Abstract
Introduction
This systematic review evaluated the use of buffered versus non-buffered lidocaine to increase the efficacy of inferior alveolar nerve block (IANB).
Materials and Methods
Randomized, double-blinded studies from PubMed, Web of Science, Cochrane Library, Embase, and ProQuest were identified. Two of the authors assessed the studies for risk of bias. Outcomes included onset time, injection pain on a visual analog scale (VAS), percentage of painless injections, and anesthetic success rate of IANB.
Results
The search strategy yielded 19 references. Eleven could be included in meta-analyses. Risk of bias was unclear in ten and high in one study. Buffered lidocaine showed 48 seconds faster onset time (95% confidence interval [CI], −42.06 to −54.40; P < 0.001) and 5.0 units lower (on a scale 0–100) VAS injection pain (95% CI, −9.13 to −0.77; P=0.02) than non-buffered. No significant difference was found on percentage of people with painless injection (P = 0.059), nor success rate (P = 0.290).
Conclusion
Buffered lidocaine significantly decreased onset time and injection pain (VAS) compared with non-buffered lidocaine in IANB. However due to statistical heterogeneity and low sample size, quality of the evidence was low to moderate, additional studies with larger numbers of participants and low risk of bias are needed to confirm these results.
Keywords: Buffered Lidocaine, Inferior Alveolar Nerve Block, Meta-Analysis, Randomized Controlled Trials, Sodium Bicarbonate
INTRODUCTION
Lidocaine has been a routine local anesthetic in dentistry since its first introduction into the market in 1948. Inferior alveolar nerve block (IANB) is the primary injection technique for achieving local anesthesia for mandibular dental procedures. To evaluate the anesthetic efficacy of lidocaine in IANB, Vreeland et al. [1] tested lidocaine in different volumes and concentrations in humans. Up to 80% failure of profound analgesic effect in the mandibular teeth were reported [1]. Other anesthetic problems, such as discomfort of solution deposition and slow onset, were also noteworthy to clinicians [1].
The local anesthetics contain spontaneously uncharged molecules (the base) and positive charged molecules (the cation) [2]. The uncharged free base form of the solution is responsible to diffuse through the nerve sheath, revert to the charged form within the axoplasm, and block the sodium channel to induce nondepolarizing nerve block [2]. The relative proportion of the base and the cation varies with the pH of the solution or the targeting tissues [2]. The higher the pH of the solution, the more free base form of the solution exists. The dissociation constant (pKa) also determines the relative proportion of ionic form [2]. When the pH of the solution equals to the pKa of the local anesthetics, there exist equal amounts of base and cation in the solution. Epinephrine is often used as an addition to local anesthetic agents to achieve prolonged anesthetic effects. However, a lower pH is required for local anesthetics to prolong the shelf life of epinephrine. The pH of local anesthetics without epinephrine is about 5.5 [2]. When epinephrine is added to lidocaine, to maintain a lower pH value, sodium bisulfite or hydrochloric acid are commonly used in the solution. Thus, the mean pH (± standard deviation, SD) of the solution of 1% lidocaine with 1:100,000 epinephrine is 4.24 (± 0.42), and 2% lidocaine with 1:100,000 epinephrine is 3.93 (± 0.43) [3]. The acidity of lidocaine increases the hydrogen ions in the local tissue environment and thus results in injection pain and increases onset time [2,4,5], which causes the discomfort for patients during IANB injection. The alkalinization of the lidocaine with sodium bicarbonate can increase the pH value of the solution. When it interacts with the hydrochloride acid in local anesthetics, sodium bicarbonate creates water and carbon dioxide (CO2). Catchlove [6] concluded that CO2 could potentiate the action of local anesthetics by serving as a direct depressant on the axon, concentrating local anesthetics inside the nerve trunk, and converting local anesthetics to active cations through its effect on pH at the site of action inside the nerve. The alkalinization of lidocaine has been widely evaluated in the medical field. In a systematic review, Davies [7] included 22 randomized clinical trials (RCTs) in humans and concluded that buffering local anesthetics with sodium bicarbonate could reduce injection pain while not affecting efficacy. A more rapid onset can be achieved by increasing the number of uncharged base molecules in the solution [2].
In 1992, Malamed [8] reported an approach of the addition of sodium bicarbonate immediately prior to anesthetic administration to increase the pH value of the solution in dentistry. After that, several RCTs were conducted to evaluate the effect of sodium bicarbonate buffered lidocaine with epinephrine [5,9,10,11,12,13,14,15,16,17,18]. However, the effect of alkalinization of lidocaine in mandibular nerve block remains controversial. This systematic review and meta-analysis aimed to focus on these types of studies to determine whether sodium bicarbonate buffered lidocaine is effective in shortening analgesic onset time, increasing success rate, and reducing injection pain in dental patients receiving an IANB.
MATERIALS AND METHODS
1. Inclusion and exclusion criteria
The search was limited to randomized controlled trials on healthy volunteers, or asymptomatic patients in need of bilateral dental treatment, or symptomatic patients in need of non-surgical endodontic treatment. The intervention under study was 1–2% buffered lidocaine with 8.4% sodium bicarbonate during IANB injection compared with non-buffered lidocaine. Studies of the effect of articaine [19], hyaluronidase [20], or using other buffer agents than sodium bicarbonate (i.e. sodium hydroxide) [21] were excluded. Studies using other routes of administration such as mental nerve block [22] or infiltration prior to IANB [23] were also excluded.
2. Outcomes
Primary outcome measures were the onset of time of anesthesia in seconds, anesthetic success rate of IANB, the percentage of patients with painless IANB injection, and the pain during IANB injection measured via VAS. Anesthetic success rate of IANB was defined as the tooth without pain or with mild pain during endodontic access [14,15]. Injection pain evaluated the level of discomfort or pain for patients when depositing lidocaine solution during the injection procedure.
