Abstract
The Japanese rock ptarmigan, Lagopus muta japonica, inhabits the alpine zone of mountainous areas at 3000 m above sea level. Since L. m. japonica is endangered due to a decline in the overall population, controlling infectious diseases such as those caused by protozoan parasites is a critical factor in the conservation of this species. Although Eimeria spp. are considered to have a negative impact on Japanese rock ptarmigan populations, the ecological interactions between the parasites and their hosts have not yet been fully clarified. We therefore conducted seasonal surveys of the prevalence of Eimeria spp. in Japanese rock ptarmigan populations. In addition, we recorded the ambient temperature in ptarmigan habitat and characterized the ability of eimerian isolates to acquire infectivity. Eimeria spp. were detected in 217 of 520 (41.7%) Japanese rock ptarmigan fecal samples in 2006 and in 177 of 308 (57.5%) fecal samples in 2007. Specifically, we observed two types of oocysts characteristic of E. uekii and type B. In adult birds and chicks, infection rates increased towards August (summer) and then decreased as the temperature decreased toward November (winter). Oocyst counts per gram (OPG) of feces peaked in August in adults and chicks, and OPG values were markedly higher in chicks than in adults. Isolated Eimeria spp. oocysts sporulated at temperatures as low as 8 °C and remained viable after being stored at 4 °C for 6 months. Our findings suggest that Eimeria spp. can complete their annual lifecycle in the cold timberline regions inhabited by the host, the Japanese rock ptarmigan, and that Eimeria spp. infection is widespread in the bird populations examined.
Keywords: Conservation, Ecological epidemiology, Eimeria, Japanese Alps, Japanese rock ptarmigan
Graphical abstract
Highlights
-
•
Two type oocysts, E. uekii and type B, highly infected Japanese rock ptarmigans.
-
•
Infection rates increased towards summer and decreased as the temperature decreased toward winter.
-
•
Parasites remained viable even after being stored at 4 °C for 6 months.
-
•
Parasites may be adapted to colder temperature to complete life cycle at the timberline regions.
1. Introduction
The rock ptarmigan, Lagopus muta (Montin, 1781) in the order Galliformes is a cold-adapted species that inhabits the alpine areas of the northern hemisphere. In terms of taxonomy, the species is currently divided into approximately 23–30 subspecies (Johnsgard, 1983; del Hoyo et al., 1994). One of these subspecies, the Japanese rock ptarmigan (L. m. japonica), inhabits the timberline regions of the Japanese alpine zone at approximately 3,000 m above sea level. This subspecies is endemic to Japan and is considered to be endangered due to a decline in the overall population (estimated population: ≤2,000 individuals) (Wildlife Division of the Ministry of the Environment, 2012). Given their relative scarcity, the Japanese rock ptarmigan was designated a special natural monument of Japan in 1955 and is listed as vulnerable in the Japanese Red Data Book (Murata et al., 2007; Wildlife Division of the Ministry of the Environment, 2017).
The conservation of small wild animal populations is difficult, as population numbers can be markedly affected by infectious agents that can cause death or interfere with breeding. Among such infectious agents, parasites can have a potential negative influence on population health as they decrease host physical condition, fecundity, and survival (Anderson and May 1981; Murata et al., 2007). Consequently, controlling host-parasite interactions is an important part of ensuring the survival of threatened animal populations. It has recently been shown that parasite infections, especially by the protozoan parasite Eimeria spp. (Phylum: Apicomplexa), are associated with a decrease in overall body condition and an increase in mortality in rock ptarmigans (L. m. islandorum) in Iceland (Stenkewitz et al., 2016). Although Eimeria infection in rock ptarmigans has not been studied extensively, preventing diseases (e.g. coccidiosis in birds) can be one of the most important means by which animal species can be conserved in addition to protecting their environment.
To date, seven Eimeria spp. have been identified in the rock ptarmigan (L. muta): E. lagopodi from Switzerland (Galli-Valerio, 1929), E. brinkmanni and E. fanthami from Canada (Levine, 1953), E. uekii and type B from Japan (Kamimura and Kodama, 1981; Ishihara et al., 2006), and E. muta and E. rjupa from Iceland (Skirnisson and Th Thorarinsdottir, 2007). Typically, Eimeria spp. infections are initiated by oral ingestion of sporulated oocysts, which form two sporozoites within four sporocysts. Each sporozoite then undergoes development in the mucosa of the host intestine before being released in the feces as oocysts. Under ideal environmental conditions (i.e. temperature, humidity and oxygen availability), these noninfectious oocysts undergo a sporulation process resulting in the formation of infectious oocysts. In Eimeria spp., the optimal sporulation temperature is generally 27–28 °C (Waldenstedt et al., 2001; Pyziel and Demiaszkiewicz, 2015), with freezing below −15 °C considered to inhibit sporulation or kill the oocysts (Landers, 1953; Lassen and Seppä-Lassila, 2014). The areas of the Japanese alpine zone that are inhabited by the Japanese rock ptarmigan typically experience snowfall from September or October until June in late spring. However, it is not known how the eimerian parasites can survive such harsh environmental conditions and infect their hosts to complete their life cycle. In this study, we examined the seasonal prevalence of Eimeria spp. in chicks and adults of the Japanese rock ptarmigan, measured the environmental temperature in the areas in which they were found, and biologically characterized the ability of eimerian isolates to acquire the infectivity.
