Abstract
The short-term effect of high-glucose (HG) treatment on store-operated Ca2+ entry in mesangial cells (MCs) is not well-known. The aim of the present study was to determine whether and how HG treatment for a short period altered protein abundance of Orai1, the channel mediating store-operated Ca2+ entry in MCs. Rat and human MCs were exposed to HG (25 mM) for 2, 4, 8, and 24 h, and the abundance of Orai1 protein was significantly decreased at the time points of 8 and 16 h. Consistently, HG treatment for 8 h significantly reduced store-operated Ca2+ entry in rat MCs. However, HG treatment for the same time periods did not alter the levels of Orai1 transcript. Cycloheximide, a protein synthesis inhibitor, did not affect the HG-induced decrease of Orai1 protein, suggesting a posttranslational mechanism was involved. However, the HG effect on Orai1 protein was significantly attenuated by MG132 (a ubiquitin-proteasome inhibitor) and NH4Cl (a lysosomal pathway inhibitor). Furthermore, HG treatment for 8 h stimulated ubiquitination of Orai1 protein. We further found that polyethylene glycol-catalase, an antioxidant, significantly blunted the HG-induced reduction of Orai1 protein. In support of involvement of reactive oxygen species in the HG effects, hydrogen peroxide (H2O2) itself significantly decreased abundance of Orai1 protein and increased the level of ubiquitinated Orai1. Taken together, these results suggest that a short-term HG treatment decreased abundance of Orai1 protein in MCs by promoting the protein degradation through the ubiquitination-proteasome and –lysosome mechanisms. This HG-stimulated posttranslational mechanism was mediated by H2O2.
Keywords: hydrogen peroxide, mesangial cells, Orai1, store-operated Ca2+ entry, ubiquitination
INTRODUCTION
Glomerular mesangial cells (MCs) and their matrix form the central stalk of the glomerulus. MCs comprise approximately one-third of the decapsulated glomerular cell population. Physiologically, these glomerular cells have many beneficial roles, which include secretion of growth factors that allow normal cell turnover, production of mesangial matrix to provide structural support for glomerular capillaries, modulation of glomerular hemodynamics through their contractile properties, and phagocytosis of apoptotic cells or immune complexes formed at or delivered to the glomerular capillaries (1). Dysfunction of MCs is closely associated with several kidney diseases, including diabetic nephropathy (46). As in most cell types, Ca2+ signaling plays a central role in mediating and regulating the physiological and pathological processes in MCs (33). Among multiple Ca2+ signaling pathways in MCs, store-operated Ca2+ channel (SOC)-mediated Ca2+ entry (SOCE) plays a pivotal role in physiology and pathology of MCs (5, 7, 34, 38, 40). SOCs are activated on depletion of Ca2+ in the endoplasmic reticulum in response to activation of G protein-coupled receptors. Although Orai2, Orai3, and several types of canonical transient receptor potential proteins may function as SOC in some cell types (10, 32, 50), Orai1 is the predominant protein constituting SOC in both excitable and nonexcitable cells (16, 51, 56).
MCs are also an important target of metabolic abnormalities in the diabetic environment. Dysfunction of MCs contributes to the development of diabetic kidney disease. Studies demonstrated that high glucose (HG) is the principal cause of MC pathogenesis in diabetes and altered MC function by HG is central to the pathogenesis of progressive diabetic glomerulopathy (2, 22, 25, 44, 53). We (8) have previously demonstrated that the long-term treatment (≥7 days) of HG increased abundance of Orai1 protein and SOCE in human MCs. However, the short-term (<1 day) effect of HG on this channel protein in the glomerular cells is not known. Because MC function could be impaired in a short period of exposure to HG, it is important to know the HG-induced early changes of intracellular Ca2+ signaling in MCs. The aim of the present study was to identify whether and how a short-term HG treatment altered the level of Orai1 protein in rat MCs.
MATERIALS AND METHODS
Mesangial cell culture.
Rat MCs immortalized with pSV3-Neo were purchased from American Type Culture Collection (ATCC CRL-2573; Manassas, VA). These cells were validated by the vendor by showing positive for desmin and vimentin and negative for cytokeratin 8. We further confirmed their origin by immunocytochemical analysis of α-smooth muscle actin. Primary human MCs were purchased from ScienCell (cat. no. 4200; Carlsbad, CA). These cells were validated by the vendor by showing immunofluorescence specific to fibronectin, Thy-1, and smooth muscle actin. Both rat and human MCs in a 75-cm2 flask were cultured in 5.6 mM glucose DMEM (GIBCO, Carlsbad, CA) supplemented with 25 mM HEPES, 4 mM l-glutamine, 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, 0.4 mg/ml G418, and 10% fetal bovine serum (FBS). When the flask reached 90% confluence, the cells were split into 60-mm (for Western blot) or 35-mm (rat MCs for RT-PCR and Ca2+ imaging experiments) culture plates. Except for rat MCs for Ca2+ imaging experiments, the cells used for all other studies were growth-arrested by 0.5% FBS overnight before they were applied to various treatments as specified in figure legends. The cell growth was arrested with 0.5% FBS medium during treatments. The culture media were replaced every 2 days with fresh media. The rat and human MCs used in the present study were with subpassages 10–20 and 4–8, respectively.
Western blot.
