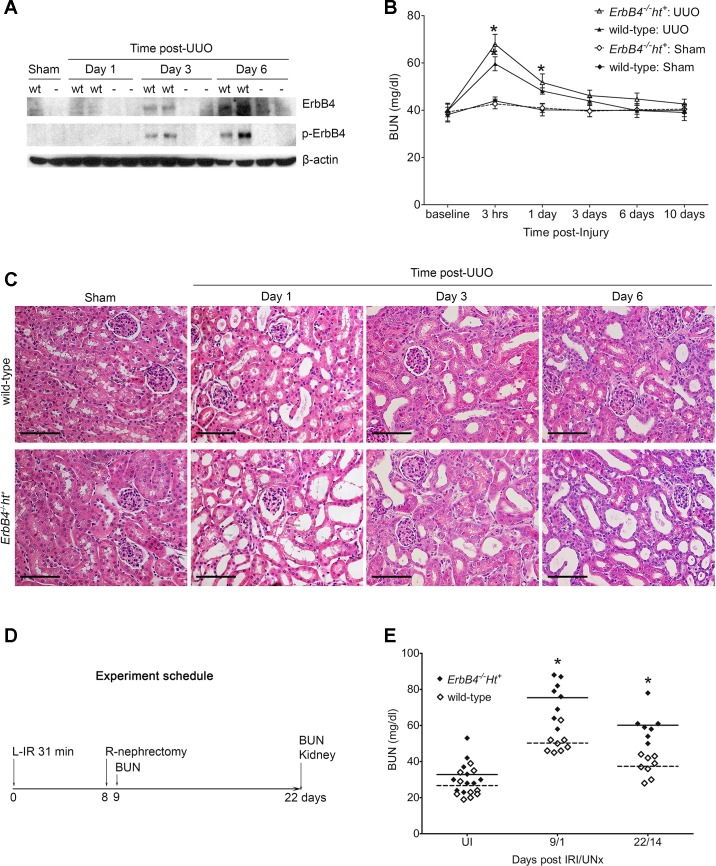
Fig. 3.
Characterization of ErbB4 deletion on changes of renal morphology and function following injury. A: expression and activation of ErbB4 levels after injury detected by immunoblotting. In wild-type sham-operated kidneys, minimum levels of ErbB4 were detected without activation form [phosphorylated (p)-ErbB4]. After unilateral ureteral obstruction (UUO), ErbB4 levels increased gradually from day 3 to day 6 post-UUO, concomitant with increased levels of p-ErbB4 in wild-type controls. ErbB4 expression was not detected in either sham or UUO kidneys from ErbB4−/−ht+ mice. wt, Wild type; −, ErbB4−/−ht+. B: blood urea nitrogen (BUN) level after UUO injury. Compared with sham, mice undergoing UUO had increased BUN levels after 3 h of injury in both groups. After UUO (1 day), only ErbB4−/−ht+ mice still had significantly elevated levels of BUN; n = 6–8 in each time point of each group. *P < 0.05 compared with sham. C: hematoxylin and eosin (H&E) staining. No morphological changes were detected in sham-operated kidneys in either group. Compared with wild-type controls, kidneys with ErbB4 deletion had more dilated tubules 1 day after UUO and increased interstitial area 3 and 6 days post-UUO. Scale bar, 100 µm. D: experimental schedule for ischemia-reperfusion injury followed by uninephrectomy (IRI/UNx). E: BUN levels post-IRI/UNx injury. Compared with the uninjured mice (UI), IRI/UNx injury increased BUN levels in both wild-type and ErbB4 deletion mice after 1 or 14 days of injury (P < 0.05). In IRI/UNx groups, compared with the wild type, ErbB4 deletion further increased BUN levels to a significant degree with slow recovery at 14 days after UNx. *P < 0.05; n = 7–8 in each group.
Measurement of BUN.
Blood was collected at different times after UUO via tail vein and was centrifuged at 2000 g for serum collection. Blood urea nitrogen (BUN) levels were determined using the QuantiChrom Urea Assay Kit (DIUR-500; BioAssay Systems, Hayward, CA).
Renal histopathology.
At the indicated time points, mice were euthanized, and kidneys were removed and fixed in 4% paraformaldehyde before paraffin embedding. Midsagittal sections of 5 μm size were stained with hematoxylin and eosin (H&E), Masson’s trichrome staining, or sirius red/fast green staining. The images were examined using a Zeiss microscope (Thornwood, NY). Blue color in Masson’s trichrome staining or red color in sirius red/fast green staining that represents collagen staining that excluded the vascular area was quantified using ImageJ software in 20 random fields.
Immunohistochemistry.
Sections (5 μm) prepared as indicated above were used for immunohistochemistry. After rehydration, antigen retrieval was performed in citrate buffer (pH 6.0) in a steamer for 20 min, followed by cooling for 20 min in an ice-cold water bath. Nonspecific staining blocking included the following: 0.3% hydrogen peroxide for blocking endogenous peroxidase, 5% normal serum from the species that the secondary antibody was generated in to block nonspecific reactive sites, and avidin-biotin for blocking endogenous biotin. Antibody diluted in antibody buffer [1× PBS containing 1% BSA (blocking, stabilizer), 5% normal serum (blocking), 0.1% Triton X-100 (penetration enhancer), and 0.1% Tween 20 (detergent and surface tension reducer)] was incubated at 4°C overnight, followed by fluorochrome-labeled secondary antibodies for fluorescence staining or biotinylated secondary antibody and an ABC kit for immunochemistry staining. The images were examined using a Zeiss microscope or a Zeiss-410 confocal microscope at the Vanderbilt Imaging Core facility.
Immunoblotting.
A quarter of the kidney was snap-frozen and stored at −80°C until use. Frozen samples were homogenized and lysed in ice-cold buffer composed of 1% Triton X-100, 10% glycerol, 20 mM HEPES, 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM Na3VO4, and immunoblotting was carried out as previously described (35).
Statistical analysis.
Data were evaluated using unpaired t-test for two groups and analysis of variance followed by a post hoc Bonferroni’s multiple-comparison test for three or more groups. Data were analyzed using GraphPad Prism 5. Values were expressed as means ± SE from at least three separate experiments. P < 0.05 was considered statistically significant.
RESULTS
Expression levels of ErbB4 in normal and fibrotic human kidneys.