2. Materials and methods
2.1. Study area and birds
The survey in the present study was conducted in the Hida Mountains of the Northern Japanese Alps from April to November in 2006 and 2007; the area extends over Toyama, Gifu, Nagano and Niigata prefectures. We collected a total of 520 fresh Japanese rock ptarmigan fecal samples, including 72 samples from chicks, in 2006, and 308 samples, including 30 chicks, in 2007. Samples were collected from 11 sites: Mt. Tateyama (36°35′N, 137°36′E), Mt. Jonendake (36°19′N, 137°43′E), Mt. Sugorokudake (36°22′N, 137°35′E), Mt. Asahidake (36° 49′N, 137° 43′E), Mt. Shiroumadake (36°45′N, 137°45′E), Mt. Chougadake (36°17′N, 137°43′E), Mt. Otenshoudake (36° 21′N, 137°42′E), Mt. Jiigatake (36°35′N, 137°45′E), Mt. Minamidake (36° 19′N, 137°39′E), Mt. Norikuradake (36°6′N, 137°33′E), Mt. Yarigadake (36°20′N, 137°38′E), as well as elsewhere in the Northern Alps. Where possible, the age (adult or chick) and sex of the ptarmigan that produced the fecal sample were recorded. Fecal samples were collected by tracking individual birds and collecting any feces that they produced. Additionally, birds could be identified based on unique identification numbers and chicks were identified by observing patterns of their feather color from short distance. In these study areas, it was relatively easy to find and chase a family of the rock ptarmigan during breeding period. Although an effort was made not to collect feces from the same ptarmigans more than twice a month, the possibility exists that some samples were collected from the same birds more than twice in a given month. In addition, the temperatures on the windward and leeward slopes of Mt. Tateyama were measured using data loggers (UA-002-64, Onset Computer Corp., MA, USA).
2.2. Fecal examinations
The fecal samples were placed in a cooler box, transported to our laboratory, and stored at 4 °C until analysis. The eimerian oocysts were examined by sucrose centrifugal flotation method (Uga et al., 2000). The number of oocysts per gram (OPG) was determined by diluting the feces after filtering through a steel mesh as reported previously (Brackett and Bliznick, 1949). Several positive samples, which contained a large number of oocysts and could be examined within 2–3 weeks after being shedded, were incubated in a 2.5% potassium dichromate (K2Cr2O7) solution at 25 °C to allow the oocysts to sporulate. Sporulated oocysts were observed under a differential interference contrast microscope under oil immersion at 1,000 × magnification. Fifty oocysts and their internal structures were then analyzed using a digital color image analysis system (Lumina Vision, Mitani Corporation, Tokyo, Japan). Fecal samples (approximately 50 pooled samples) containing a large number of oocysts (mainly, morphologically E. uekii) were filtered by steel mesh and incubated at a range of temperatures (4–45 °C) using 90 cm petri dishes and observed at 24-h intervals over 18 days to determine the timing of sporulation by counting 100 oocysts. In addition, some oocysts were stored at 4 °C for 6 months and then incubated at room temperature to evaluate sporulation.
2.3. Statistical analyses
The statistical tests were performed by the Pearson's Chi-square for comparison of sexes, and the Student's t-test for the comparison of the prevalence between adult birds and chicks and seasonal OPG of E. uekii and type B. Seasonal comparison of the prevalences, e.g. between spring (April and May) and summer (Jun, July, and August) or autumn (September, October, and November), could not be conducted because of few sample numbers. Statistical significance was set at p < 0.05.
