An imbalance in protein production is common to several diseases. Endoplasmic reticulum (ER) stress occurs when proteins misfold during biosynthesis, resulting in reduced production of cell surface and secreted proteins. Misfolding activates cellular stress pathways including the unfolded protein response (UPR), the production of reactive oxygen and nitrogen species and a state of oxidative stress. Oxidative stress and ER stress are integrally entwined states, implicated in the aetiology of many diseases, diabetes and obesity1, arthritis and spondyloarthropathies, multiple forms of respiratory inflammation2, inflammatory bowel diseases, infections3 and cancer4. Consequently, there is substantial interest in therapeutic targeting of stress in a variety of disorders.
Major intracellular stress response pathways triggered by oxidative stress and ER stress (UPR) in turn regulate inflammatory pathways. Oxidative stress results from an imbalance between oxidants [reactive oxygen species (ROS) and reactive nitrogen species (RNS)] derived from the local environment or produced within cells and cellular antioxidants. Protein folding in the ER results in production of ROS, which are deactivated by endogenous antioxidants such as glutathione peroxidase and superoxide dismutase. Despite ER‐resident chaperones and enzymes that promote correct protein folding, a proportion of all proteins misfold. Factors known to increase misfolding include ROS and RNS, increased protein synthesis, microbial toxins, viral infection and cytokines. Prolonged or severe ER stress can induce inflammation, autophagy and apoptosis (Figure 1). The mechanisms by which ER stress influences pathology, and is interlinked with inflammation and oxidative stress, are covered by the reviews in this Special Feature of Clinical & Translational Immunology.
Figure 1.
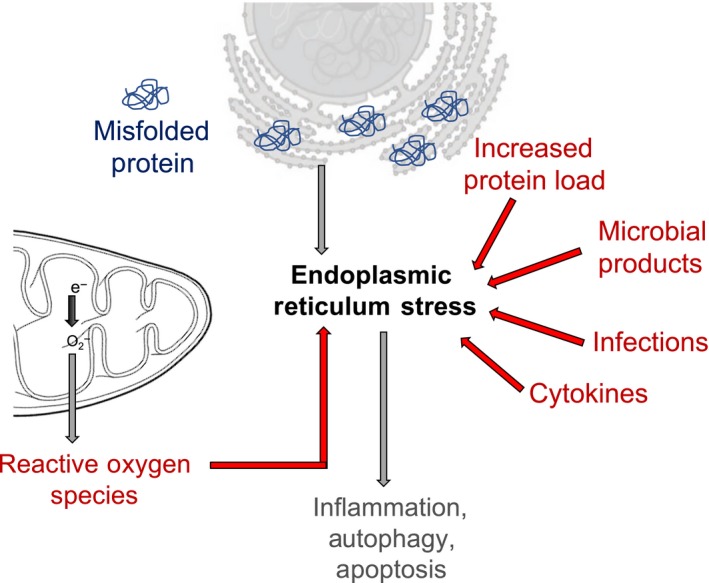
Schematic representation of different factors that can induce ER stress, including reactive oxygen species, increased protein load within the ER, microbial products, infections and cytokine. Acute ER stress response activates pathways to rectify the accumulation of misfolded proteins. However, chronic activation of the ER stress pathways in disease can lead to inflammation, autophagy and apoptosis.