The whole cell lysates were fractionated by 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with primary Orai1 (cat. no. O8264; Sigma-Aldrich) and α-tubulin (cat. no. sc-5286; Santa Cruz Biotechnology) antibodies. Bound antibodies were visualized with SuperSignal West Femto or Pico Luminol/Enhancer Solution (Thermo Scientific, Rockford, IL). The specific protein bands were visualized and captured using the AlphaEaseFC imaging system (Alpha Innotech, San Leandro, CA). The integrated density value (IDV) of each band was measured by drawing a rectangle outlining the band using AlphaEaseFC software with auto-background subtraction. The anti-Orai1 primary antibody used in this study recognized nonglycosylated Orai1 at ~35 kDa and glycosylated Orai1 at ~50 kDa. Therefore, we used a total IDV of Orai1 protein bands, i.e., summation of the IDV of each Orai1 band, for analysis. The abundance of Orai1 protein was evaluated by normalizing the total IDV of Orai1 bands to that of α-tubulin band in the same group on the same blot. The anti-Orai1 primary antibody was validated in our (8) previous study.
Quantitative real-time RT-PCR.
The total RNA was isolated from cultured rat MCs using PerfectPure RNA Cultured Cell Kit (5 Prime, Hamburg, Germany) following the manufacturer’s protocol. Rat orai1 primers (forward: 5′-CCA TAA GAC GGA CCG ACA GT-3′; reverse: 5′-GGG AAG GTG AGG ACT TAG GC-3′) and β-actin primers (forward: 5′-AGC CAT GTA CGT AGC CAT CC-3′; reverse: 5′-ACC CTC ATA GAT GGG CAC AG-3′) were synthesized by Integrated DNA Technologies (IDT, Coralville, IA). A total of 1.0-μg RNA in a final volume of 20 μl was used for RT reactions using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) following the manufacturer’s reaction protocol. A total of 0.2-μg RT product and 100 nM primer was used for real-time PCR, which was performed using iQ SYBR Green Supermix (Bio-Rad) in a final volume of 20 μl. The PCR mix was denatured at 95°C for 10 min, followed by 45 cycles of melting at 95°C for 15 s, annealing at 57°C for 10 s, and elongation at 72°C for 15 s. After amplification, a melting curve analysis from 65 to 95°C with a heating rate of 0.02°C/s with a continuous fluorescence acquisition was made. The assay was run on a C1000 Thermal Cycler (Bio-Rad). The average threshold cycle (Ct) of fluorescence unit was used to analyze mRNA levels. The Orai1 mRNA level was normalized by the corresponding β-actin mRNA level. Quantification was calculated as follows: mRNA levels = 2ΔCt, where ΔCt = Ct,Orai1 − Ct,actin.
Fluorescence measurement of [Ca2+]i.
Measurements of intracellular Ca2+ concentration ([Ca2+]i) in human MCs using fura 2 were performed using dual-excitation wavelength fluorescence microscopy. MCs grown on a coverslip (22 × 22 mm) were loaded with 2 μM acetoxymethyl ester of fura 2 (fura 2-AM) and 0.018 g/dl Pluronic F-127 (Invitrogen, Grand Island, NY) for 50 min at room temperature followed by additional 20-min incubation in fura 2 free physiological saline solution. The coverslip was then placed in a perfusion chamber (model RC-2OH; Warner Instruments) mounted on the stage of a Nikon Diaphot inverted microscope. Fura 2 fluorescence was monitored at 340- and 380-nm excitation wavelengths and at 510-nm emission wavelength using NIS-Elements Advanced Research software (Nikon Instruments, Melville, NY) at room temperature. [Ca2+]i was calculated using the software following the manufacturer’s instructions. Calibrations were performed at the end of each experiment, and conditions of high [Ca2+]i were achieved by addition of 5 μM ionomycin, whereas conditions of low [Ca2+]i were obtained by addition of 5 mM EGTA.
Ubiquitination assay.
Ubiquitinated Orai1 protein was evaluated using the UBI-QAPTURE-Q kit from Enzo Life Science (Farmingdale, NY) by following the manufacturer’s instructions. The kit facilitates pull-down of both mono- and polyubiquitinated proteins (independent of lysine residue chain linkage) from cell extracts through use of a high-binding-affinity matrix. Whole cell lysates of rat MCs were prepared in RIPA buffer. The samples with 500-µg protein content and control binding solution (control ubiquitinated protein lysate, provided by the manufacturer) were incubated with the affinity matrix to capture all ubiquitinated proteins. After a thorough wash, the proteins were eluted in SDS-PAGE gel loading buffer and subjected to regular Western blot analysis using the Orai1 primary antibody. The control ubiquitinated protein lysates were extracted from HeLa cells, which contain endogenous Orai1 protein (9). Therefore, we used the control lysates as the positive control of ubiquitinated Orai1 in Western blot. For an internal loading control (input), 50-µg protein of lysates (before ubiquitin enrichment) from different groups of samples was applied to the regular Western blot analysis for probing α-tubulin.
Hydrogen peroxide detection.
Hydrogen peroxide (H2O2) generated by rat MCs was determine by Amplex Red Hydrogen Peroxide Assay Kit (cat. no. A22188; Invitrogen) following the manufacturer’s instruction. In brief, rat MCs were plated in 35-mm cell culture dishes. When ~90% confluence was reached, cell growth was stopped by 0.5% FBS cell culture medium overnight. The cells were then treated with 0.5% FBS phenol-free medium containing 5 mM d-glucose and 20 mM α-mannitol or 25 mM d-glucose for the time periods as indicated (Fig. 6A). After treatment, 50 µl of culture medium was collected. All samples and H2O2 standards were prepared with Amplex Red assay kits following the manufacturer’s instruction. The fluorescence intensity was measured at 530 nm for excitation and emission in the range of 560–590 nm using the PerkinElmer 2030 Multilabel Plate Reader (VICTOR X3; PerkinElmer Singapore, Life and Analytical Sciences). The standard curve of H2O2 was established using SigmaPlot software (version 11.0), and the concentrations of H2O2 in samples were calculated from the H2O2 standard curve.