3. Search methods for identification of studies
Five electronic databases were searched using the following strategies:
-
MEDLINE via PubMed (searched on 03/22/2017; updated on 04/01/2018) limited to English language and Humans:
(“IANB” OR ((“mandible”[MeSH Terms] OR “mandible” OR “mandibular”) AND “block”[All Fields]) OR (“mandibular nerve”[MeSH Terms] OR (“mandibular” AND “nerve”) OR “mandibular nerve” OR (“inferior” AND “alveolar” AND “nerve”) OR “inferior alveolar nerve”)) AND ((“buffers”[MeSH Terms] OR “buffers” OR “buffered” OR “sodium bicarbonate” OR (sodium AND bicarbonate) OR “alkalinized”) AND (“lidocaine” [MeSH Terms] OR “lidocaine”))
-
The Web of Science (searched on 03/22/2017; updated on 04/01/2018):
TS=((inferior alveolar block OR mandibular block) AND (buffered lidocaine OR (lidocaine AND (buffers OR buffered))))
-
The Cochrane Library (searched on 03/22/2017; updated on 04/01/2018):
(inferior alveolar block or mandibular block) and (buffered lidocaine or (lidocaine and (buffers or buffered)))
-
The Embase (searched on 03/22/2017; updated on 04/01/2018):
(‘lidocaine’/exp OR lidocaine) AND (buffer OR buffered) AND ((inferior AND alveolar AND block) OR (mandibular AND block))
-
The ProQuest (searched on 04/01/2018):
(“buffered lidocaine”) AND (“inferior alveolar nerve block”) limited to scholarly journals and dissertations and theses
4. Data collection and analysis
Two independent reviewers (J.G., K.Y) screened titles and abstracts of the articles identified by the search strategy for inclusion and exclusion criteria. Full-text articles were obtained for those references that fulfilled the inclusion criteria. Full-text was also obtained for those references that could not be excluded just based on the title and abstract. Two independent reviewers (J.G., K.Y.) scanned all reference sections of the included articles for additional relevant articles.
5. Data extraction and management
Table 1 shows the data extracted from the full-text articles eligible for inclusion by two independent reviewers (J.G., R.E.). Data extraction included the authors and years of recruitment, demographics of participants and sample size, intervention methods for the study and control groups, study design, and the outcome results for each study (Table 2). The two independent reviewers (J.G., R.E.) resolved any disagreements by open discussion of the issue until an agreement was reached.
Table 1. Summary of eligible studies.
| Reference | Recruitment years, Country, Study Type | BUFFERED Local anesthetic with buffer and concentration | NON-BUFFERE D group Local anesthetic | Co-Interventions | Inclusion / Exclusion criteria | Gender Average Age [Range] | Summary of Risk of Bias |
|---|---|---|---|---|---|---|---|
| Chopra et al. [9] 2016 | N/A | 1.8 ml 2% lidocaine with 1:200,000 epi and 8.4% Sodium bicarbonate (10:1 ratio) | 1.8 ml 2% lidocaine with 1:200,000 epi | N/A | Frankl's behavior rating grade three or four | 18F/12M | Unclear |
| India, | Not require any sedation | [6–8 years](n = 17) | |||||
| Split mouth | No analgesics for 24 hrs before the appointment | [9–12 years](n = 13) | |||||
| N = 30 | |||||||
| Comerci et al. [10] 2015 | N/A | 1.7 ml 2% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 1.7 ml 2% lidocaine with 1:100,000 epi | Long buccal nerve block | Aged 18 years or older | 5F/15M | Unclear |
| USA, | Treatment requiring bilateral IA and LB nerve blocks | 46 years | |||||
| Split mouth | Good general health ASA I or ASA II | [27–81 years] | |||||
| N = 20 | Baseline pain level of all patients was 0 | ||||||
| Donaldson [11] 2006 (not included in meta-analysis) | N/A | 1.8ml of 2% lidocaine HCl with 1:100,000 epi and 8.4% sodium bicarbonate(17:1 ratio) | 1.8ml of 2% lidocaine HCl with 1:100,000 epi | N/A | ASA I | 20F/24M | High |
| USA, | > 80 pounds | Age: N/A | |||||
| splint mouth | Presence of bilateral non-carious, vital permanent canines | ||||||
| (n = 44) | No large restorations, periodontal disease or previous trauma in the lower arch/No soft tissue pathology at the injection site/No medication that could interact with the lidocaine or could alter pain perception | ||||||
| Kashyap et al. [12] 2011 | N/A | 2.5 ml 2% lidocaine with 1:80,000 epi and 8.4% sodium bicarbonate (10:1 ratio) | 2.5 ml 2% lidocaine with 1:80,000 epi | Lingual nerve block | 100 healthy patients who needed procedures under local anesthesia in the mandibular region | Not stated | Unclear |
| India | Long buccal block | ||||||
| Parallel RCT | |||||||
| (n = 100) | |||||||
| (n = 50 in each group) | |||||||
| Malamed et al. [5] 2013 | N/A | 1.8 ml 2% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 1.8 ml 2% lidocaine with 1:100,000 epi | N/A | Healthy volunteers | Gender: N/A | Unclear |
| USA | 41.6 years | ||||||
| Split mouth | [23–76 years] | ||||||
| n = 18 | |||||||