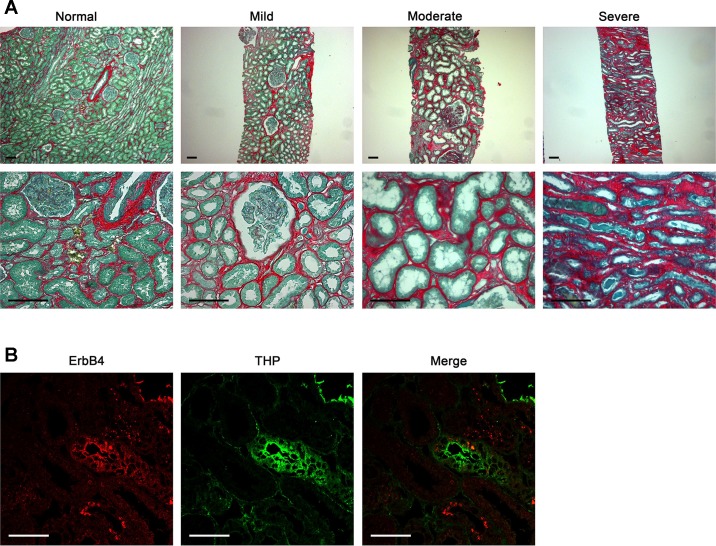
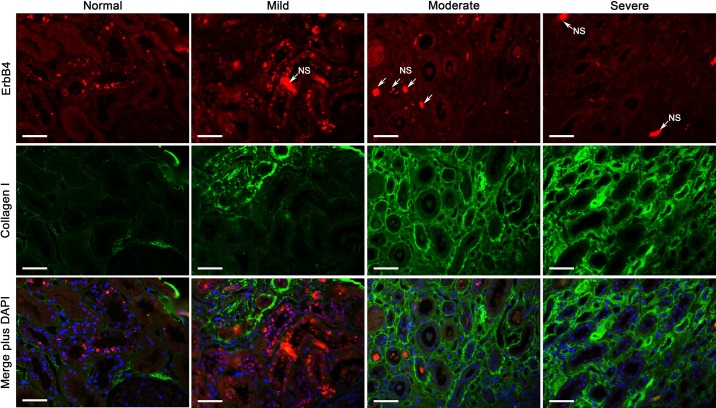
Kidney interstitial fibrosis from biopsy samples of patients with different stages of injury was evaluated by sirius red/fast green staining (Fig. 1A), with sirius red staining for collagen I and IV and fast green for noncollagen tissues. ErbB4 expression and localization were investigated using immunostaining. Our results showed that ErbB4 is expressed in the cytoplasm and perinuclear of tubular epithelial cells at low levels in normal human kidneys, which are localized at the thick ascending limb as indicated by the colocalization with THP (Fig. 1B) and collecting ducts as we reported previously (35). The ErbB4 expression levels slightly increased in the mild renal fibrosis but decreased or diminished in kidneys with moderate or severe fibrosis shown by collagen I immunostaining (Fig. 2). Those results suggested inverse correlation between levels of ErbB4 expression and the extent of renal fibrosis.
Fig. 1.
Validation of the degree of renal fibrosis and avian erythroblastic leukemia viral oncogene homolog 4 (ErbB4) localization in human kidney biopsies. A: sirius red/fast green staining to validate the degree of renal fibrosis in human samples. Scale bar, 100 µm. B: low levels of ErbB4 (red) were expressed in normal human kidneys; some was detected in thick ascending limb, as indicated by the colocalization with Tamm-Horsfall glycoprotein (THP). Scale bar, 50 µm.
Fig. 2.
ErbB4 expression in normal and fibrotic human kidneys. Low levels of ErbB4 (red) were expressed in normal human kidneys, with increased expression in the mild renal fibrotic kidneys but decreased expression in the moderate and severe fibrotic kidneys, as indicated by collagen I immunostaining (green). NS, nonspecific staining. Representative images from n = 3 mice in each group are shown. Scale bar, 50 µm.
Delayed serum BUN level recovery in ErbB4 deletion mice.
Levels of ErbB4 expression and activation following UUO in mice were investigated using immunoblotting and indicated low levels of ErbB4 and essentially undetectable p-ErbB4 (indicative of activated receptor) in sham-operated wild-type kidneys, but expression of both total and phosphorylated receptor increased markedly at 3 and 6 days after UUO in wild-type kidneys. As expected, we did not detect ErbB4 expression in either group of ErbB4−/−ht+ kidneys (Fig. 3A).
Compared with sham-operated mice, UUO induced elevations in BUN levels within 3 h in both wild-type and ErbB4−/−ht+ mice, followed by rapid recovery. However, 1 day after UUO only ErbB4−/−ht+ mice still had significantly elevated BUN levels compared with sham, although by 3 days after UUO BUN had returned to the normal range in both groups (Fig. 3B). H&E staining showed no morphological changes in the contralateral kidneys or kidneys that underwent sham operation in either ErbB4 deletion or the wild type. However, in UUO kidneys from ErbB4−/−ht+ mice, tubulointerstitial changes were seen at day 3 and were more pronounced by day 6 after UUO, whereas minimal tubulointerstitial changes can only be detected at day 6 post-UUO in wild-type mice (Fig. 3C). These results suggested ErbB4 deletion may affect the ability of the contralateral kidney to compensate following injury.
In the mouse IRI/UNx model (Fig. 3D), ErbB4 deletion led to significantly higher BUN levels at both 1 day and 14 days postuninephrectomy compared with the wild-type mice (Fig. 3E).
ErbB4 deletion worsened tubular injury in UUO kidneys.