3. Results
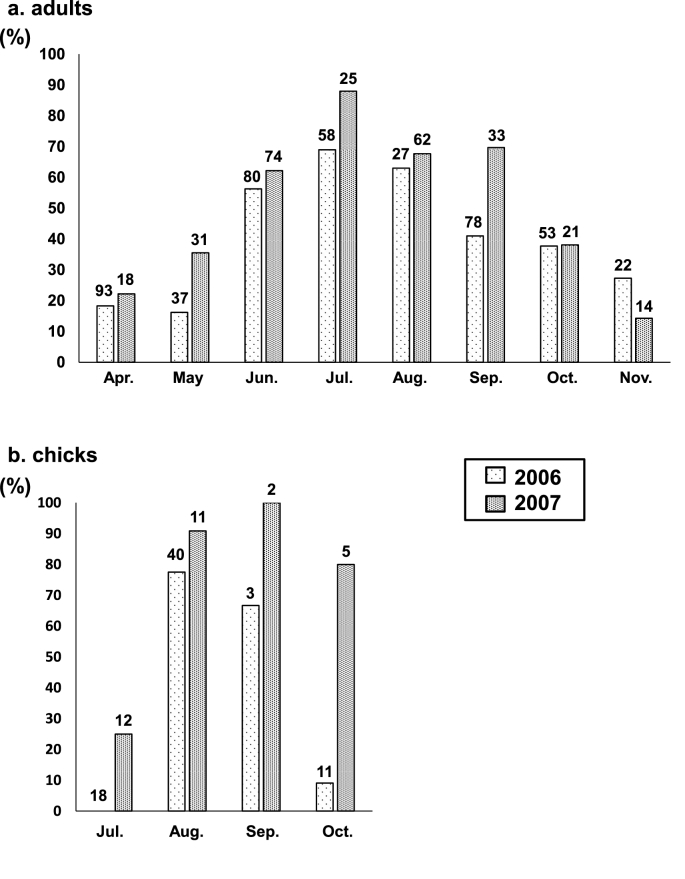
We detected Eimeria spp. in 217 (41.7%) of 520 Japanese rock ptarmigan samples in 2006, and 177 (57.5%) of 308 samples in 2007 (Table 1). No significant difference was observed in infection prevalence between adults and chicks (P > 0.05 in 2006 and 2007). Except for one site (Minamidake), Eimeria spp. were found in fecal samples from all of the sampled sites. Regarding to the infection prevalence over time, in adult birds, the rate of infection increased from April to July (early summer), peaking at 69.0% in 2006 and 88.0% in 2007, and then decreased toward November (winter) (Fig. 1). In chicks, infection rates increased after August (summer) (77.5% in 2006 and 90.9% in 2007) and decreased in October (early winter) (9.1% in 2006 and 80.0% in 2007). Chicks generally hatch on June and July, and they are cared by parents for 3–4 months. Infection rates of male and female were 46 of 116 (39.7%) and 49 of 106 (46.2%) in 2006, and 44 of 83 (53.0%) and 26 of 52 (50.0%) in 2007, respectively. Significant differences were seen between the prevalence of male and female only in 2006 (P < 0.05).
Table 1.
Summary of the prevalence of Eimeria spp. in Japanese rock ptarmigan in the present study.
| Mountains | Total No. | Positive No. | 2006* |
2007* |
||
|---|---|---|---|---|---|---|
| Adult | Chicks | Adult | Chicks | |||
| Tateyama | 469 | 219 (46.7) | 121/292 (41.4) | 20/40 (50) | 61/118 (51.7) | 17/19 (89.5) |
| Jiigatake | 340 | 194 (47.1) | 6/12 (50) | 11/20 (55) | 54/71 (76.1) | 1/10 (10) |
| Asahidake | 109 | 64 (58.7) | 9/22 (40.9) | 0/6 (0) | – | – |
| Norikuradake | 58 | 22 (37.9) | 21/54 (38.9) | 1/4 (25) | – | – |
| Sugorokudake | 53 | 22 (41.5) | 21/47 (44.7) | – | – | – |
| Jonendake | 41 | 19 (46.3) | 0/2 (0) | – | – | – |
| Shiroumadake | 37 | 20 (54.1) | 3/4 (75) | 2/2 (100) | 1/5 (20) | 0/1 (0) |
| Yarigadake | 31 | 15 (48.4) | – | – | 15/31 (48.4) | – |
| Otenshoudake | 15 | 10 (66.7) | 2/2 (100) | – | – | – |
| Minamidake | 9 | 0 (0) | 0/9 (0) | – | – | – |
| Chougadake | 6 | 1 (16.7) | 0/4 (0) | – | 1/2 (50) | – |
| Other or unknown | 52 | 27 (51.9) | – | – | 26/51 (51.0) | 1/1 (100) |
| Total | 828 | 394 (47.6) | 183/448 (40.8) | 34/72 (47.2) | 158/278 (56.8) | 19/30 (63.3) |
Parentheses; positive percentage (%), *; positive No./examined No.
No significant difference was observed in infection prevalence between adults and chicks (P > 0.05 in 2006 and 2007).
Fig. 1.
Seasonal prevalence of Eimeria spp. infection in Japanese rock ptarmigans from April to November in 2006 and 2007. (a) and (b) show the prevalence of infection in adult birds and chicks, respectively. Numbers above bars indicate the total number of fecal samples analyzed.