| Phero et al. [13] 2017 | N/A | 4 ml 2% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 4 ml 2% lidocaine with 1:100,000 epi | No co-intervention | Two sessions at the UNC Oral and Maxillofacial Surgery Clinic, ASA I | 11F/12M | Unclear |
| USA, | Exclusion: Allergy to lidocaine class of anesthetic drugs, local anesthetic drug use in past week, current symptoms teeth or oral mucosa | Median = 21 years | |||||
| split mouth | [18–30 years] | ||||||
| (n = 23) | |||||||
| Saatchi et al. [14] 2015 | N/A | 3.6 ml of 2% lidocaine with 1:80,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 3.6 ml 2% lidocaine with 1:80,000 epi in which 0.36ml lidocaine was replaced by distilled water | N/A | Vital mandibular posterior tooth with active pain and a long response to cold testing with Endo-Frost cold spray | 46F/34M | Unclear |
| Iran, | 35 years in buffered group | ||||||
| Parallel RCT | 36 years in nonbuffered group | ||||||
| (n = 80) | [20–55 years] | ||||||
| (40 in each group) | |||||||
| Schellenberg et al. [15] 2015 | N/A | 2.8ml 4% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) (n = 50) | 2.8ml 4% lidocaine with 1:100,000 epinephrine (n = 50) | 0.9 ml non-buffered 2% lidocaine with 1:100,000 epi buccal soft tissue anesthesia | Emergency patients | 61F/39M | Unclear |
| USA, | Good health | 35 years in buffered group | |||||
| RCT | Have a vital mandibular posterior tooth with actively experiencing moderate to severe pain Had a prolonged response to cold testing with Endo-Ice | 36 years in non-buffered group | |||||
| (n = 100) | [18–64 years] | ||||||
| (50 in each group) | |||||||
| Tavaka [16] 2013 | N/A | 1.7 ml 2% lidocaine, with 1:100,000 epi and 8.4% sodium bicarbonate (ratio not stated, the onset system) | 1.7 ml 2% lidocaine, with 1:100,000 epi | Long buccal nerve block with separated VAS units for this injection | Informed consent provided 9-12 years of age | 10F/10M | Unclear |
| USA, | Comprehend the visual analog scale, numeric rating scale, and verbal rating scale | [9–12 years] | |||||
| Split mouth | Present moderate mandibular dental disease bilaterally Have 4 to 7 natural teeth present in each mandibular quadrant with moderate dental disease on at least one tooth | ||||||
| (n = 20) | Be willing to attend the clinic for 3 or more appointments | ||||||
| Exclusion: Received an anesthetic, analgesic or sedative within 24 hours prior to the therapy appointments | |||||||
| Warren et al. [17] 2017 | N/A | 4.4 ml of 1% lidocaine with 1:100,000 epi and 8.4% sodium bicarbonate (9:1 ratio) | 4 ml 2% lidocaine with 1:100,000 epi | Lingual and Long buccal nerve block | Treated at the oral and maxillofacial surgery clinic 2 weeks apart, ASA I | 12F/11M | Unclear |
| USA, | Exclusion: Allergy to lidocaine class of anesthetic drugs, local anesthetic drug use in past week, current symptoms teeth or oral mucosa | Median = 25 years | |||||
| Splint mouth | [18–30 years] | ||||||
| (n = 23) | |||||||
| Whitcomb et al. [18] 2010 | N/A | 3.6 ml 2% lidocaine with 1 : 100,000 epi and 8.4% sodium bicarbonate (5:1 ratio) | 3.6 ml 2% lidocaine with 1:100,000 epi | N/A | Good health | 12 F/ 28 M | Unclear |
| USA, | Not taking any medications that would alter their perception of pain | 26 years | |||||
| Split mouth | [18–38 years] | ||||||
| (n = 40) |
Table 2. Summary of outcomes included in meta-analyses.
| Reference | Sample Size Per Group | Outcomes Reported | pH value of the solution before / after buffering [range] | Outcome in Tx Group | Outcome in Control Group |
|---|---|---|---|---|---|
| Chopra et al. [9] 2016 | Buffered: n = 30 | Onset time (sec), mean ± SD | 4.33 / 7.32 | 84.2 ± 28.9 | 86 ± 27.8 |
| Non-buffered: n = 30 | Injection pain using Heft-Parker VAS score (0–170mm scale), mean ± SD | 36.8 ± 17.7 | 39.5 ± 18.2 | ||
| 21.65 ± 10.41* | 23.24 ± 10.71* | ||||
| Comerci et al. [10] 2015 | Buffered: n = 20 | Injection pain for the IANB (0–10 units), mean ± SD | [3.3–5.5]¶/ [7.3–7.6]∥ | 2.7 ± 1.3 | 2.7 ± 1.9 |
| Non-buffered: n = 20 | 27 ± 13* | 27 ± 19* | |||
| Donaldson [11] 2006 (not included in meta-analysis) | Buffered: n = 44 | Injection pain using Heft-Parker VAS score (0-170 mm scale), mean ± SD | 4.11 / 6.83 | 29.77 ± 4.07 | 27.42 ± 3.86 |
| Non-buffered: n = 44 | 17.5 ± 2.39* | 16.1 ± 2.3* | |||
| Onset time (min) by EPT, mean ± SD | 12.73 ± 1.27 | 14.98 + 1.42 | |||
| 763.8±76.2† | 898.8±56.8† | ||||
| Kashyap et al. [12] 2011 | Buffered: n = 50 | Percentage of painless injection, (%) | 3.05 / 7.38 | 50/50 (100%) | 11/50 (22%) |
| Non-buffered: n = 50 | Onset time (sec), mean ± SD | 34.4 ± 9.8 | 109.8 ± 31.6 | ||
| Malamed et al. [5] 2013 | Buffered: n = 18 | Percentage of painless injection, (%) | 3.85 / 7.31 [7.29–7.33] | 8/18 (44%) | 1/18 (6%) |
| Non-buffered: n = 18 | |||||