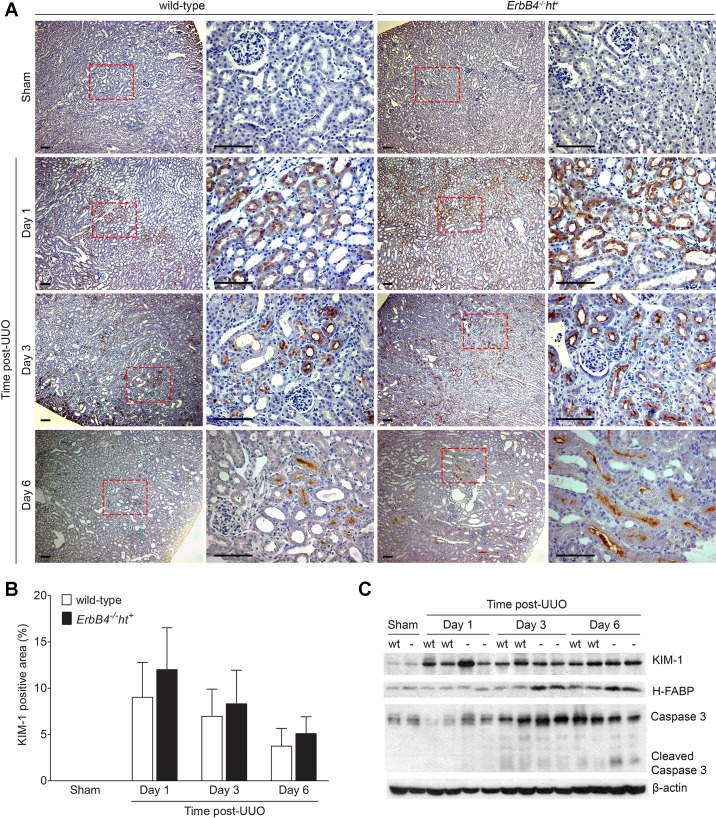
Compared with sham-operated kidneys, the UUO kidneys in both wild-type and ErbB4−/−ht+ mice demonstrated increased immunostaining of KIM-1, an established marker for proximal tubular injury (7), with highest expression levels at 1 day post-UUO compared with 3 and 6 days post-UUO (Fig. 4A). One day post-UUO, KIM-1 immunostaining was mainly detected in the S3 segment in the outer medulla but was also detectable in S1 and S2 segments in the renal cortex at 3 and 6 days post-UUO. There were no statistically significant differences in relative KIM-1 immunostaining (Fig. 4B) or immunoblotting (Fig. 4C) between the UUO kidneys from wild-type and ErbB4−/−ht+ mice.
Fig. 4.
Effects of ErbB4 deletion on tubular injury after UUO. A: kidney injury molecule-1 (KIM-1) immunohistochemistry staining. In sham-operated kidneys, no KIM-1 immunostaining was detected in either group. Post-UUO injury (1 day), KIM-1 immunostaining was dramatically increased in the S3 segments and was also detected in S1 and S2 segments at 3 and 6 days post-UUO in both groups. Scale bar, 100 µm. B: quantification of KIM-1-positive immunostaining area of A using Image J software. Values represent means ± SE from 20 random fields of 4–6 kidneys in each group. C: expression levels of kidney injury marker proteins by immunoblotting. KIM-1 expression levels increased early in the UUO injury by 1 day post-UUO and remained elevated at days 3 and 6 post-UUO with no significant differences between wild-type and ErbB4−/−ht+. Elevated heart fatty acid-binding protein (H-FABP) levels were detected 3 days post-UUO injury and remained elevated at 6 days post-UUO in ErbB4−/−ht+ mouse kidneys compared with wild-type kidneys at the same time points. Baseline caspase-3 expression was detected in sham-operated kidneys. After UUO, kidneys from ErbB4−/−ht+ mice showed slightly higher levels of total caspase-3 at 1 and 3 days post-UUO, whereas elevated cleaved caspase-3 was only detected at 6 days post-UUO. Images represent 3 independent experiments of 6 samples from each group.
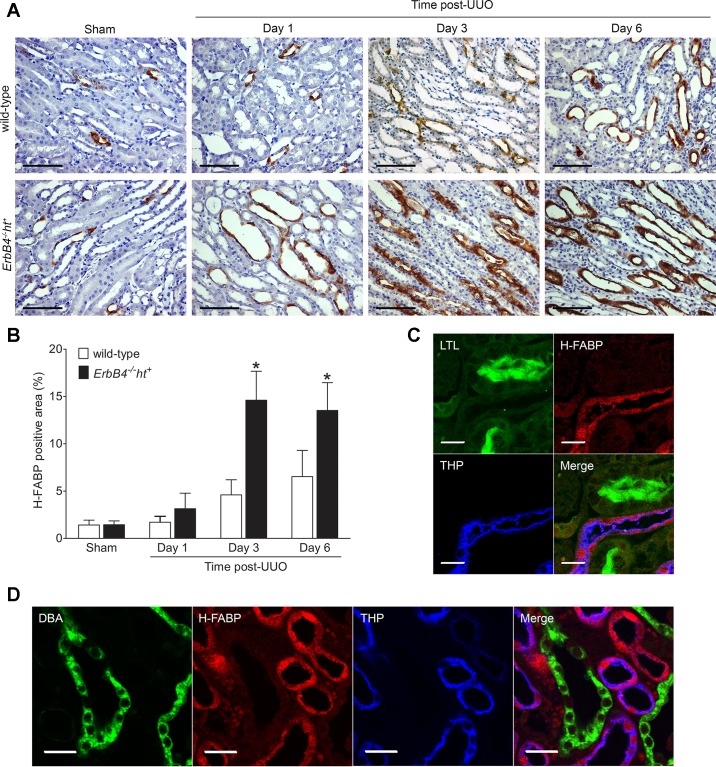
H-FABP has been previously described as a marker for distal nephron and collecting duct injury (16). In sham-operated kidneys, H-FABP was only sparsely expressed in tubules. Its levels increased at 1 day post-UUO, peaked at 3 days post-UUO, and remained at high levels at 6 days post-UUO. Compared with wild-type mice, UUO kidneys from ErbB4−/−ht+ mice expressed significantly higher levels of H-FABP at days 3 and 6 post-UUO (Fig. 5, A and B), which was further confirmed by immunoblotting (Fig. 4C). Immunofluorescent localization of H-FABP in 3 days post-UUO kidneys from ErbB4−/−ht+ mice indicated that H-FABP was expressed predominantly in thick ascending limb and distal tubules, as indicated by colocalization with THP. H-FABP did not colocalize with either DBA, a marker for collecting duct, or LTL, a marker for the proximal tubule (Fig. 5, C and D). Therefore, these studies indicated that H-FABP serves as a marker for thick ascending limb and distal tubule, with increased expression levels after injury. Cleaved caspase-3 was moderately increased in 3 days post-UUO kidneys of both wild-type and ErbB4−/−ht+ mice, but at 6 days post-UUO the ErbB4−/−ht+ mice had markedly increased levels of cleaved caspase-3 compared with the wild type (Fig. 4C).
Fig. 5.