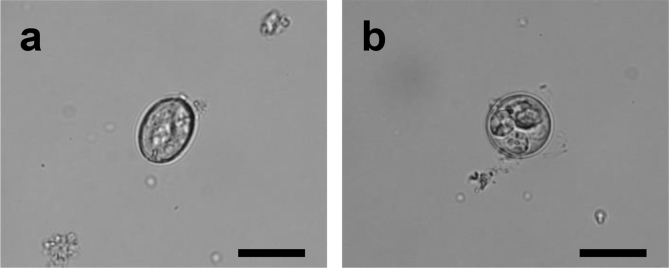
Microscopic observations revealed two morphologically distinct types of oocysts (Fig. 2): one that was typical of E. uekii and one that was similar to the type B oocyst reported previously (Kamimura and Kodama, 1981; Ishihara et al., 2006). The E. uekii-type oocysts were ellipsoidal in shape with a smooth colorless wall, no oocyst residuum, and one to three ovoid polar granules (1.6–3.1 μm). The micropyle was indistinct or absent. Sporulated oocysts (n = 50; length × width) measured 23.8 ± 1.7 μm (20.3–28.9 μm) × 15.7 ± 1.3 μm (13.8–18.8 μm), and had a shape index (L/W) of 1.5 ± 0.1 (1.3–1.8). Sporocysts (n = 50) measured 12.4 ± 0.8 μm (9.8–14.4 μm) × 6.7 ± 0.5 μm (5.9–7.8 μm) and had a shape index of 1.9 ± 0.2 (1.5–2.3). Stieda body and sporocyst residuum were present and two refractile bodies measuring 1.3–4.1 μm in diameter were observed in sporozoites. The type B-like oocysts were subspherical with a smooth colorless wall, no oocyst residuum and micropyle, and one to two ovoid polar granules (1.5–2.8 μm). Sporulated oocysts (n = 50; length × width) measured 21.4 ± 2.4 μm (13.6–26.0 μm) × 19.2 ± 2.2 μm (13.1–24.6 μm), and had a shape index (L/W) of 1.1 ± 0.1 (1.0–1.4). Sporocysts (n = 50) measured 12.1 ± 1.1 μm (9.7–13.8 μm) × 7.5 ± 0.7 μm (5.9–9.2 μm) and had a shape index of 1.6 ± 0.2 (1.2–2.1). Stieda body and sporocyst residuum were present. Two refractile bodies measuring 1.5–4.1 μm in diameter were observed in sporozoites. After comparisons against previous studies (Kamimura and Kodama, 1981; Ishihara et al., 2006), we identified the oocysts isolated in this study as E. uekii and type B oocysts.
Fig. 2.
Photomicrograph of eimerian oocysts detected in the feces of Japanese rock ptarmigans; (a) E. uekii and (b) type B. The scale bar indicates 20 μm.
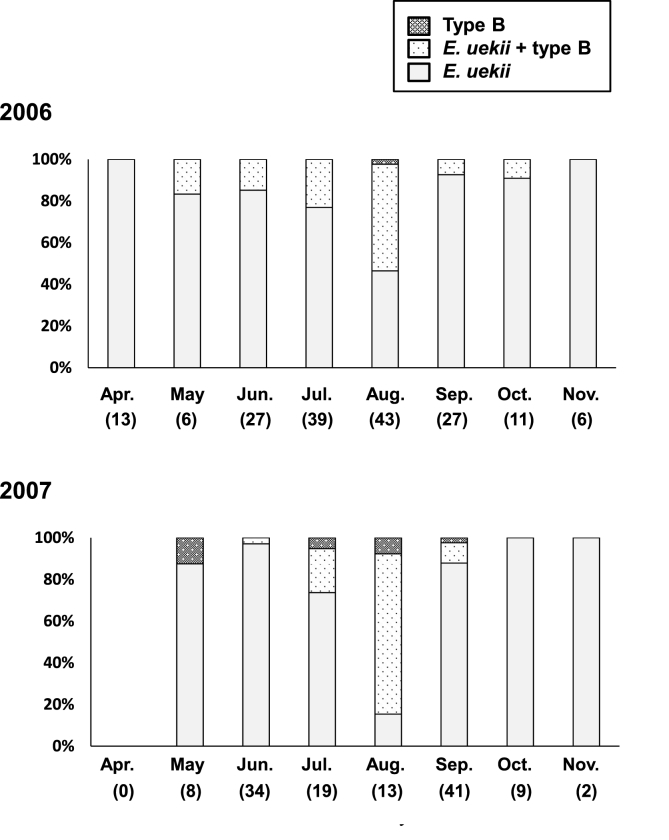
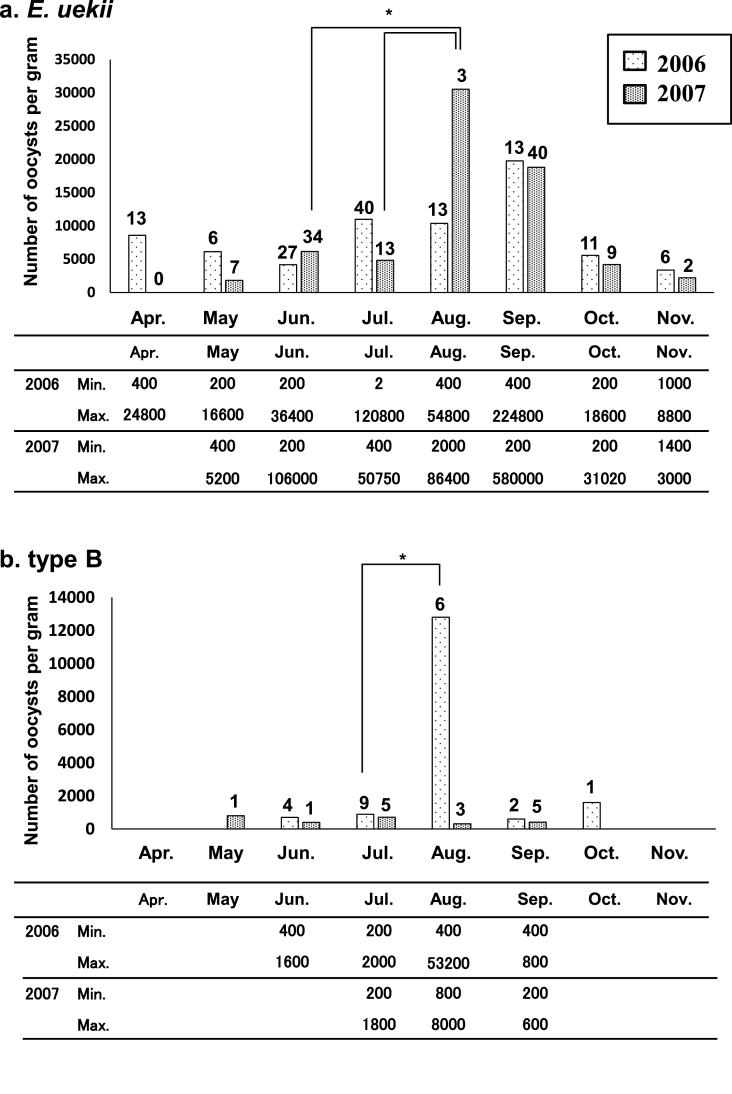
Detection rates for the two Eimeria spp. in adults and chicks are shown in Fig. 3. Mixed infection by E. uekii and type B oocysts was frequently observed, with type B oocysts being most abundant in August. The infection rate by type B oocysts in chicks was high; 17 of 23 (73.9%) positive birds were observed in August 2006 and 8 of 11 (72.7%) positive birds were observed in August 2007. We calculated the