| Phero et al. [13] 2017 | Buffered: n = 23 | Injection pain using score (0–10 units), difference in medians (95% CI) | 3.5 / neutral pH (as described in paper) | −2/3 unit (95% CI, −1.46 to 0.13) | |
| Non-buffered: n = 23 | −6.7 ± 18.22‡ | ||||
| Saatchi et al. [14] 2015 | Buffered: n = 40 | Successful IANB (%) | N/A / [7.3–7.6]∥ | 25/40 (62.5%) | 15/40 (47.5%) |
| Non-buffered: n = 40 | |||||
| Schellenberg et al. [15] 2015 | Buffered: n = 50 | Successful IANB (%) | 4.51 / 7.05 | 16/50 (32%) | 20/50 (40%) |
| Non-buffered: n = 50 | |||||
| Tavaka [16] 2013 | Buffered: n = 20 | Injection pain using VAS score (0–100 mm scale), mean ± SD | [3.3–5.5]¶ / up to human physiologic pH (as described in paper) | 33.05 ± 24.80 | 43 ± 27.01 |
| Non-buffered: n = 20 | |||||
| Warren et al. [17] 2017 | Buffered: n = 23 | Injection pain using score (0–10 units), difference in medians (95% CI) | 3.5 / neutral pH (as described in paper) | −1 unit (95% CI, 0.37–1.71) | |
| Non-buffered: n = 23 | −10 ± 14.8§ | ||||
| Whitcomb et al. [18] 2010 | Buffered: n = 40 | Percentage of painless injection, (%) | 3.37 / 7.50 | 29/40 (72%) | 23/40 (58%) |
| Non-buffered: n = 40 | |||||
SD = Standard deviationIQR = interquartile range CI = confidence interval
*After converting original VAS to 100mm scale
†After converting from minutes to seconds
‡After converting median difference and IQR to mean difference ± SD
§After converting median difference and 95% CI to mean difference ± SD
∥Based on Frank et al. [12]
¶Based on the manufacturer DENTSPLY International
6. Assessment of risk of bias in RCTs
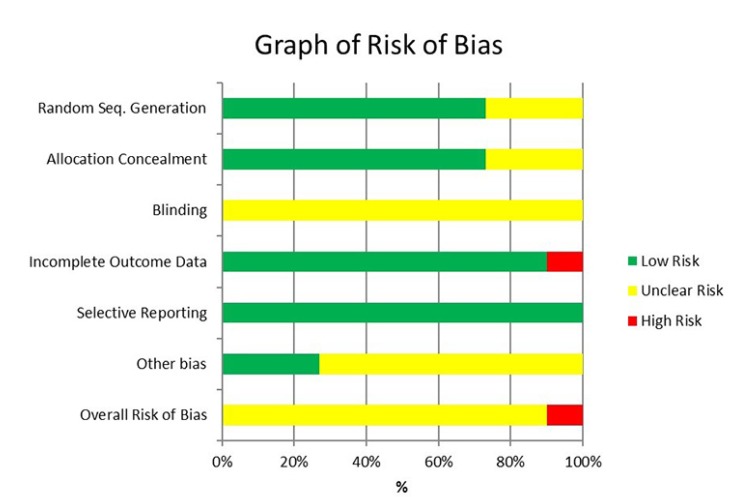
The risk of bias tool described in the Cochrane Handbook for Systematic Reviews of Interventions [24] was used to assess the risk of bias of each eligible study. Two independent reviewers (J.G., R.E.) assessed the risk of bias for each included study. Disagreements were resolved by discussion. Table 3 and Fig. 1 show the summary of the risk of bias for each study.
Table 3. Summary of risk of bias for eligible RCT studies.
| Study | Random Seq. Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Reporting | Other potential bias | Overall Bias |
|---|---|---|---|---|---|---|---|
| Chopra et al. [9] 2016 | - | - | ? | - | - | ? | ? |
| Comerci et al. [10] 2015 | - | ? | ? | - | - | - | ? |
| Donaldson [11] 2006 | - | - | ? | + | - | ? | + |
| Kashyap et al. [12] 2011 | - | ? | ? | - | - | ? | ? |
| Malamed et al. [5] 2013 | ? | - | ? | - | - | ? | ? |
| Phero et al. [13] 2017 | - | - | ? | - | - | ? | ? |
| Saatchi et al. [14] 2015 | ? | - | ? | - | - | ? | ? |
| Schellenberg et al. [15] 2015 | - | - | ? | - | - | - | ? |
| Tavaka [16] 2013 | - | - | ? | - | - | ? | ? |
| Warren et al. [17] 2017 | - | - | ? | - | - | - | ? |
| Whitcomb et al. [18] 2010 | ? | ? | ? | - | - | ? | ? |
KEY: + High risk of bias
− Low risk of bias
? Unclear risk of bias
Fig. 1. Summary of risk of bias of eligible RCT's.
7. Statistical analyses
Studies were pooled into a meta-analysis only when investigators had used similar interventions and measured similar outcomes. For dichotomous outcomes, risk ratio (RR) with 95% CI were used to express treatment effects. For continuous data, the authors used difference in means with 95% CIs. Whenever parallel design and split-mouth design crossover studies were included, the authors conducted paired and independent tests with the same results. For the two studies [13,17] that provided median, 95% CIs and/or interquartile range (IQR), the methods described by Wan et al. [25] were used to estimate mean and SD. For two studies [9,11] that reported the VAS score using 170mm scale and two studies [13,17] using 10 units scales, the authors converted the measurements to 100 mm scale VAS for the meta-analysis. Ph value range was either provided in the included studies or was calculated as described by Frank et al. [3], and reported in Table 2. The authors used a random-effects model on combined estimates of effect except when only two studies were included in a meta-analysis, and then the fixed-effect model will be used. Statistics reported were the Cochrane Q test [26] and the I2 statistic [27]. Asymptomatic patients who did not take any medication and required dental work and healthy volunteers were separated in the analysis from symptomatic patients. Separate subgroup analyses were conducted for pediatric and adult patients for injection pain (VAS scores). Comprehensive Meta-Analysis software Version 3 (Biostat Solutions, Inc; USA) was used to conduct statistical analyses.
8. Levels of evidence and summary of the review findings
The quality of evidence assessment and summary of the review findings were conducted using the software GRADEprofiler (GRADEPro), which follows the Cochrane Collaboration and Grading of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group recommendations [24].