H-FABP localization and its expression levels after UUO injury. A: expression of H-FABP after UUO injury. Compared with the wild type, ErbB4−/−ht+ kidneys showed increased H-FABP immunoreactivity 1 day after UUO and had further increases at 3 and 6 days post-UUO. Scale bar, 100 µm. B: quantification of H-FABP-positive immunostaining area of A using Image J software. Values represent means ± SE from 20 random fields of 4–6 kidneys in each group. *P < 0.05 compared with the wild type at the same time point. C and D: immunofluorescence staining of H-FABP with marker proteins. H-FABP colocalized with THP, a marker for thick ascending limb and distal convoluted tubule. H-FABP did not colocalize with Lotus tetragonolobus lectin (LTL), a marker for proximal tube, or DBA, a marker for collecting duct. Scale bar, 20 µm.
ErbB4 deletion promoted renal fibrogenesis in both UUO and IRI/UNx kidney injuries.
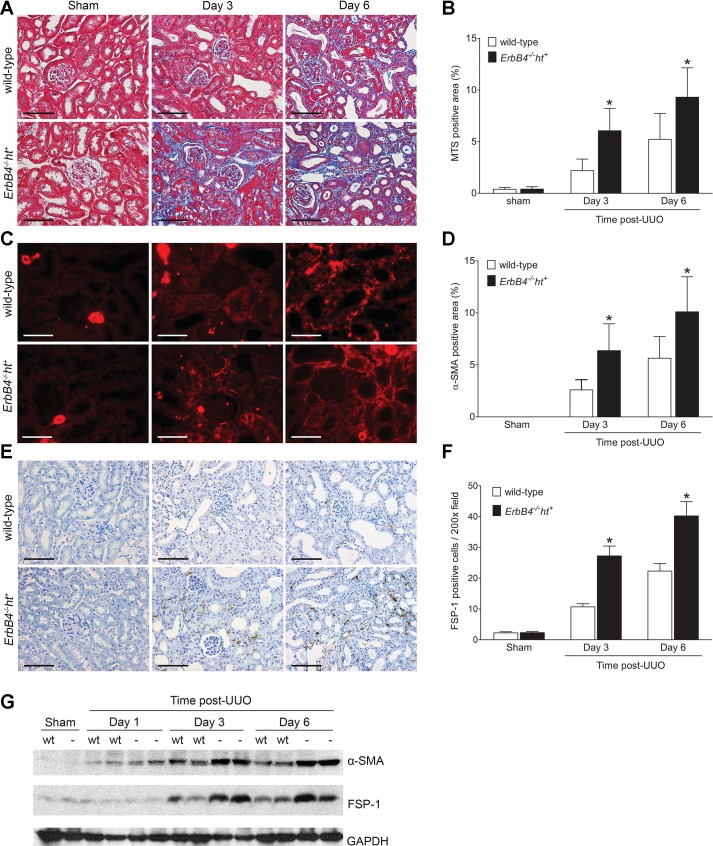
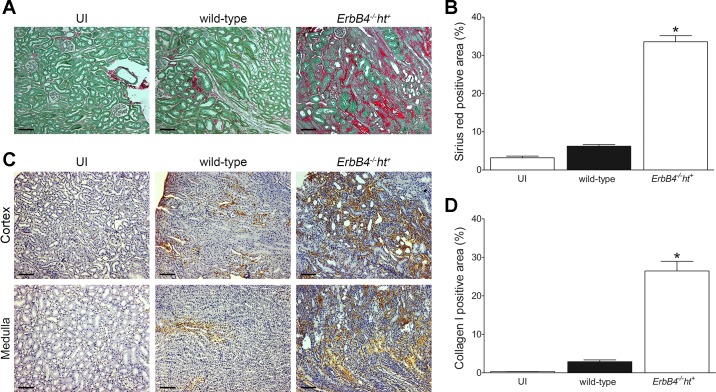
Renal fibrosis indicated by collagen staining with Masson’s trichrome stain was detected as early as 3 days post-UUO injury in ErbB4−/−ht+ mice, whereas it was only apparent at 6 days post-UUO in wild-type mice (Fig. 6, A and B). Those results were further confirmed by both immunostaining and immunoblotting for α-SMA (Fig. 6, C, D, and G), a marker for myofibroblast activation in the interstitium (3), and FSP-1 (Fig. 6, E–G), a marker for fibroblasts during renal fibrogenesis (8). In the IRI/UNx mouse model, ErbB4 deletion mouse kidneys showed severe renal fibrosis at 2 wk after uninephrectomy as indicated by sirius red/fast green and collagen I staining, whereas only minimum sirius red or collagen I staining was seen in wild-type kidneys (Fig. 7, A–D).
Fig. 6.
ErbB4 deletion accelerated renal interstitial fibrosis after UUO injury. Paraffin-embedded kidney sections from wild-type (n = 6) or ErbB4−/−ht+ (n = 8) mice were stained with Masson’s trichrome (A) to identify collagen fibers (scale bar, 100 µm), α-smooth muscle actin (α-SMA, C) to identify activated interstitial myofibroblasts (scale bar, 50 µm), and fibroblast-specific protein 1 (FSP-1, E), a marker for fibroblasts (scale bar, 100 µm). Representative images are shown, and positive staining was quantified from 20 random fields using ImageJ software. The corresponding results are shown in B, D, and F. Values represent means ± SE. *P < 0.05 compared with the wild type at the same time point. G: α-SMA and FSP-1 expression by immunoblotting. Compared with kidneys from the wild type, kidneys with ErbB4 deletion showed elevated expression levels of α-SMA and FSP-1 starting at 3 days post-UUO injury and remained high at 6 days post-UUO.
Fig. 7.
ErbB4 deletion accelerated renal interstitial fibrosis after IRI/UNx injury. A: sirius red/fast green staining on renal kidney post-22 days of initial IRI/UNx injury. Compared with the uninjured or wild-type kidneys, ErbB4 deletion kidney showed extensive sirius red staining. Scale bar, 100 µm. C: collagen I immunostaining in IRI/UNx kidneys. Scale bar, 100 µm. Representative images are shown, and positive staining was quantified from 20 random fields using ImageJ software. The corresponding results are shown in B and D. Values represent means ± SE. *P < 0.05 compared with the wild type and uninjured.
ErbB4 deletion induced G2/M phase arrest in UUO kidneys.