OPG for some fecal samples and summarized the findings in Fig. 4. In adults, the OPG values for E. uekii oocysts increased in August and September (Fig. 4a), while values for type B oocysts peaked in August (Fig. 4b), although the number total of type B oocysts examined was limited. Average OPG values could be determined for some positive chicks in August; OPG values for E. uekii oocysts were 106,532 (400–742,000 (minimum-maximum); n = 29)) in 2006 and 161,800 (800-608,000; n = 9) in 2007. Similarly, average OPG values for type B oocysts were 43,979 (2000–148,000; n = 17) in 2006 and 4338 (800-12,000; n = 8) in 2007. The OPG values obtained for chicks in 2006 were significantly higher than those of adults (P < 0.05).
Fig. 3.
Seasonal detection rate for E. uekii, type B, and mixed Eimeria spp. oocyst infection in both adults and chicks in 2006 and 2007. Numbers in parentheses below months indicate the total number of fecal samples analyzed. Data for the number of chicks (23 in 2006 and 11 in 2007) were only available for August.
Fig. 4.
Average seasonal OPG values for (a) E. uekii and (b) type B in adult birds in 2006 and 2007. Numbers above bars indicate the total number of fecal samples analyzed. Tables below graphs show the maximum and minimum OPG values. Significant differences of OPG indicate as (*) between the months (P < 0.05).
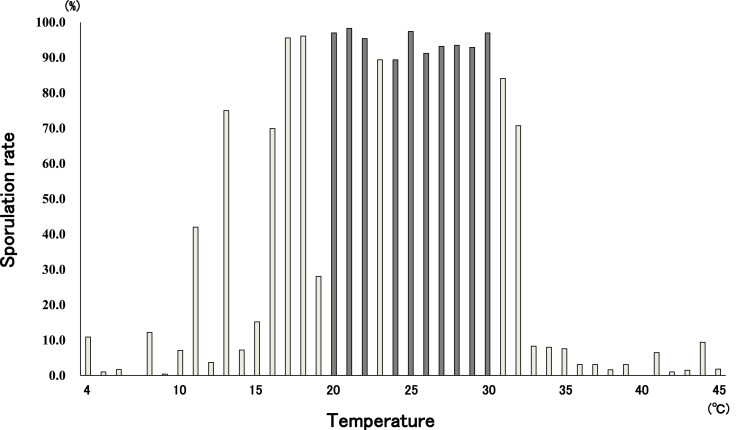
We also examined the sporulation characteristics of the isolated Eimeria spp. (mainly E. uekii) and how they were affected by temperature. More than 80% of oocysts sporulated after 24-h incubation at temperatures of between 20 and 30 °C, except at 23 °C. After 48-h incubation, oocysts sporulated at slightly lower temperatures (17 and 18 °C) and higher (31 °C) temperatures (Fig. 5). However, oocysts did not sporulate at temperatures above 33 °C and the morphology of most oocysts was observed to change, especially at temperatures above 38 °C. Statistically, positive correlation was observed between temperatures (4–30 °C) and sporulation, y = 4.5081x - 21.051, R2 = 0.7227, correlation coefficient: 0.85. At incubation temperatures between 8 and 16 °C, oocyst sporulation occurred from 72 to 384 h. After storage at 0 °C for 6 months, 82.0% of oocysts sporulated successfully after incubation at room temperature.