RESULTS
1. Results of the search
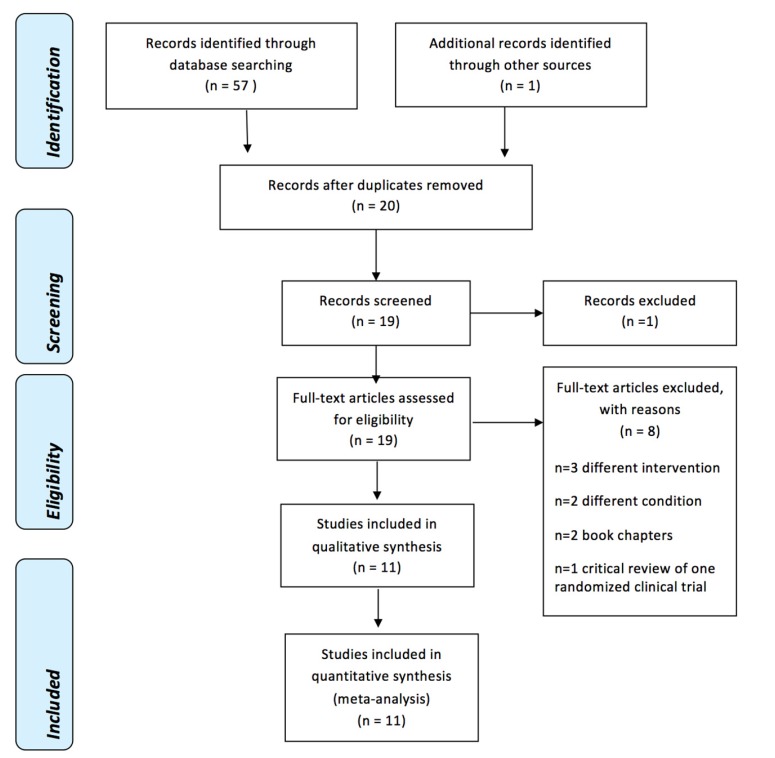
The initial search strategy yielded 57 references, which were reduced to 20 references after removing duplicates. Twenty studies were assessed independently by two review authors (J.G., K.Y.), and based on the abstracts and titles, one reference [28] was excluded because it was a conference abstract.
All of the 19 manuscripts identified were searched for full-text and analyzed for inclusion independently by two review authors (J.G., K.Y.). Eleven manuscripts were relevant for inclusion [5,9,10,11,12,13,14,15,16,17,18]. Of these 19 manuscripts, eight were identified for exclusion. Three studies [19,20,21] were excluded since they used different intervention: articaine [19], buffered with hyaluronidase [20], and buffered with sodium hydroxide [21]. Two studies [22], [23] were excluded due to different conditions: mental nerve block [22], and supplemental buccal infiltration [23]. Two were book chapters [29,30] and one study [31] was a critical review of one RCT. The preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flowchart (Fig. 2) shows a summary of the results. Table 1 shows a list of the included studies.
Fig. 2. PRISMA Flow Diagram [33].
2. Included studies
Eleven RCTs evaluating the effect of buffered and non-buffered 1%, 2%, or 4% lidocaine with epinephrine on the effectiveness of IANB were included [5,9,10,11,12,13,14,15,16,17,18]. Eight of the eleven trials used split mouth technique (the patients' contralateral side was used as the control group) [5,9,10,11,13,16,17,18], while three of the eleven studies were parallel RCTs [12,14,15]. Inclusion criteria varied among the studies. Two parallel RCTs included patients with symptomatic irreversible pulpitis [14,15]. Four studies had only healthy volunteers participating in their clinical trials [5,10,11,18]. In two pediatric studies [9,16], participants who did not receive any analgesic medication before the appointment and could behave during the procedures were included. One parallel RCT [12] and two split-mouth studies [13,17] included only patients who required bilateral mandibular treatment.
2.1. Risk of bias in included studies
One study demonstrated high risk [11] while the remaining studies showed unclear risk of bias (Fig. 2, Table 3).
3. Effects of interventions
The eleven included studies comparing buffered lidocaine to non-buffered lidocaine were all incorporated in the meta-analyses as the authors had reported similar outcomes of onset time [9,11,12], VAS scores on injection pain [9,10,11,13,15,16,17], success rate [14,15], and the percentages of painless injection [5,18].
Onset time.
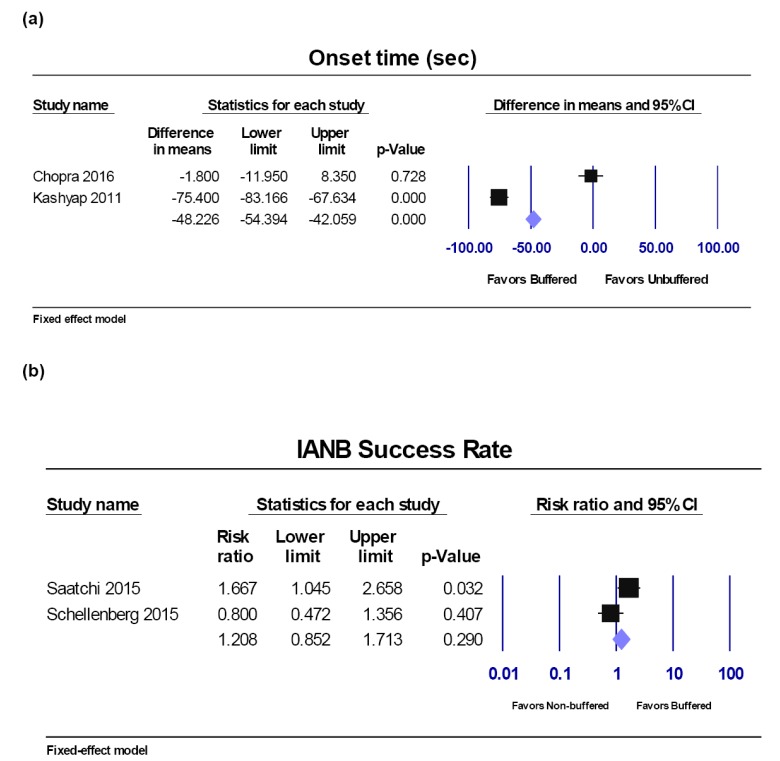
Four studies [9,11,12,18] reported onset time. Two studies [11,18] used an electric pulp test (EPT), one testing on mandibular canines [11] and one on mandibular incisors, molars and premolars [18]. These two studies were not combined into a meta-analysis with those checking for gingival probing onset time [9,12]. Two studies were included in the meta-analysis [9,12], as authors checked patients' symptoms and their reactions to gingival probing. The units of the onset time were all converted to seconds for the meta-analysis. The Q-value is 127.4 with 1 degree of freedom and a P-value < 0.001; I2 = 99.215%. Pooled results showed a faster onset time (48 seconds) using buffered lidocaine compared to non-buffered lidocaine using the random-effects model (95% CI, −42.06 to −54.40; P < 0.001) (Fig. 3a).