Fig. 6.

HG-induced decrease in Orai1 protein level was associated with increased H2O2 generation. A: the concentration of H2O2 in culture medium of rat MCs detected by Amplex Red assay. Confluent cells were treated with 0.5% FBS phenol-free medium containing either 5.6 mM d-glucose and 20 mM α-mannitol (−HG) or 25 mM d-glucose for the time periods indicated. *P < 0.05, compared with HG group at the same time point. n Indicates the number of independent experiments. B and C: Western blot showing Orai1 protein level in rat MCs with and without HG treatment for 8 h in the presence and absence of polyethylene glycol-catalase (300 U/ml). A representative immunoblot is shown in B, and the summary data are presented in C. In B: TB, α-tubulin, the loading control. In C: *P < 0.05, compared with all other groups. n Indicates the number of independent Western blot.
Materials.
Thapsigargin (TG; T9033), polyethylene glycol-catalase (C4963), MG132 (M7449), NH4Cl (A9434), and urea H2O2 (289132) were purchased from Sigma-Aldrich. Cycloheximide was purchased from Cell Signaling Technology (cat. no. 2112). The source of all other materials is indicated in other places.
Statistical analyses.
Data are reported as means ± SE. The one-way repeated measures of ANOVA and Student-Newman-Keuls post hoc analysis were used to analyze the difference among multiple groups. The unpaired Student’s t-test was used to analyze the difference between two groups. In Fig. 1, B and D, and Figs. 3A, 5B, and 7D, the data in both control (“−HG” or “−H2O2”) and treatment (“+HG” or “+H2O2”) groups were normalized by dividing each individual value of control and treatment samples by the mean of the control samples. P < 0.05 was considered statistically significant. Statistical analysis was performed using SigmaStat (Jandel Scientific, San Rafael, CA).
Fig. 1.
HG treatment for a short term decreased Orai1 protein abundance in rat and human MCs. A and C: representative Western blot showing time-course effect of HG treatment on Orai1 protein content in rat (A) and human (C) MCs. Confluent MCs were cultured in 0.5% FBS media containing either 5 mM d-glucose (−HG) or 25 mM d-glucose (+HG) for 2, 4, 8, and 24 h. The medium for the cells in –HG group also contained 20 mM α-mannitol for the osmotic control. The Orai1 primary antibody recognized 2 bands, nonglycosylated Orai1 at ~35 kDa and glycosylated Orai1 at ~50 kDa. L, protein ladder; TB, α-tubulin, the loading control. B and D: summary data from experiments shown in A and C, respectively. The level of Orai1 protein in –HG group at each time point was considered as 100%, and the level of Orai1 protein in +HG groups was normalized to that in –HG group at the same time point. “n” Indicates the number of independent experiments. *P < 0.05, +HG group vs. –HG group at the same time point.
Fig. 3.

Effect of short-term HG treatment on expression level of Orai1 mRNA and Orai1 protein stability in rat MCs. A: quantitative real-time RT-PCR showing Orai1 mRNA expression in rat MCs with and without HG treatment. Confluent rat MCs were cultured in 0.5% FBS containing 5.6 mM d-glucose and 20 mM α-mannitol (−HG) or 25 mM d-glucose (+HG) for 8 h. n Indicates the number of independent experiments. B: representative Western blot showing Orai1 protein stability in response to short-term HG treatment in rat MCs. Confluent cells were growth-arrested by 0.5% FBS overnight and then were treated with 0.5% FBS medium containing 5.6 mM d-glucose and 20 mM α-mannitol (−HG) or 25 mM d-glucose (+HG) in the presence of 200 µM cycloheximide (Cyc) for the indicated time periods. Levels of Orai1 protein in cell lysates were measured using Western blot analysis. L, protein ladder; TB, α-tubulin, the loading control. C: summary data for the experiments presented in B. n Indicates the number of independent experiments. *P < 0.05, compared with 0- and 2-h time point in +HG group; †P < 0.05, compared with 8-h time point in –HG group.
Fig. 5.
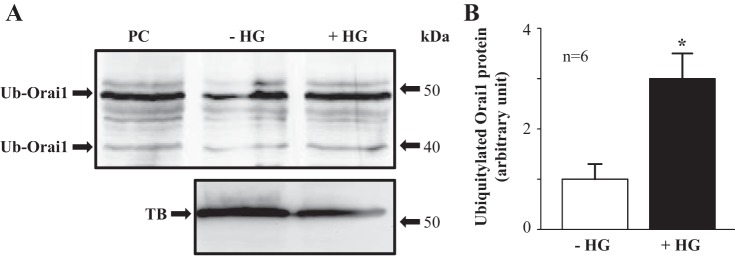
HG treatment increased the level of ubiquitinated Orai1 protein in rat MCs. A: representative Western blot showing abundance of ubiquitinated Orai1 protein in rat MCs with and without HG treatment. Confluent rat MCs were cultured in 0.5% FBS medium containing 5.6 mM d-glucose and 20 mM α-mannitol (−HG) or 25 mM d-glucose (+HG) for 8 h. PC, positive control, provided by the manufacturer; Ub-Orai1, ubiquitinated Orai1; TB, α-tubulin, the loading control. B: summary densitometric data from experiments presented in A. *P < 0.05, +HG vs. –HG. n Indicates the number of independent experiments.