Fig. 5.
Sporulation rate for Eimeria spp. (mainly E. uekii) after incubation at different temperatures for 48 h. Dark bars indicate sporulation rates of >85% after incubation for 24 h.
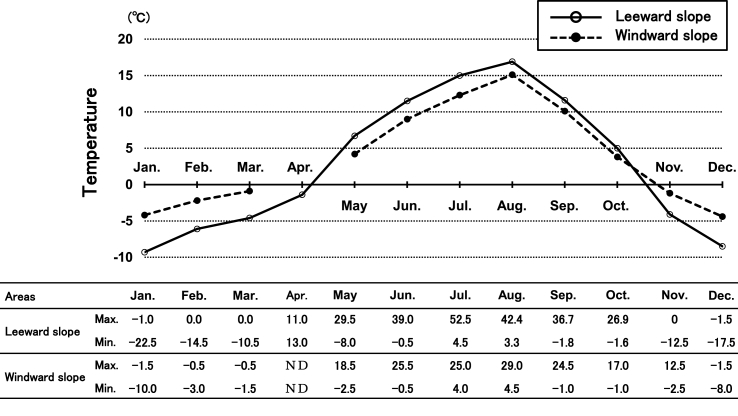
We measured the average, maximum and minimum environmental temperatures on the windward and leeward slopes of Mt. Tateyama (Fig. 6). Temperatures peaked in August, and temperatures on the leeward slopes of the mountain sites were lower than those on windward slopes between May and October.
Fig. 6.
Average monthly environmental temperatures on the windward and leeward slopes of Mt. Tateyama from 2006 to 2007. The temperatures on the windward slopes were not measured in April. The table below the graph shows monthly maximum and minimum temperatures.
4. Discussion
To date, two reports have been published on Eimeria spp. infection in the Japanese rock ptarmigan (Kamimura and Kodama, 1981; Ishihara et al., 2006). The prevalence of parasites described as E. uekii was 61–85% in adult birds and 84% in chicks on Mt. Tateyama from 1971 to 1974, increasing toward September and decreasing in October and November (Kamimura and Kodama, 1981). In the other report, the prevalence of E. uekii and type B oocysts at 13 sites including Mt. Tateyama was 60.9% and 29.7%, respectively, from 2003 to 2005 (Ishihara et al., 2006). The findings of these studies suggested that Eimeria spp. are widely distributed in Japanese rock ptarmigan populations. We examined 828 birds at more than 11 sites and determined the prevalence to be 47.6%, with infections increasing toward summer and peaking in July. Among examined sites, only one (Minamidake) was found to be negative. There is less possibility that birds stay at the limited area because birds including chicks after hatching easily moved to other places. The reason remains unknown but the number of oocysts shedded in feces of the examined birds could not be the peak. Oocysts of E. uekii were detected more frequently than those of type B, which corroborates the findings of previous studies.
Japanese rock ptarmigans are restricted to the alpine zone at around 3,000 m above sea level. They are typically found on the windward slopes of mountains after winter in March to May where their evergreen food plants are more accessible as the snow thaws relatively quickly in these areas (Nakamura, 2007). Males then compete for females and the pairs establish a breeding territory on the leeward slopes where they feed on sprouts and arthropods. Between June and September, the birds lay and brood their eggs, and the precocial young remain with their parents for 3–4 months after hatching. It is considered that the transmission of Eimeria parasites occurs when adult birds come into close contact with each other during breeding, with infections among birds likely occurring most often in August and September when temperatures are optimal for sporulation. The increase in the density of the birds as the temperature increases may facilitate completion of the Eimeria lifecycle.
Like in adult birds, the prevalence of Eimeria spp. in chicks also peaked in August and September. However, OPG values among chicks were markedly higher than among adult birds. During breeding, female birds primarily take care of chicks. Although no clear tendency in Eimeria prevalence was observed between sexes, the parents are the most likely source of infection to chicks. However, since adults are expected to have acquired immunity against Eimeria spp., it is considered that the number of oocysts shed by mature birds would not have been large. In addition, coprophagy is commonly found in vertebrates, and the intestinal microbial flora acquired in this way can provide significant benefits to nutrition and growth performance due to the beneficial effect that the flora have on the gut (Soave and Brand, 1991; Barrow, 1992). Although coprophagy is uncommon in birds (Hurd et al., 1991), we observed that some Japanese rock ptarmigan chicks consumed their parents’ feces (data not shown). Such behavior would facilitate infection by Eimeria spp. Furthermore, gut microflora in chickens has been shown to mitigate the symptoms associated with Eimeria spp. infection (Dalloul et al., 2003; Lee et al., 2007; Giannenas et al., 2012), and however, further studies are necessary to evaluate that changes in the microbiome transmitted from parents to chicks might reduce the number of oocysts that are shed by the adults.