Fig. 3. Buffered lidocaine versus non-buffered lidocaine. Forest plot comparisons: a) Onset time in seconds and b) IANB success rate. CI: confidence interval.
Success rate of IANB.
Only symptomatic patients were included in the meta-analysis. Two studies [14,15] reported the success rate during IANB using buffered and non-buffered lidocaine in patients with symptomatic irreversible pulpitis. The Q-value was 4.171 with 1 degree of freedom and a P-value of 0.041; I2 was 76% and the T2 = 0.205. Pooled results showed no statistical difference in the success rate of IANB when applying buffered lidocaine compared to non-buffered lidocaine using the fixed-effect model (RR, 1.208; 95% CI, 0.852 to 1.713; P = 0.290) (Fig. 3b).
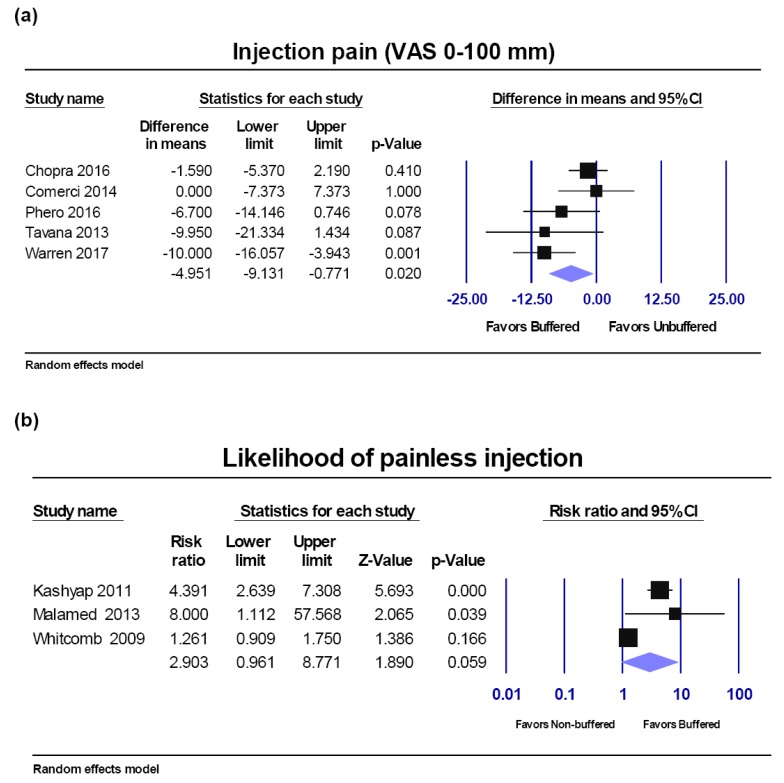
VAS score injection pain.
Among seven studies reporting VAS injection pain, two studies were excluded from the meta-analysis (Fig. 4a), and analyzed in sensitivity analyses, due to the heterogeneity of the patients or due to high risk of bias and low pH.
Fig. 4. Buffered lidocaine versus non-buffered lidocaine. Forest plot comparisons: a) Injection pain (VAS score) and b) percentage of painless injection. CI: confidence interval.
Five studies [9,10,13,16,17] including healthy volunteers and asymptomatic patients who required bilateral dental treatment (i.e. restoration, third molar extraction, etc.) were included in the meta-analysis. The Q-value was8.010 with 4 degrees of freedom and a p-value of 0.091; I2 was 50.061. Pooled results showed a significant decrease in injection pain with buffered compared to non-buffered lidocaine of −4.951 units on a 0–100 scale (random-effects model; 95% CI, −9.131 to −0.771; P = 0.02) (Fig. 4a).
VAS pain sensitivity analyses:
a) High risk study and low pH:
One study by Donaldson et al. 2006 [11] was assessed at high risk of bias and was using low pH solution in the buffered group (6.83). The pH values of the buffered local anesthetics in the five studies [9,10,13,16,17] reporting VAS pain were within physiological pH value range (Table 2). Sensitivity analysis including six studies [9,10,11,13,16,17], had similar results (Q-value = 11.752; P = 0.038; I2 = 57.454). Pooled results showed a significant decrease in injection pain with buffered compared to non-buffered lidocaine of −3.6 VAS pain units on a 0–100 scale (random-effects model; 95% CI, −6.582 to −0.623; P = 0.018).
b) Symptomatic patients:
Only one study [15] included patients with symptomatic irreversible pulpitis, compared to pain-free patients in the other six studies [9,10,11,13,16,17]. Sensitivity analysis results including seven studies were similar (random-effects model; difference in means = −3.124; 95% CI, −5.908 to −0.341; P = 0.028).
c) Pediatric patients:
There were two studies [9,13] that had pediatric patients. In order to evaluate whether patients' ages affect the VAS pain score on injection, another meta-analysis was conducted which grouped the studies by population (results not shown). When subgrouping the studies into adults and pediatric patients, there was neither statistically significant difference for adults (difference in means, −4.132; 95% CI, −8.716 to 0.453; P = 0.077), nor pediatric patients (difference in means, −3.861; 95% CI, −10.927 to 3.206; P = 0.284) for injection pain on VAS scales.