Fig. 7.
H2O2 decreased Orai1 protein abundance and increased ubiquitination of Orai1 protein in rat MCs. A: representative Western blot showing H2O2 effect on Orai1 protein abundance. Confluent rat MCs were treated with 300 µM H2O2 for the time indicated. TB, α-tubulin, loading control. B: summary densitometric analyses of ratios of Orai1 to α-tubulin from the experiments presented in A. The values in all groups were normalized to that in 0-min group. *P < 0.05, compared with 0-min group. n Indicates the number of independent experiment. C: representative Western blot showing H2O2 effect on Orai1 protein ubiquitination. Confluent rat MCs were with or without 300 µM H2O2 treatment for 60 min. PC, positive control, provided by the manufacturer; Ub-Orai1, ubiquitinated Orai1. D: summary densitometric data from experiments presented in C. *P < 0.05, +H2O2 vs. –H2O2. n Indicates the number of independent experiments.
RESULTS
HG treatment for a short period decreased abundance of Orai1 protein in rat and human MCs.
Orai1 is the pore-forming protein of SOC (16, 51, 56). We have previously demonstrated that Orai1 protein was present and functioned as SOC in MCs (8, 48). To determine a time-course effect of HG on Orai1 protein content, rat MCs were treated with and without HG (25 mM) for different time periods and Western blots were carried out to evaluate the level of Orai1 protein. In the cells without HG treatment, 20 mM α-mannitol was added to the culture media for the osmotic control. As shown in Fig. 1, the level of Orai1 protein was decreased by HG treatment with time and a significant reduction was observed in the time point of 8 h, which sustained at least for additional 16 h.
To confirm the HG effects, we repeated the experiments in primary human MCs. As shown in Fig. 1, C and D, the HG effects on Orai1 protein in rat MCs were reproduced in human MCs. These results suggest that a short-term HG treatment (≤24 h) decreased abundance of Orai1 protein in MCs.
Short-term HG treatment decreased SOCE in rat MCs.
Orai1 has been shown to mediate SOCE in MCs (8, 48). Because the short-term treatment of HG reduced the amount of Orai1 protein (Fig. 1), we expected that SOCE was decreased accordingly. Ca2+ imaging experiments were carried out to assess the functional consequence of the short-term HG treatment. In rat MCs with and without HG treatment for 8 h, we measured fura 2 fluorescence-indicated Ca2+ entry response using a classic “Ca2+ add-back” protocol described in our (22, 23) previous publications. TG (1 µM) was used to activate SOCE. As shown in Fig. 2, TG evoked a rapid and transient rise of [Ca2+]i in 1 mM Ca2+ bathing solution in MCs both with and without HG treatment. This initial response was due to depletion of the internal Ca2+ stores, a process for opening SOC. Readdition of 2 mM Ca2+ to the Ca2+-free bath resulted in an increase in [Ca2+]i. This elevation of [Ca2+]i represents SOCE. The TG-stimulated SOCE response was significantly diminished in MCs with HG treatment compared with the cells without HG treatment (Fig. 2). These fura 2 data provided functional evidence supporting an inhibitory effect of a short-term HG treatment on SOCE in rat MCs.
Fig. 2.
HG treatment for 8 h decreased SOCE. Fura 2 fluorescence ratiometry was used to assess the intracellular Ca2+ concentration ([Ca2+]i). SOCE was evaluated using a Ca2+-readmission protocol. A and B: representative traces showing TG (1 μM)-evoked Ca2+ response in rat MCs with (+HG) and without (−HG) HG treatment for 8 h. Ca2+ traces were smoothed using SigmaPlot (version 11.0). [Ca2+]B represents the Ca2+ concentration in bathing solution. SOCE was the increase in [Ca2+]i on switching the bathing solution from Ca2+-free to 2 mM Ca2+ solution. The numbers inside the horizontal bar on the top of each graph indicate the Ca2+ concentration in bathing solution. C: summary data showing SOCE in rat MCs with (+HG) and without (−HG) HG treatment. *P < 0.05, compared with −HG group. n Indicates the number of cells analyzed in each group.
Short-term HG treatment decreased Orai1 protein stability in rat MCs.
To determine whether the HG-induced decrease of Orai1 protein abundance was due to suppression of orai1 transcription, we conducted quantitative real-time RT-PCR and evaluated the mRNA expression level of orai1 in rat MCs with and without HG treatment for different time periods. As shown in Fig. 3A, HG treatment for a time period ranging from 2 to 8 h did not reduce the level of Orai1 transcript.
We then further measured the effect of HG treatment on the protein stability of Orai1. Rat MCs were treated with HG (25 mM) or normal glucose (5 mM) and 20 mM α-mannitol (osmotic control) in the presence of 200 µM cycloheximide for 2, 4, and 8 h. Western blot was conducted to evaluate the abundance of Orai1 protein at different time points. As shown in Fig. 3, B and C, HG treatment significantly accelerated degradation of Orai1 protein. In contrast, the abundance of Orai1 protein in the cells without HG treatment did not have a significant change with time. Taken together, the data from quantitative real-time RT-PCR and protein stability studies suggest that the HG-associated decrease of Orai1 protein abundance did not involve transcriptional and translational mechanisms.
Posttranslational mechanisms mediated the HG-induced decrease in Orai1 protein abundance.