After being shed from the host, oocysts obtain infectivity by sporulation under favorable conditions. In bovine and ovine Eimeria spp., freezing at −10 to −18 °C has been shown to kill the oocysts (Landers, 1953; Lassen and Seppä-Lassila, 2014). The surface temperature of the soil when there is snow cover will not drop far below 0 °C; however, soil temperatures can fall below −15 °C in the absence of snow (Sharratt et al., 1992). Based on our finding that oocysts remained viable after storage at 0 °C for 6 months, it is possible that the oocysts could have survived in the soil over winter and that they then sporulated after June when the environmental temperatures in the study area exceeded 0 °C. Although several studies have examined the effect of low temperature on eimerian oocyst sporulation in other animals, incubating eimerian oocysts at 8 °C for 8 weeks did not make these oocysts sporulate (Edgar, 1954). However, in our study, eimerian oocysts from Japanese rock ptarmigans were capable of sporulating at 8 °C if they were incubated for more than 48-h. It is therefore considered possible that Eimeria spp. from cold-adapted birds may be relatively tolerant to, and capable of sporulating at low temperatures.
Although it has been reported that E. uekii may cause lesions in the host (Kamimura and Kodama, 1981), the virulence of Eimeria spp. in the Japanese rock ptarmigan has not been examined in detail. Since collecting diarrhea samples or finding weak or dead birds in the field is rare, clarifying the pathogenicity of the parasites is difficult. In addition, the number of ptarmigans in Japan is decreasing and several populations have been extirpated. Within the context of ptarmigan conservation, further studies are therefore necessary in order to evaluate the ecology, biology and pathogenicity of Eimeria spp.
Acknowledgements
The authors gratefully acknowledge Dr. Hiroshi Nakamura (Professor Emeritus of Shinshu University), Dr. Takaaki Sakanakura (The Institute for Alpine Environment and Biota), Mr. Tsutomu Matsuda (Toyama Raicho Kenkyukai: Toyama Rock Ptarmigan Research Association), and Ms. Yoko Ichikawa, Ms. Asumi Tamada, Ms. Rina Nagai, Ms. Mio Hagihara, and Ms. Mizuki Takahashi (Former College of Bioresource Sciences, Nihon University) for assistance with conducting surveys in the present study. A part of this study was supported by grants from the Life Science Center of Nihon University, JSPS KAKENHI (Grant No. JP16510179) and the Environment Research and Technology Development Fund (No. 4-1604).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijppaw.2018.03.004.
Contributor Information
Makoto Matsubayashi, Email: matsubayashi@vet.osakafu-u.ac.jp.
Sayaka Tsuchida, Email: sayaka07249250@gmail.com.
Kazunari Ushida, Email: k_ushida@kpu.ac.jp.
Koichi Murata, Email: k-murata@brs.nihon-u.ac.jp.
Ethics statement
All experiments were carried out without using live animals, and the collection of feces was conducted in a non-invasive manner. Thus, ethical approval for animal experimentation was not necessary. All of the examinations in this study were permitted by the Ministry of the Environment, Government of Japan. No animals were sacrificed for the purpose of this study and the study did not have any human participants.