Percentage of painless injection.
Three studies [5,12,18] reported whether patients experienced painless injection during IANB using buffered and non-buffered lidocaine. The Q-value was 18.429 with 2 degrees of freedom and a P-value < .0001; I2 was 89.148%. Pooled results showed no significant increase of the percentage of patients with painless injection when applying buffered lidocaine compared to non-buffered lidocaine using the random effect model (RR, 2.903; 95% CI, 0.961 to 8.771; P = 0.059) (Fig. 4b).
Levels of evidence and summary of the review findings (according to the GRADE recommendation).
The level of evidence for injection pain for IANB using VAS scores was moderate owing to risk of bias. The level of evidence for onset time in seconds was low, due to inconsistency (statistically significant heterogeneity and I2 larger than 50%) and imprecision (small number of studies included and small sample sizes). The level of evidence for the percentage of patients with painless IANB injection was moderate, due to inconsistency. The level of evidence for success rate of IANB was low, due to inconsistency and imprecision, with only two studies included (Table 4).
Table 4. Summary of the evidence and quality of the findings (GRADE).[34].
| Buffered lidocaine compared to non-buffered lidocaine for injection pain, onset time, percentage of painless injection, and success rate in mandibular nerve block | |||||
|---|---|---|---|---|---|
| Outcomes | No. of participants(studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with non-buffered lidocaine | Risk difference with buffered lidocaine | ||||
| Injection pain for IANB using VAS | 232 (5 RCTs) | ⊕⊕⊕⊖ | N/A | The mean difference in injection pain was 5.0 VAS Units lower in buffered group (9.13 lower to 0.77 lower) | |
| MODERATEa | |||||
| Onset time (seconds) | 160 (2 RCTs) | ⊕⊕⊖⊖ | N/A | The mean difference in onset time was 48.23 seconds lower in buffered group (42.06 lower to 54.40 lower) | |
| LOWa,b | |||||
| The percentage of patients with painless IANB injection | 216 (3 RCTs) | ⊕⊕⊕⊖ | RR 2.903 (0.961 to 8.771) | 324 per 1,000 | 617 more participants per 1,000 (13 fewer to 2,518 more) in buffered group than in the non-buffered group |
| MODERATEa | |||||
| Anesthetic success rate | 180 (2 RCTs) | ⊕⊕⊖⊖ | RR 1.208 (0.852 to 1.713) | 389 per 1,000 | 81 more participants per 1,000 (58 fewer to 277 more) in buffered group than in the non-buffered group |
| LOWa,b | |||||
| CI: Confidence interval; RR: Risk ratio N/A: Not applicable | |||||
| GRADE Working Group grades of evidence | |||||
| High certainty: Authors are very confident that the true effect lies close to that of the estimate of the effect | |||||
| Moderate certainty: Authors are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different | |||||
| Low certainty: Authors' confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect | |||||
| Very low certainty: Authors have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aHigh magnitude of statistical heterogeneity I2 > 50%, statistically significant heterogeneity Q P-value < .10
bSmall number of studies, small sample size.
DISCUSSION
This systematic review included eleven studies with 508 participants. Of these eleven studies, one study had high risk of bias [11] while the remaining ten studies had unclear risk of bias. Meta-analysis showed faster onset time of 48 seconds in average, and a decrease in injection pain of 5 units (on a scale 0–100) in the buffered group compared to non-buffered lidocaine.
A recent systematic review [32] has been published in 2018, and has a different PICO question compared to the current systematic review. The aim of the previous systematic review was to investigate the efficacy of buffered local anesthetics in reducing infiltration pain and anesthesia onset time in dentistry. Three IANB studies as well as infiltration studies in adult patients were included. No statistically significant differences in VAS pain for the IANB studies (P = 0.21) and the infiltration studies (P = 0.22) were found. In that systematic review, it is unclear how VAS pain data was obtained for two studies [12,18] and pediatric patients were not included.
The results of our review are applicable to people aged from 6 years to 81 years, of both genders, who received IANB injection with 1–2% lidocaine with epinephrine. There was significant heterogeneity in all conducted meta-analyses. Dosage varied from 1.7 mL to 4 mL. The percentage of lidocaine varied among 1% [17], 4% [15], and 2% in the remaining studies. There was difference in the concentration of epinephrine from 1:80,000 to 1:200,000. Another potential confounder is the preparation of the non-buffered lidocaine in control groups. Warren et al. [17] used 4.4 ml 1% lidocaine with 1:100,000 epinephrine in the buffered group, while 4 ml 2% lidocaine with 1:100,000 epinephrine in the control group. On the other hand, only one study by Saatchi et al. [14] replaced the same amount of lidocaine with distilled water in the control group to compensate for the amount of lidocaine that was replaced by buffer solution in the experimental group. In other words, all other studies [5,9,10,11,12,13,15,16,17,18] had unbalanced dosage of lidocaine in experimental and control groups, which may affect the outcome of IANB.
The populations included varied as well. There were two studies [9,13] that had pediatric patients, aging from 6–12 years, and subgroup analyses were conducted by age for VAS injection pain. Two parallel RCTs [14,15] assessed patients with symptomatic irreversible pulpitis. As this could be a source of bias because the effectiveness of local anesthetic can be affected by local tissue inflammation [2], the authors conducted a sensitivity analysis including symptomatic and asymptomatic patients with similar results. The overall strength of the evidence, according to the GRADE system, was moderate for injection pain for IANB using VAS scores and percentage of patients with painless IANB injection, and low for success rate of IANB and onset time.