We then examined whether the HG effect on Orai1 protein was mediated by a posttranslational mechanism. The ubiquitin-proteasome system and lysosomal pathway are the two major mechanisms for protein degradation (31, 43). Recently, ubiquitin-mediated Orai1 degradation has been reported in HEK-293 and HeLa cells (30). To gain further insight into the potential mechanism(s) by which Orai1 is posttranslationally regulated by HG in MCs, we examined Orai1 protein levels in the presence of the ubiquitin-proteasome inhibitor carbobenzoxy-Leu-Leu-leucinal (MG132; Refs. 30, 41, 43) and the lysosomal inhibitor ammonium chloride (NH4Cl; Refs. 43, 47). As shown in Fig. 4, HG treatment for 8 h significantly reduced the abundance of Orai1 protein. However, either MG132 (10 µM) or NH4Cl (10 mM), but not DMSO (vehicle control for MG132), significantly blunted the HG-induced response, resulting in a significantly greater level of Orai1 compared with HG treatment alone or the vehicle treatment (Fig. 4). These data suggest that the HG-induced decrease of Orai1 protein abundance is mediated by the ubiquitin-proteasome and -lysosome systems.
Fig. 4.
Effect of MG132 and NH4Cl treatment on Orai1 protein abundance in rat MCs with and without HG treatment. A: representative Western blot. Confluent rat MCs were incubated with 0.5% FBS medium containing 5.6 mM d-glucose and 20 mM α-mannitol (osmotic control) or 25 mM d-glucose (HG) in the presence or absence of MG132 (10 µM) or NH4Cl (10 mM) or DMSO (vehicle control for MG132) for 8 h. TB, α-tubulin, loading control. B: densitometric analyses of Orai1 protein levels. The values of Orai1 to α-tubulin in all groups were normalized to the value in the group without treatment of HG, DMSO, MG132, and NH4Cl. *P < 0.05, compared with the groups indicated. n Indicates the number of independent experiments.
HG treatment increased the levels of ubiquitinated Orai1 protein in rat MCs.
If HG promoted Orai1 protein degradation through the ubiquitination pathway, then the level of ubiquitinated Orai1 protein should be elevated by HG treatment. We next evaluated the level of ubiquitinated Orai1 protein in rat MCs with and without HG treatment. As shown in Fig. 5, HG treatment for 8 h significantly increased the level of ubiquitinated Orai1. These results are consistent with the data from MG132 and NH4Cl experiments and further support the ubiquitination mechanism mediating the HG-associated Orai1 protein degradation in rat MCs.
H2O2 mediated HG-induced decrease in Orai1 protein abundance in rat MCs.
Numerous studies demonstrate strong associations of HG-induced pathological changes with increased reactive oxygen species in MCs (18–20, 23). We therefore sought to determine whether increased H2O2 level contributed to the HG-induced Orai1 protein degradation. In rat MCs, HG significantly increased H2O2 level in all time periods (1, 4, and 8 h) we examined. Importantly, the antioxidant polyethylene glycol-catalase, which metabolizes H2O2, significantly blunted the HG-induced decrease of Orai1 protein abundance (Fig. 6, B and C). Taken together, these data suggest that production of H2O2 is a downstream mechanism for HG-stimulated Orai1 protein degradation.
H2O2 decreased Orai1 protein abundance and stimulated ubiquitination of Orai1 in rat MCs.
If H2O2 mediated the HG-stimulated Orai1 protein degradation, then H2O2 treatment could recapitulate the HG effects. Thus we next examined the effects of H2O2 treatment on Orai1 protein level and ubiquitination of the protein in rat MCs. As shown in Fig. 7, A and B, treating MCs with H2O2 (300 µM) for various time periods from 5 min to 4 h reduced the level of Orai1 protein. A significant decrease occurred at 1 h and sustained for at least an additional 3 h. Furthermore, treatment of rat MCs with 300 µM H2O2 for 1 h significantly increased the level of ubiquitinated Orai1 protein (Fig. 7, C and D). These results in conjunction with the data presented in Fig. 6 further suggest a H2O2 mechanism underlying the HG-stimulated Orai1 protein degradation.
DISCUSSION
Earlier studies from several groups have demonstrated a regulatory role of HG on SOCE in MCs (8, 17, 36, 37, 40). Interestingly, all of those studied the long-term effect of HG treatment (≥5 days) on this Ca2+ signaling. Because HG can impair intracellular signaling of MCs in a short period of time (2, 13, 24, 29, 39, 55), understanding the short-term effect of HG treatment on SOCE is important. In the present study, we provided evidence that HG could significantly reduce the Orai1 protein level and SOCE as early as 8 h after treatment, and this HG response was sustained at least up to 1 day. The inhibitory effect of HG in a short-term treatment found in this study is opposite to the stimulatory effect of HG in a long-term treatment reported in our (8) previous study. This suggests that the effect of HG on Orai1 protein abundance and SOCE in MCs is time course dependent. Although the mechanism(s) underlying the time-course-dependent difference is not known from the present study, it has been reported that the changes in several intracellular signaling proteins in MCs, such as various isoforms of protein kinase C and transforming growth factor-β1 (TGF-β1), were dependent on the time periods of exposure to HG (27, 53). It is possible that the early and late responses of Orai1 protein and SOCE to HG treatment are mediated by separate intracellular pathways.