Our understanding of the crosstalk between different cellular stress pathways and inflammation is constantly evolving. Pickering et al.1 describe the increase in hyperglycaemia‐induced ROS is associated with increased low‐grade chronic inflammation resulting in diabetic complications such as cardiovascular disease, chronic kidney disease and retinopathy. They raise an interesting possibility of utilising anti‐inflammatory drugs that have dual effects – on inflammation and on oxidative stress – and that potentially a combination of the two might be an approach to improve disease outcomes in diabetic complications more effectively.1
Excessive production of monoclonal immunoglobulin is a key feature of multiple myeloma. Nikesitch et al.4 describe that this increased protein production induces ER stress and activates the proteosomal degradation pathways to avoid accumulation of the protein in the ER and the consequent cell death. Proteosomal inhibitors have been quite effective, but unfortunately, there is increased resistance to therapies such as bortezomib. There is a need for development of treatments that could include autophagy inhibitors, heat‐shock protein inhibitors or ubiquitin ligase inhibitors. However, resistance to such drugs might be a possibility; therefore, drugs that could specifically target resistance to proteasome inhibitors or resensitise multiple myeloma to proteasome inhibitors should be developed. The mechanisms around resistance and the exact involvement of the UPR are unclear in multiple myeloma, and a better understanding of the pathways is required prior to the development of effective therapies.
Chen et al.2 discuss the influences of intrinsic cell and environmental factors that have the potential to contribute to oxidative stress and ER stress in a range of respiratory diseases, including cystic fibrosis, asthma and idiopathic pulmonary fibrosis.2 Even though proof‐of‐concept studies in animals show that blocking ER and oxidative stress is effective in models of respiratory inflammation, the use of antioxidants, in particular N‐acetyl‐cysteine, in respiratory disease‐focussed clinical trials have been largely disappointing. It is clear that ER and oxidative stress are an integral part of exacerbating the inflammation which leads to pathophysiology in respiratory disease; however, there is a growing need for specific inhibitors with reduced toxicity.
In order to replicate, viruses must utilise the host ER. By selectively regulating parts of the UPR, particularly elements that block translation, some viruses have evolved mechanisms to manipulate host stress responses in order to maintain viral replication. Cytokines can modulate ER stress, although the underlying purpose of this is still unknown. Wang et al. 3 hypothesise that, despite the ability of viruses to regulate the UPR, innate cytokines are released quickly and dynamically to either limit or disturb viral protein biosynthesis via the induction of oxidative stress. Because ROS and RNS directly cause proteins to misfold in the ER, the viral inhibition of the UPR allowing translation to proceed will be ineffective, as the translated viral proteins will misfold as a result of the inappropriate oxidative state in the ER. Dysregulation of the cellular stress pathways can contribute to pathology during viral infection and chronic noninfectious inflammation, and therefore, alleviating cellular stress has therapeutic potential. One such cytokine that has been shown to modulate the cellular stress pathways is Interleukin‐22 (IL‐22). Alabbas et al.5 discuss the reported pro‐ and anti‐inflammatory roles of IL‐22 and its role in modulating the oxidative stress and ER stress response. The potential use of IL‐22 as therapy is dependent on the timing of treatment, dosing and the underlying disease pathology, for example IL‐22 is pro‐inflammatory in psoriasis, whereas it is anti‐inflammatory in diabetes.
Together, the reviews in this Special Feature highlight the need for understanding the complex relationship between the cellular stress pathways and inflammation. It is clear that ER stress and inflammation are implicated in the pathophysiology of several infectious and inflammatory diseases. The ultimate aim of the research in the field is to devise new strategies to alleviate or prevent inflammatory diseases, and a deeper understanding of these pathways may identify new and complementary targets for therapies for a range of chronic diseases.
Conflict of interest
The author declares no conflict of interest.
References
- 1. Pickering RJ, Rosado CJ, Sharma A et al Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunol 2018; 7: e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen AC‐H, Burr L, McGuckin MA. Oxidative and endoplasmic reticulum stress in respiratory disease. Clin Transl Immunol 2018; 7: e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang R, Moniruzzaman MD, Shuffle E et al Immune regulation of the unfolded protein response at the mucosal barrier in viral infection. Clin Transl Immunol 2018; 7: e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nikesitch N, Lee JM, Ling S et al Endoplasmic reticulum stress in the development of multiple myeloma and drug resistance. Clin Transl Immunol 2018; 7: e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alabbas SY, Begun J, Florin TH et al The role of IL‐22 in the resolution of sterile and nonsterile inflammation. Clin Transl Immunol 2018; 7: e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]