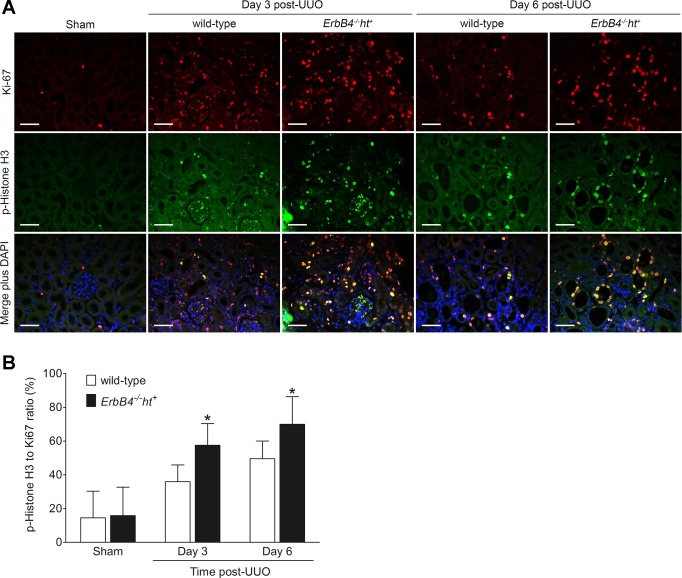
Previous studies reported that tubule G2/M phase arrest is one of the mechanisms underlying development of tubulointerstitial fibrosis (12, 31), whereas ErbB4 may play a role in cell cycle progression (10). Therefore, cell cycle status was analyzed using p-Histone H3, a marker for G2/M phase, and Ki-67, a general marker of proliferating cells. The p-Histone H3-to-Ki-67 ratio was used to evaluate cells in G2/M. Our results showed that, in both groups of sham-operated mice, p-Histone H3 and Ki-67 were very rarely expressed in either tubular epithelial cells or interstitial cells and were not significantly increased 1 day after UUO (data not shown). On day 3 post-UUO, both Ki-67- and p-Histone H3-positive cells were increased significantly in obstructed kidneys, with increased p-Histone H3 and p-Histone H3/Ki-67 ratio in obstructed kidneys from ErbB4−/−ht+ mice. A similar pattern was seen in day 6 post-UUO kidneys, albeit with less cell proliferation, as indicated by a reduced number of Ki-67-positive cells (Fig. 8, A and B).
Fig. 8.
ErbB4 deletion increased G2/M arrest after UUO injury. A: representative images from sham-operated kidneys in either group (n = 6) and 3 and 6 days post-UUO kidneys from wild-type (n = 6) and ErbB4−/−ht+ mice (n = 8) stained with Ki-67 (red), p-Histone H3 (green), and DAPI (blue). Scale bar, 50 µm. B: p-Histone H3 and Ki-67-positive cells were counted using ImageJ software from at least 20 random fields. p-Histone H3-to-Ki-67 ratio was used to evaluate cells in G2/M phase. Values represent means ± SE. *P < 0.05 compared with the wild type at the same time point.
ErbB4 deletion led to epithelial dedifferentiation in UUO kidneys.
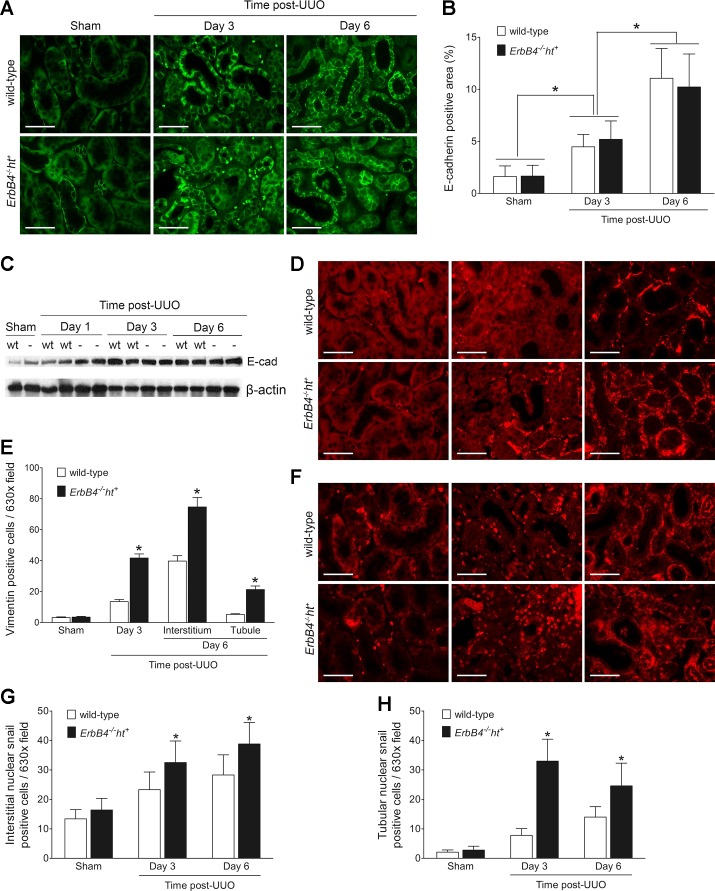
Epithelial dedifferentiation has been associated with G2/M arrest and with development of tubulointerstitial fibrosis (12, 31). In sham-operated kidneys, E-cadherin was not expressed in proximal tubule, which normally expresses predominantly N-cadherin (22). Following UUO, the expression of E-cadherin increased comparably in proximal tubules in both wild-type and ErbB4−/−ht+ mice (Fig. 9, A and B), which was further confirmed by Western blot (Fig. 9C). The increased E-cadherin expression levels may be caused by the dilatation of the tubules as reported by other investigators (5). Therefore, other markers of epithelial dedifferentiation, vimentin and snail1, were examined after UUO. The expression of vimentin increased significantly in UUO kidneys from ErbB4−/−ht+ mice compared with wild-type mice at 3 days after UUO injury. At 6 days following UUO, significantly increased levels of vimentin immunostaining were detected in both interstitium and tubular epithelium (Fig. 9, D and E). Expression levels of nuclear snail1 were also increased significantly in both interstitium and tubules in UUO kidneys from ErbB4−/−ht+ mice compared with the wild type at both 3 and 6 days after UUO (Fig. 9, F–H).
Fig. 9.
ErbB4 deletion induced renal tubular epithelial dedifferentiation after UUO injury. Sham or UUO-operated kidney sections were stained with anti-E-cadherin (E-cad, A), an epithelial marker, and anti-vimentin (D) and Snail 1 (F), mesenchymal marker. Scale bar, 50 µm. Positive immunostaining areas or cell numbers were calculated using ImageJ software and represented as means ± SE (B, E, G, and H). *P < 0.05 compared with the wild type at the same time point. C: E-cadherin expression levels by immunoblotting. Images represent results from 3 independent experiments.