Orai1-mediated SOCE is essential for many physiological and pathological processes, including muscle physiology and diseases (42), immunity (15), thrombus formation (4), and cardiac function and pathology (11). It is also demonstrated that SOCE regulates cellular processes in cell-type-specific and/or cell-context-dependent manners. For instance, in glomerular MCs, activation of SOCE abrogates HG- and TGF-β1-induced fibronectin production and inhibition of SOCE significantly increased extracellular matrix proteins (55). On the contrary, in the proximal tubular epithelial cells, SOCE promoted TGF-β1-stimulated fibronectin production (35). Accumulation of extracellular matrix proteins in the glomerulus is one of the consistent pathological changes initiated at the early stage of diabetic nephropathy (28, 45). MCs are one of the major sources of extracellular matrix proteins (1, 25). Because SOCE in MCs decreases the level of extracellular matrix proteins, it is possible that the early attenuation of SOCE in MCs caused by HG contributes to the early pathological changes in diabetic kidney (deposition of extracellular matrix proteins and mesangial expansion). Therefore, restoring SOCE in MCs may be a therapeutic option for patients with diabetic kidney disease.
One important finding in this study was that a short-term treatment of HG decreased Orai1 protein abundance by stimulating the protein degradation. Intracellular protein degradation can be achieved in several ways, the major one of which is to ubiquitinate proteins leading to proteasome degradation or proteolysis within lysosomes of those proteins. This posttranslational mechanism for regulation of Orai1 has been reported previously. For instance, the Orai1 protein abundance in HEK-293 cells stably expressing Orai1 was significantly decreased by Nedd4–2, a ubiquitin ligase, and increased by knocking down Nedd4–2 (14). Consistently, Nedd4–2 is activated by AMP-activated kinase (AMPK), and T cells from AMPKα−/− animals showed an increased abundance of Orai1 protein within the plasma membrane (3). Furthermore, ubiquilin 1, a protein linking the ubiquitination machinery to the proteasome, interacts with Orai1, leading to ubiquitination and lysosomal degradation of Orai1 (30). Our data suggest that the HG-stimulated ubiquitination-mediated Orai1 degradation involves both proteasome and lysosomal pathways. It is not known whether the HG effect is mediated by Nedd4–2 and/or ubiquilin 1. However, different from the findings in the present study, the study by Lee et al. (30) showed that the proteasome mechanism was not involved in Orai1 degradation. This discrepancy may result from the difference in cell system between their and our studies. The study by Lee et al. (30) was conducted in HEK-293 cell line that overexpressed Orai1 and ubiquilin 1. However, we used rat MCs and studied endogenous Orai1.
Our study further suggests that H2O2 mediates the HG-induced Orai1 protein degradation. Numerous studies have demonstrated that Ca2+ channel function can be regulated by reactive oxygen species (26, 49). We (23, 52) previously demonstrated that reactive oxygen species reduced abundance of canonical transient receptor potential 6 (TRPC6), a Ca2+-permeable channel, through a transcriptional mechanism in human MCs. We (12, 21) also demonstrated that H2O2 can acutely stimulate membrane trafficking of TRPC6, resulting in an increase in cell surface expression of TRPC6 channel. The present study provided evidence that reactive oxygen species (H2O2) can modulate Ca2+ channel function, such as SOCE, by altering abundance of channel proteins through regulation of channel protein ubiquitination/degradation. Considering a wide range of SOCE function and firm associations of reactive oxygen species with cellular physiological and pathological processes, the findings from the present study may advance our understanding of the molecular mechanisms for reactive oxygen species-associated cell function and dysfunction.
It is noted that a significant decrease in Orai1 protein abundance by HG treatment required a time period of >4 h but occurred within 1 h by H2O2 treatment (Figs. 1 and 7). We showed that HG significantly elevated cellular H2O2 level within 1 h (Fig. 6). If H2O2 mediated the HG effect on Orai1 protein degradation, then why did HG treatment need an additional >2 h to reduce the level of Orai1 protein? One possibility is that H2O2-stimulated ubiquitination and degradation of Orai1 is concentration dependent. Although HG significantly elevated cellular H2O2 level in 1 h, it might not reach the level that significantly accelerated Orai1 degradation. Therefore, additional time is needed to allow accumulation of H2O2. In the present study, we treated the cells with 300 µM H2O2. Although the Amplex Red assay showed a trend of time-dependent increase in HG-generated H2O2, it was not possible to detect the actual concentration of H2O2 inside cell.
In summary, we showed a posttranslational regulation of SOCE signaling by a short-term treatment of HG in MCs. HG is the initiator and principal cause of renal pathogenesis in diabetes (2, 22, 25, 44, 53). SOCE in MCs has been demonstrated to protect kidney from diabetic injury (6, 54, 55). Therefore, impairment to SOCE pathway in MCs by HG in the early phase may contribute to renal injury in diabetes mellitus and consequently promote the development of diabetic nephropathy.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1-R01-DK-115424-01 (R. Ma), American Heart Association SouthWest Affiliate Grant-in-Aid 16GRNT27780043 (R. Ma), and a Harry S. Moss Heart Trust Award (R. Ma).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M. conceived and designed research; H.J. and S.Z. performed experiments; H.J., S.Z., and R.M. analyzed data; H.J., S.Z., and R.M. interpreted results of experiments; H.J., S.Z., and R.M. prepared figures; R.M. drafted manuscript; H.J., S.Z., and S.C. edited and revised manuscript; H.J., S.Z., and S.C. approved final version of manuscript.