Conflicts of interest
The authors declare that they have no conflict of interest.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Anderson R.M., May R.M. The population dynamics of microparasites and their invertebrate hosts. Philos. T. R. Soc. B. 1981;291:451–524. [Google Scholar]
- Barrow P. Probiotic for chickens. In: Fuller R., editor. Probiotics, the Scientific Basis. Chapman and Hall; London, UK: 1992. pp. 225–257. [Google Scholar]
- Brackett S., Bliznick A. The effect of small doses of drugs on oocyst production of infections with Eimeria tenella. Ann. N. Y. Acad. Sci. 1949;52:595–610. [Google Scholar]
- Dalloul R.A., Lillehoj H.S., Shellem T.A., Doerr J.A. Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poultry Sci. 2003;82:62–66. doi: 10.1093/ps/82.1.62. [DOI] [PubMed] [Google Scholar]
- del Hoyo J., Elliott A., Sargatal J. Lynx Edicions; Barcelona: 1994. Hand-book of the Birds of the World (Volume 2): New World Vul-tures to Guineafowl; p. 638. [Google Scholar]
- Edgar S.A. Effect of temperature on the sporulation of oocysts of the protozoan, Eimeria tenella. Trans. Am. Microsc. Soc. 1954;73:237–242. [Google Scholar]
- Galli-Valerio B. Notes de Parasitologie. Zentralblatt für Bakteriologie und Parasitenkunde, I. Abteilung Orginale. 1929;112:54–59. [Google Scholar]
- Giannenas I., Papadopoulos E., Tsalie E., Triantafillou E., Henikl S., Teichmann K., Tontis D. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet. Parasitol. 2012;188:31–40. doi: 10.1016/j.vetpar.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Hurd P.L., Weatherhead P.J., McRae S.B. Parental consumption of nestling feces: good food or sound economics? Behav. Ecol. 1991;2:69–72. [Google Scholar]
- Ishihara S., Shiibashi T., Sato Y., Murata K., Nogami S. Two Eimeria species isolated from wild Japanese rock ptarmigans (Lagopus mutus japonicus) in Japan. J. Vet. Med. Sci. 2006;68:991–993. doi: 10.1292/jvms.68.991. [DOI] [PubMed] [Google Scholar]
- Johnsgard P.A. The University of Nebraska Press; USA: 1983. The Grouse of the World; p. 413. [Google Scholar]
- Kamimura K., Kodama H. Eimeria uekii sp. n. from Lagopus mutus japonicus (Clark) in Mts. Tateyama, the Japan Alps. Jap. J. Parasitol. 1981;30:467–470. [Google Scholar]
- Landers E.J. The effect of low temperatures upon the viability of unsporulated oöcysts of ovine coccidia. J. Parasitol. 1953;39:547–552. [PubMed] [Google Scholar]
- Lassen B., Seppä-Lassila L. Recovery and sporulation of bovine Eimeria oocysts after exposure to sub-zero temperature. Vet. Med. Zoot. 2014;66:35–39. [Google Scholar]
- Lee S.G.H., Lillehoj H.S., Dalloul R.A., Park D.W., Hong Y.H., Lin J.J. Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poultry Sci. 2007;86:63–66. doi: 10.1093/ps/86.1.63. [DOI] [PubMed] [Google Scholar]
- Levine N.D. A review of the coccidia from the avian orders Galliformes, Anseriformes and Charadriiformes, with a description of three new species. Am. Midl. Nat. 1953;49:247–252. [Google Scholar]
- Murata K., Tamada A., Ichikawa Y., Hagihara M., Sato Y., Nakamura H., Nakamura M., Sakanakura T., Asakawa M. Geographical distribution and seasonality of the prevalence of Leucocytozoon lovati in Japanese rock ptarmigans (Lagopus mutus japonicus) found in the alpine regions of Japan. J. Vet. Med. Sci. 2007;69:171–176. doi: 10.1292/jvms.69.171. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Rock ptarmigan Lagopus mutus japonicus. Jap. J. Ornithol. 2007;56:93–114. [in Japanese] [Google Scholar]
- Pyziel A.M., Demiaszkiewicz A.W. Observations on sporulation of Eimeria bovis (Apicomplexa: eimeriidae) from the European bison Bison bonasus: effect of temperature and potassium dichromate solution. Folia Parasitol. (Praha) 2015;62 doi: 10.14411/fp.2015.020. pii: 2015.020. [DOI] [PubMed] [Google Scholar]
- Sharratt B.S., Baker D.G., Wall D.B., Skaggs R.H., Ruschy D.L. Snow depth required for near steady-state soil temperature. Agric. For. Meteorol. 1992;57:243–251. [Google Scholar]
- Skirnisson K., Th Thorarinsdottir S. Two new Eimeria species (Protozoa: eimeriidae) from wild rock ptarmigans, Lagopus muta islandorum, in Iceland. Parasitol. Res. 2007;101:1077–1081. doi: 10.1007/s00436-007-0589-5. [DOI] [PubMed] [Google Scholar]
- Soave O., Brand C.D. Coprophagy in animals: a review. Cornell Vet. 1991;81:357–364. [PubMed] [Google Scholar]
- Stenkewitz U., Nielsen Ó.K., Skírnisson K., Stefánsson G. Host-parasite interactions and population dynamics of rock ptarmigan. PLoS One. 2016;11:e0165293. doi: 10.1371/journal.pone.0165293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga S., Matsuo J., Kono E., Kimura K., Inoue M., Rai S.K., Ono K. Prevalence of Cryptosporidium parvum infection and pattern of oocyst shedding in calves in Japan. Vet. Parasitol. 2000;94:27–32. doi: 10.1016/s0304-4017(00)00338-1. [DOI] [PubMed] [Google Scholar]
- Waldenstedt L., Elwinger K., Lundén A., Thebo P., Uggla A. Sporulation of Eimeria maxima oocysts in litter with different moisture contents. Poultry Sci. 2001;80:1412–1415. doi: 10.1093/ps/80.10.1412. [DOI] [PubMed] [Google Scholar]
- Wildlife Division of the Ministry of the Environment . 2012. Report of Protection Propagation Program of Japanese Rock Ptarmigans, Tokyo; p. 1. [Google Scholar]
- Wildlife Division of the Ministry of the Environment . 2017. P5 Red Data List: Birds, Ministry of the Environment of Japan, Tokyo; p. 5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.