Effect of ErbB4 deletion on signaling pathways.
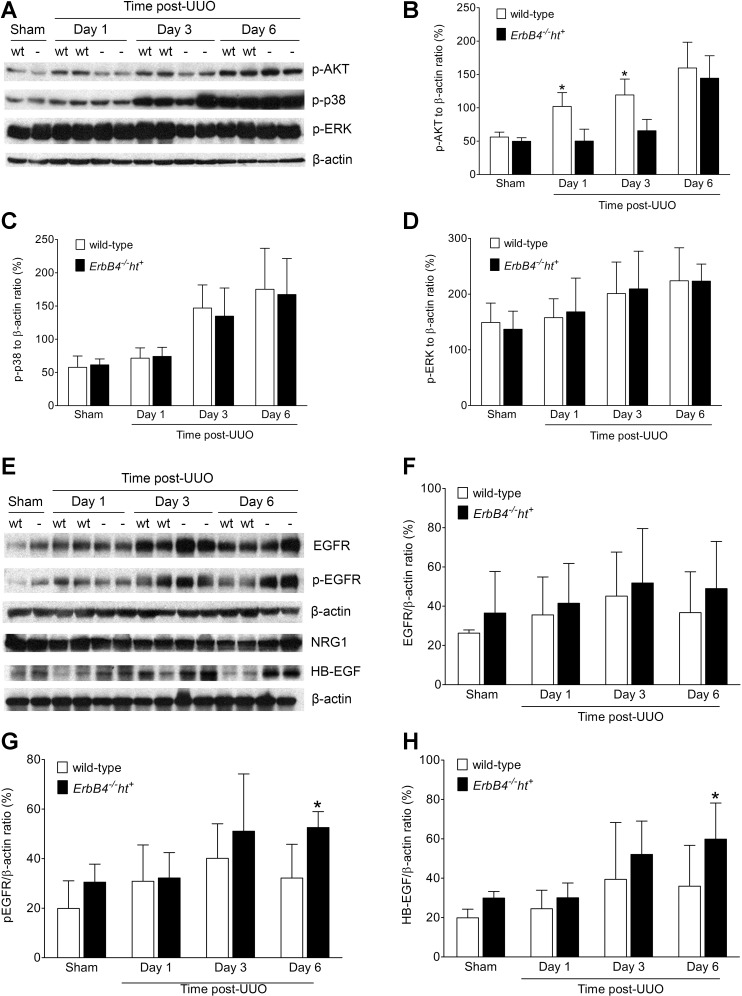
MAP kinases have been reported to play a role in renal fibrosis (13). Significantly elevated p-p38 levels were detected 3 days and later post-UUO, with similar extent in kidneys of wild-type or ErbB4−/−ht+ mice. A similar pattern was detected for p-ErK1/2 activation. In contrast, p-AKT levels significantly decreased in kidneys from ErbB4−/−ht+ mice at both 1 and 3 days following UUO compared with wild-type mice and reached similar levels at day 6 post-UUO (Fig. 10, A–D). To investigate further whether this was because of EGFR compensation after ErbB4 deletion, EGFR expression and activation levels were studied by immunoblotting. The results showed that EGFR levels were elevated in ErbB4 deletion groups in both sham and UUO mice compared with the wild type, but no significant differences were detected. The same pattern was seen with p-EGFR levels, except that significantly higher levels of EGFR phosphorylation were detected at day 6 post-UUO in the ErbB4 deletion groups (Fig. 10, E–G).
Fig. 10.
Signaling alterations after UUO injury. A: representative images from 3 independent experiments of MAP kinase protein expression levels by immunoblotting. B–D: densitometric analysis of immunoblotting in A expressed as ratios to β-actin. Values represent means ± SE. *P < 0.05 compared with the wild type. E: representative images of epidermal growth factor receptor (EGFR) expression, activation, and ligand level changes by Western blots. F–H: densitometric analysis of immunoblotting in E expressed as ratios to β-actin. Values represent means ± SE. *P < 0.05 compared with the wild type.
In addition, we did not detect significant changes in the expression levels of NRG1, a ligand for ErbB4, in either the sham or UUO group in kidneys with ErbB4 deletion compared with the wild type. However, the expression levels of HB-EGF, a ligand for both EGFR and ErbB4 receptors, were increased after UUO injury, with highest levels detected at day 6 post-UUO in ErbB4 deletion mice (Fig. 10, E and H). This is consistent with our recent studies showing that HB-EGF overexpression led to renal epithelial dedifferentiation and a profibrotic phenotype resulting from increased EGFR activation (21).
ErbB4 deletion elevated YAP and the TGF-β/p-Smad3 signaling pathways in UUO kidneys.
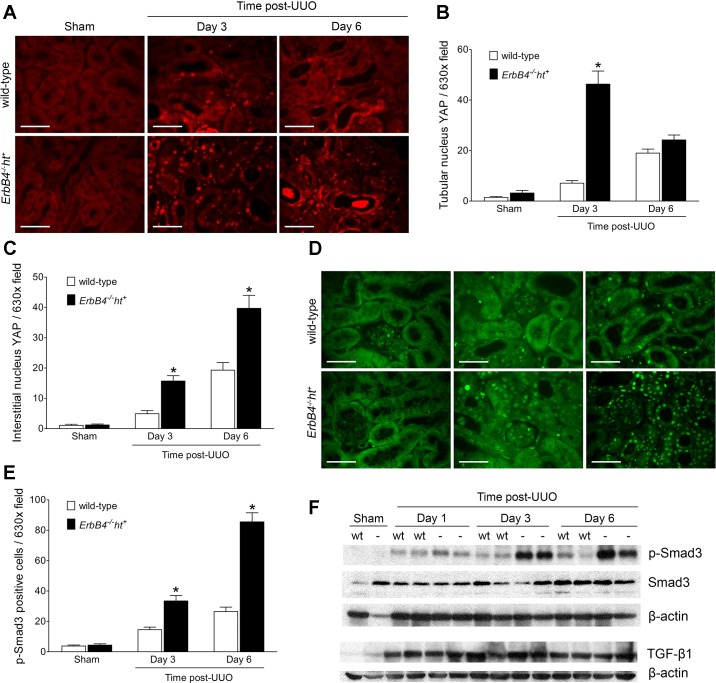
Recent studies have indicated that activation of the Hippo/YAP pathway occurs in response to UUO and may mediate tubulointerstitial fibrosis (26). Activated YAP translocates to the nucleus where it can mediate transcription of profibrotic factors. Because AKT phosphorylation can retain YAP in the cytosol through activation of 14–3-3, which binds to YAP (1), YAP signaling was investigated. In the sham-operated kidneys, low levels of YAP immunostaining were only detected in the cytoplasm and not in the nucleus. In UUO kidneys, YAP immunostaining was increased in both cytoplasm and nucleus, with more cytoplasmic staining in wild-type mice and more nuclear staining in ErbB4−/−ht+ mice. Nuclear YAP staining peaked at day 3 in tubular epithelium from ErbB4−/−ht+ mice, whereas it was further increased in interstitial cells at day 6 post-UUO (Fig. 11, A–C). Another known mediator of fibrogenesis, p-Smad3, also increased following UUO, with significantly greater expression in kidneys from ErbB4−/−ht+ mice (Fig. 11, D–F), whereas no changes in total TGF-β1 levels were detected (Fig. 11F).