REFERENCES
- 1.Abboud HE. Mesangial cell biology. Exp Cell Res 318: 979–985, 2012. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Ayo SH, Radnik R, Garoni JA, Troyer DA, Kreisberg JI. High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am J Physiol Renal Physiol 261: F571–F577, 1991. doi: 10.1152/ajprenal.1991.261.4.F571. [DOI] [PubMed] [Google Scholar]
- 3.Bhavsar SK, Schmidt S, Bobbala D, Nurbaeva MK, Hosseinzadeh Z, Merches K, Fajol A, Wilmes J, Lang F. AMPKα1-sensitivity of Orai1 and Ca2+ entry in T - lymphocytes. Cell Physiol Biochem 32: 687–698, 2013. doi: 10.1159/000354472. [DOI] [PubMed] [Google Scholar]
- 4.Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bösl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 113: 2056–2063, 2009. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 5.Campos AH, Calixto JB, Schor N. Bradykinin induces a calcium-store- dependent calcium influx in mouse mesangial cells. Nephron 91: 308–315, 2002. doi: 10.1159/000058409. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhari S, Li W, Wang Y, Jiang H, Ma Y, Davis ME, Zuckerman JE, Ma R. Store-operated calcium entry suppressed the TGF-β1/Smad3 signaling pathway in glomerular mesangial cells. Am J Physiol Renal Physiol 313: F729–F739, 2017. doi: 10.1152/ajprenal.00483.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhari S, Ma R. Store-operated calcium entry and diabetic complications. Exp Biol Med (Maywood) 241: 343–352, 2016. doi: 10.1177/1535370215609693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhari S, Wu P, Wang Y, Ding Y, Yuan J, Begg M, Ma R. High glucose and diabetes enhanced store-operated Ca2+ entry and increased expression of its signaling proteins in mesangial cells. Am J Physiol Renal Physiol 306: F1069–F1080, 2014. doi: 10.1152/ajprenal.00463.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YJ, Chang CL, Lee WR, Liou J. RASSF4 controls SOCE and ER-PM junctions through regulation of PI(4,5)P2. J Cell Biol 216: 2011–2025, 2017. doi: 10.1083/jcb.201606047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr 71: 149–179, 2013. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins HE, Zhu-Mauldin X, Marchase RB, Chatham JC. STIM1/Orai1-mediated SOCE: current perspectives and potential roles in cardiac function and pathology. Am J Physiol Heart Circ Physiol 305: H446–H458, 2013. doi: 10.1152/ajpheart.00104.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 286: 31799–31809, 2011. doi: 10.1074/jbc.M111.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dlugosz JA, Munk S, Ispanovic E, Goldberg HJ, Whiteside CI. Mesangial cell filamentous actin disassembly and hypocontractility in high glucose are mediated by PKC-ζ. Am J Physiol Renal Physiol 282: F151–F163, 2002. doi: 10.1152/ajprenal.0055.2001. [DOI] [PubMed] [Google Scholar]
- 14.Eylenstein A, Gehring EM, Heise N, Shumilina E, Schmidt S, Szteyn K, Münzer P, Nurbaeva MK, Eichenmüller M, Tyan L, Regel I, Föller M, Kuhl D, Soboloff J, Penner R, Lang F. Stimulation of Ca2+-channel Orai1/STIM1 by serum- and glucocorticoid-inducible kinase 1 (SGK1). FASEB J 25: 2012–2021, 2011. doi: 10.1096/fj.10-178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev 231: 189–209, 2009. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 17.Frecker H, Munk S, Wang H, Whiteside C. Mesangial cell-reduced Ca2+ signaling in high glucose is due to inactivation of phospholipase C-β3 by protein kinase C. Am J Physiol Renal Physiol 289: F1078–F1087, 2005. doi: 10.1152/ajprenal.00434.2004. [DOI] [PubMed] [Google Scholar]
- 18.Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci (Lond) 125: 361–382, 2013. doi: 10.1042/CS20130065. [DOI] [PubMed] [Google Scholar]
- 19.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 20.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285: F219–F229, 2003. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 21.Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 285: 23466–23476, 2010. doi: 10.1074/jbc.M109.093500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham S, Ding M, Sours-Brothers S, Yorio T, Ma JX, Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol 293: F1381–F1390, 2007. doi: 10.1152/ajprenal.00185.2007. [DOI] [PubMed] [Google Scholar]
- 23.Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, Ding Y, Ma R. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol 301: C304–C315, 2011. doi: 10.1152/ajpcell.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB. Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol 13: 894–902, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Heilig CW, Liu Y, England RL, Freytag SO, Gilbert JD, Heilig KO, Zhu M, Concepcion LA, Brosius FC 3rd. d-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes 46: 1030–1039, 1997. doi: 10.2337/diab.46.6.1030. [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 1275–1312, 2008. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN. Transcriptional activation of transforming growth factor-β1 in mesangial cell culture by high glucose concentration. Kidney Int 54: 1107–1116, 1998. doi: 10.1046/j.1523-1755.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 28.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 233: 4–11, 2008. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 29.Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int 67: 1762–1771, 2005. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee JE, Jeon IS, Han NE, Song HJ, Kim EG, Choi JW, Song KD, Lee HK, Choi JK. Ubiquilin 1 interacts with Orai1 to regulate calcium mobilization. Mol Cells 35: 41–46, 2013. doi: 10.1007/s10059-013-2268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebl MP, Hoppe T. It’s all about talking: two-way communication between proteasomal and lysosomal degradation pathways via ubiquitin. Am J Physiol Cell Physiol 311: C166–C178, 2016. doi: 10.1152/ajpcell.00074.