Fig. 11.
ErbB4 deletion increased yes-associated protein (YAP) and p-Mothers against decapentaplegic homolog 3 (Smad3) nuclear expression after UUO. A: immunofluorescence staining of YAP. Scale bar, 50 µm. B and C: quantification of YAP-positive cells from at least 20 random fields using ImageJ. Compared with the wild type, obstructed kidneys with ErbB4 deletion showed more YAP nuclear staining in both tubular epithelium and interstitium as early as 3 days post-UUO. At 6 days post-UUO, much more YAP nuclear-positive cells were located in the interstitium. Values represent means ± SE. *P < 0.05 compared with the wild type. D: immunofluorescence staining of p-Smad3. Scale bar, 50 µm. E: quantification of p-Smad3-positive cells. Compared with the wild type, obstructed kidneys with ErbB4 deletion showed more p-Smad3 nuclear staining starting 3 days post-UUO and increased continuously at 6 days post-UUO. Values represent means ± SE from at least 20 random fields. *P < 0.05 compared with the wild type. F: p-Smad3, Smad3, and TGF-β1 expression by immunoblotting. Images represent results from 3 independent experiments.
DISCUSSION
In the current study, we found that, in human kidney biopsy samples, ErbB4 expression levels are inversely correlated to the degree of renal fibrosis. By using mouse injury models, we found that deletion of ErbB4 promoted renal fibrosis after renal injury. There was a markedly earlier onset of tubulointerstitial fibrosis in ErbB4−/−ht+ mice compared with wild-type mice, which was accompanied by evidence of G2/M arrest and tubule dedifferentiation and activation of YAP and p-Smad3, two putative mediators of tubulointerstitial fibrosis. Although ErbB4 deletion did not alter increased activation of the ERK and p38 pathways following UUO, it did lead to a striking early decrease in activation of AKT, which is notable given the potential role of AKT in cytoprotection. Of note, the effect of ErbB4 appeared to be predominantly in nephron segments distal to proximal tubule, which is consistent with the expression pattern of ErbB4 in the adult mouse kidney (25). ErbB4 is expressed in the adult kidney at a lower level compared with the fetal kidney, with highest expression in the loop of Henle, distal convoluted tubule, and collecting duct (25, 35). In the present studies, distal tubular injury, indicated by H-FABP3 expression levels, was more severe in ErbB4-deleted obstructed kidneys compared with wild-type mice.
Prolonged tubular G2/M arrest has been shown to induce a profibrotic phenotype, with upregulation of profibrogenic growth factors, including TGF-β1 and connective tissue growth factor (31). Compared with wild-type mice, UUO kidneys with ErbB4 deletion had more tubule cells in the G2/M phase by day 3, indicated by an increased p-Histone H3/Ki-67 ratio, which may have contributed to the earlier accumulation of the interstitial matrix and fibroblast activation. Intriguingly, EGFR activation also contributes to arrest of epithelial cells in the G2/M phase after chronic injury (11, 36). Whether the absence of ErbB4 leads to more EGFR activation will be the subject of future investigation. Of note, we did not detect significantly increased cleaved caspase-3 levels in obstructed kidneys with ErbB4 deletion until day 6 after UUO, when renal fibrosis was already abundant. Those results suggested that apoptosis of the tubular epithelium was not the proximate cause of fibrosis in this setting.
Our previous studies found that ErbB4 expression was elevated in the cystic tubular epithelium of congenital polycystic kidney mice, a murine ARPKD model, and ErbB4 deletion accelerated renal function decline in association with the development of pericystic fibrosis (32). More recently, it has been reported that NRG1, an ErbB4 ligand, attenuated the development of nephropathy in a type 1 diabetes mouse model through activating an ErbB4 signaling pathway, which prevented glomerular matrix formation and glomerulosclerosis (28). These studies are consistent with genome-wide association studies of type 1 diabetes-associated nephropathy that provided suggestive, but not conclusive, evidence (odds ratio 0.66, P = 2.1 × 10−7) that an intronic SNP in ERBB4 might somehow be involved in progression of diabetic nephropathy (2, 14).
The ErbB4 receptor can be proteolytically cleaved at a juxtamembrane segment, with release of the intracellular domain of two cytoplasmic isoforms (CYT-1 and CYT-2) that are either degraded through ubiquitination (CYT-1) or translocated to the nucleus and mediate transcriptional activity (CYT-2) (19, 34). ErbB4 CYT-1, which is expressed in the kidney (35), possesses a phosphatidylinositol 3-kinase-binding domain in its COOH-terminus. Activation of ErbB4 has been shown to initiate a phosphatidylinositol 3-kinase/AKT signaling pathway that can further phosphorylate YAP at Ser127, leading to its binding to 14–3-3 in the cytoplasm. This will promote YAP localization to the cytoplasm (inactive form) and result in an inability to traffic to the nucleus, where it can function as a coactivator of transcription factors, including the ErbB4 intracellular domain (CYT-2) itself (1, 9). Furthermore, nuclear YAP and TAZ (transcriptional coactivator with PDZ-binding motif) are transcription cofactors that promote TGF-β signaling via retaining activated Smad2/3 in the nucleus (26, 29). In the present studies ErbB4 deletion led to decreased AKT activation and increased nuclear YAP and p-Smad3 expression, suggesting that the decreased AKT activation may have mediated increased YAP and TGF-β activation and promotion of tubulointerstitial fibrosis. However, it has also been reported that the phosphatidylinositol 3-kinase-AKT pathway contributes to renal fibrogenesis through promoting cell proliferation and interstitial extracellular matrix deposition (23, 36), so further studies will be necessary to determine the role of AKT activation following UUO.
In conclusion, ErbB4 deletion accelerates the development and progression of renal fibrosis following renal injury. Increased expression of ErbB4 in kidneys with chronic injury may actually reflect a compensatory effect to prevent development of tubulointerstitial injury.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-051265, DK-062794, and DK-095785 (R. C. Harris) and by funds from the Department of Veterans Affairs. Normal and different-stage human renal fibrosis biopsy samples were kindly provided by Dr. Agnes B. Fogo, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.Z. and R.C.H. conceived and designed research; F.Z., T.M., and L.A.K. performed experiments; F.Z., T.M., and L.A.K. analyzed data; F.Z., T.M., and L.A.K. interpreted results of experiments; F.Z. and T.M. prepared figures; F.Z. drafted manuscript; R.C.H. edited and revised manuscript; R.C.H. approved final version of manuscript.
REFERENCES
- 1.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11: 11–23, 2003. doi: 10.1016/S1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 2.Böger CA, Sedor JR. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet 8: e1002989, 2012. doi: 10.1371/journal.pgen.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boukhalfa G, Desmoulière A, Rondeau E, Gabbiani G, Sraer JD. Relationship between alpha-smooth muscle actin expression and fibrotic changes in human kidney. Exp Nephrol 4: 241–247, 1996. [PubMed] [Google Scholar]
- 4.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty NG, Calvo IF, Quinlan MR, Pérez-Barriocanal F, McGuire BB, Fitzpatrick JM, Watson RW. Increased E-cadherin expression in the ligated kidney following unilateral ureteric obstruction. Kidney Int 75: 205–213, 2009. doi: 10.1038/ki.2008.482. [DOI] [PubMed] [Google Scholar]
- 6.Forrester SJ, Kawai T, O’Brien S, Thomas W, Harris RC, Eguchi S. Epidermal growth factor receptor transactivation: mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Annu Rev Pharmacol Toxicol 56: 627–653, 2016. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 8.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem 278: 33334–33341, 2003. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 10.Liang X, Ding Y, Zhang Y, Chai YH, He J, Chiu SM, Gao F, Tse HF, Lian Q. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis 6: e1765, 2015. doi: 10.1038/cddis.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Zhuang S. Role of receptor tyrosine kinase signaling in renal fibrosis. Int J Mol Sci 17: 17, 2016. doi: 10.3390/ijms17060972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21: 998–1009, 2015. doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma FY, Sachchithananthan M, Flanc RS, Nikolic-Paterson DJ. Mitogen activated protein kinases in renal fibrosis. Front Biosci (Schol Ed) 1: 171–187, 2009. doi: 10.2741/s17. [DOI] [PubMed] [Google Scholar]
- 14.Maeda S, Imamura M, Kurashige M, Araki S, Suzuki D, Babazono T, Uzu T, Umezono T, Toyoda M, Kawai K, Imanishi M, Hanaoka K, Maegawa H, Uchigata Y, Hosoya T. Replication study for the association of 3 SNP loci identified in a genome-wide association study for diabetic nephropathy in European type 1 diabetes with diabetic nephropathy in Japanese patients with type 2 diabetes. Clin Exp Nephrol 17: 866–871, 2013. doi: 10.1007/s10157-013-0797-5. [DOI] [PubMed] [Google Scholar]
- 15.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res 66: 6412–6420, 2006. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 16.Nauta FL, Boertien WE, Bakker SJ, van Goor H, van Oeveren W, de Jong PE, Bilo H, Gansevoort RT. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 34: 975–981, 2011. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neelisetty S, Alford C, Reynolds K, Woodbury L, Nlandu-Khodo S, Yang H, Fogo AB, Hao CM, Harris RC, Zent R, Gewin L. Renal fibrosis is not reduced by blocking transforming growth factor-β signaling in matrix-producing interstitial cells. Kidney Int 88: 503–514, 2015. doi: 10.1038/ki.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemo R, Murcia N, Dell KM. Transforming growth factor alpha (TGF-alpha) and other targets of tumor necrosis factor-alpha converting enzyme (TACE) in murine polycystic kidney disease. Pediatr Res 57: 732–737, 2005. doi: 10.1203/01.PDR.0000159513.51898.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science 294: 2179–2181, 2001. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 20.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167, 2000. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overstreet JM, Wang Y, Wang X, Niu A, Gewin LS, Yao B, Harris RC, Zhang MZ. Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis FASEB J pii: 16, 2017. doi: 10.1096/fj.201601359RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol 4: 10, 2004. doi: 10.1186/1472-6793-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Peña AB, Grande MT, Eleno N, Arévalo M, Guerrero C, Santos E, López-Novoa JM. Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int 74: 196–209, 2008. doi: 10.1038/ki.2008.160. [DOI] [PubMed] [Google Scholar]
- 24.Skrypnyk NI, Voziyan P, Yang H, de Caestecker CR, Theberge MC, Drouin M, Hudson B, Harris RC, de Caestecker MP. Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. Am J Physiol Renal Physiol 311: F268–F277, 2016. doi: 10.1152/ajprenal.00056.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol 185: 236–245, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol 27: 3117–3128, 2016. doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA 100: 8281–8286, 2003. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandekerckhove L, Vermeulen Z, Liu ZZ, Boimvaser S, Patzak A, Segers VF, De Keulenaer GW. Neuregulin-1 attenuates development of nephropathy in a type 1 diabetes mouse model with high cardiovascular risk. Am J Physiol Endocrinol Metab 310: E495–E504, 2016. doi: 10.1152/ajpendo.00432.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 10: 837–848, 2008. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 30.Wang YN, Yamaguchi H, Hsu JM, Hung MC. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene 29: 3997–4006, 2010. doi: 10.1038/onc.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice. Kidney Int 86: 538–547, 2014. doi: 10.1038/ki.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng F, Xu J, Harris RC. Nedd4 mediates ErbB4 JM-a/CYT-1 ICD ubiquitination and degradation in MDCK II cells. FASEB J 23: 1935–1945, 2009. doi: 10.1096/fj.08-121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng F, Zhang MZ, Singh AB, Zent R, Harris RC. ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol Cell 18: 4446–4456, 2007. doi: 10.1091/mbc.E07-03-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang S, Liu N. EGFR signaling in renal fibrosis. Kidney Int Suppl (2011) 4: 70–74, 2014. doi: 10.1038/kisup.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]