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A, Penner R. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol 17: 794–800, 2007. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma R, Pluznick JL, Sansom SC. Ion channels in mesangial cells: function, malfunction, or fiction. Physiology (Bethesda) 20: 102–111, 2005. doi: 10.1152/physiol.00050.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ma R, Sansom SC. Epidermal growth factor activates store-operated calcium channels in human glomerular mesangial cells. J Am Soc Nephrol 12: 47–53, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Mai X, Shang J, Liang S, Yu B, Yuan J, Lin Y, Luo R, Zhang F, Liu Y, Lv X, Li C, Liang X, Wang W, Zhou J. Blockade of Orai1 store-operated calcium entry protects against renal fibrosis. J Am Soc Nephrol 27: 3063–3078, 2016. doi: 10.1681/ASN.2015080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menè P, Pugliese G, Pricci F, Di Mario U, Cinotti GA, Pugliese F. High glucose inhibits cytosolic calcium signaling in cultured rat mesangial cells. Kidney Int 43: 585–591, 1993. doi: 10.1038/ki.1993.86. [DOI] [PubMed] [Google Scholar]
- 37.Menè P, Pugliese G, Pricci F, Di Mario U, Cinotti GA, Pugliese F. High glucose level inhibits capacitative Ca2+ influx in cultured rat mesangial cells by a protein kinase C-dependent mechanism. Diabetologia 40: 521–527, 1997. doi: 10.1007/s001250050710. [DOI] [PubMed] [Google Scholar]
- 38.Menè P, Teti A, Pugliese F, Cinotti GA. Calcium release-activated calcium influx in cultured human mesangial cells. Kidney Int 46: 122–128, 1994. doi: 10.1038/ki.1994.251. [DOI] [PubMed] [Google Scholar]
- 39.Nasrallah R, Hébert RL. Reduced IP receptors in STZ-induced diabetic rat kidneys and high-glucose-treated mesangial cells. Am J Physiol Renal Physiol 287: F673–F681, 2004. doi: 10.1152/ajprenal.00025.2004. [DOI] [PubMed] [Google Scholar]
- 40.Nutt LK, O’Neil RG. Effect of elevated glucose on endothelin-induced store-operated and non-store-operated calcium influx in renal mesangial cells. J Am Soc Nephrol 11: 1225–1235, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Palumbo S, Shin YJ, Ahmad K, Desai AA, Quijada H, Mohamed M, Knox A, Sammani S, Colson BA, Wang T, Garcia JG, Hecker L. Dysregulated Nox4 ubiquitination contributes to redox imbalance and age-related severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 312: L297–L308, 2017. doi: 10.1152/ajplung.00305.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Z, Brotto M, Ma J. Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 47: 69–79, 2014. doi: 10.5483/BMBRep.2014.47.2.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW. Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem 278: 30005–30014, 2003. doi: 10.1074/jbc.M300614200. [DOI] [PubMed] [Google Scholar]
- 44.Rossert J, Terraz-Durasnel C, Brideau G. Growth factors, cytokines, and renal fibrosis during the course of diabetic nephropathy. Diabetes Metab 26, Suppl 4: 16–24, 2000. [PubMed] [Google Scholar]
- 45.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16, Suppl 1: S30–S33, 2005. doi: 10.1681/ASN.2004110970. [DOI] [PubMed] [Google Scholar]
- 46.Scindia YM, Deshmukh US, Bagavant H. Mesangial pathology in glomerular disease: targets for therapeutic intervention. Adv Drug Deliv Rev 62: 1337–1343, 2010. doi: 10.1016/j.addr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seglen PO, Grinde B, Solheim AE. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem 95: 215–225, 1979. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- 48.Shen B, Zhu J, Zhang J, Jiang F, Wang Z, Zhang Y, Li J, Huang D, Ke D, Ma R, Du J. Attenuated mesangial cell proliferation related to store-operated Ca2+ entry in aged rat: the role of STIM 1 and Orai 1. Age (Dordr) 35: 2193–2202, 2013. doi: 10.1007/s11357-013-9511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trebak M, Ginnan R, Singer HA, Jourd’heuil D. Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid Redox Signal 12: 657–674, 2010. doi: 10.1089/ars.2009.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trebak M, Putney JW Jr. ORAI calcium channels. Physiology (Bethesda) 32: 332–342, 2017. doi: 10.1152/physiol.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223, 2006. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Ding M, Chaudhari S, Ding Y, Yuan J, Stankowska D, He S, Krishnamoorthy R, Cunningham JT, Ma R. Nuclear factor κB mediates suppression of canonical transient receptor potential 6 expression by reactive oxygen species and protein kinase C in kidney cells. J Biol Chem 288: 12852–12865, 2013. doi: 10.1074/jbc.M112.410357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whiteside CI, Dlugosz JA. Mesangial cell protein kinase C isozyme activation in the diabetic milieu. Am J Physiol Renal Physiol 282: F975–F980, 2002. doi: 10.1152/ajprenal.00014.2002. [DOI] [PubMed] [Google Scholar]
- 54.Wu P, Ren Y, Ma Y, Wang Y, Jiang H, Chaudhari S, Davis ME, Zuckerman JE, Ma R. Negative regulation of Smad1 pathway and collagen IV expression by store-operated Ca2+ entry in glomerular mesangial cells. Am J Physiol Renal Physiol 312: F1090–F1100, 2017. doi: 10.1152/ajprenal.00642.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu P, Wang Y, Davis ME, Zuckerman JE, Chaudhari S, Begg M, Ma R. Store-operated Ca2+ channels in mesangial cells inhibit matrix protein expression. J Am Soc Nephrol 26: 2691–2702, 2015. doi: 10.1681/ASN.2014090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA 103: 9357–9362, 2006. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]