The use of buffered lidocaine has raised some clinical questions that need further research to be answered: Will buffered lidocaine reduce the injection pain and onset time of IANB in patients with symptomatic irreversible pulpitis or acute apical abscess? Additional studies also are needed to eliminate other sources of variability previously described in the literature. Common problems associated with the included studies were as follows: varies dosage and percentage with anesthesia, unbalanced dosage of lidocaine in experimental and control groups, different concentration of epinephrine, supplemental administration of anesthesia other than IANB, small sample size, and inconsistent method of outcome assessments. Future studies might look at the effectiveness of buffered lidocaine in both symptomatic and asymptomatic patients with different routes of anesthetic administration. More standardized clinical trials are needed to provide higher level of evidence to determine the benefits of buffered lidocaine for IANB local anesthesia in dental treatment.
CONCLUSIONS
There is moderate quality of evidence to support the use of buffered lidocaine in IANB local anesthesia to decrease injection pain by 5 units on a scale of 0–100 and low quality of evidence to support the effectiveness in reducing onset time. Due to the small sample size and the small number of included studies, further studies are needed to confirm these results. Thus, there is inadequate evidence at this point to recommend the buffered lidocaine for IANB local anesthesia in patients in need of dental treatment.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Gayle Macdonald, PhD at Herman Ostrow School of Dentistry of USC.
Footnotes
DISCLOSURE: The authors have no funding and no conflicts of interest.
References
- 1.Vreeland DL, Reader A, Beck M, Meyers W, Weaver J. An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. J Endod. 1989;15:6–12. doi: 10.1016/S0099-2399(89)80091-3. [DOI] [PubMed] [Google Scholar]
- 2.Malamed SF. Handbook of Local Anesthesia-E-Book. 6th edition. Elsevier Health Sciences; 2014. [Google Scholar]
- 3.Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71–73. doi: 10.1177/229255031202000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208:191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 5.Malamed SF, Tavana S, Falkel M. Faster onset and more comfortable injection with alkalinized 2% lidocaine with epinephrine 1:100,000. Compend Contin Educ Dent. 2013;34(Spec No):10–20. [PubMed] [Google Scholar]
- 6.Catchlove RF. The influence of CO 2 and pH on local anesthetic action. J Pharmacol Exp Ther. 1972;181:298–309. [PubMed] [Google Scholar]
- 7.Davies RJ. Buffering the pain of local anaesthetics: A systematic review. Emerg Med (Fremantle) 2003;15:81–88. doi: 10.1046/j.1442-2026.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 8.Malamed SF. What's new in local anesthesia? Anesth Prog. 1992;39:125–131. [PMC free article] [PubMed] [Google Scholar]
- 9.Chopra R, Jindal G, Sachdev V, Sandhu M. Double-Blind Crossover Study to Compare Pain Experience During Inferior Alveolar Nerve Block Administration Using Buffered Two Percent Lidocaine in Children. Pediatr Dent. 2016;38:25–29. [PubMed] [Google Scholar]
- 10.Comerci AW, Maller SC, Townsend RD, Teepe JD, Vandewalle KS. Effect of a new local anesthetic buffering device on pain reduction during nerve block injections. Gen Dent. 2015;63:74–78. [PubMed] [Google Scholar]
- 11.Donaldson TV. Sodium bicarbonate buffered 2% lidocaine 1:100,000 epinephrine versus unbuffered 2% lidocaine 1:100,000 epinephrine for inferior alveolar nerve block injections. The Texas A&M University System Health Science Center, ProQuest Dissertations Publishing; 2006. [Google Scholar]
- 12.Kashyap VM, Desai R, Reddy PB, Menon S. Effect of alkalinisation of lignocaine for intraoral nerve block on pain during injection, and speed of onset of anaesthesia. Br J Oral Maxillofac Surg. 2011;49:e72–e75. doi: 10.1016/j.bjoms.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 13.Phero JA, Nelson B, Davis B, Dunlop N, Phillips C, Reside G, et al. Buffered Versus Non-Buffered Lidocaine With Epinephrine for Mandibular Nerve Block: Clinical Outcomes. J Oral Maxillofac Surg. 2017;75:688–693. doi: 10.1016/j.joms.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 14.Saatchi M, Khademi A, Baghaei B, Noormohammadi H. Effect of sodium bicarbonate-buffered lidocaine on the success of inferior alveolar nerve block for teeth with symptomatic irreversible pulpitis: a prospective, randomized double-blind study. J Endod. 2015;41:33–35. doi: 10.1016/j.joen.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Schellenberg J, Drum M, Reader A, Nusstein J, Fowler S, Beck M. Effect of Buffered 4% Lidocaine on the Success of the Inferior Alveolar Nerve Block in Patients with Symptomatic Irreversible Pulpitis: A Prospective, Randomized, Double-blind Study. J Endod. 2015;41:791–796. doi: 10.1016/j.joen.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Tavaka SP. Pain experiences in pediatric dental patients to buffered and conventional local anesthesia. University of California, San Francisco ProQuest Dissertations Publishing; 2013. 1541898. [Google Scholar]
- 17.Warren VT, Fisher AG, Rivera EM, Saha PT, Turner B, Reside G, et al. Buffered 1% Lidocaine With Epinephrine Is as Effective as Non- Buffered 2% Lidocaine With Epinephrine for Mandibular Nerve Block. J Oral Maxillofac Surg. 2017;75:1363–1366. doi: 10.1016/j.joms.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 18.Whitcomb M, Drum M, Reader A, Nusstein J, Beck M. A prospective, randomized, double-blind study of the anesthetic efficacy of sodium bicarbonate buffered 2% lidocaine with 1:100,000 epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2010;57:59–66. doi: 10.2344/0003-3006-57.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shurtz R, Nusstein J, Reader A, Drum M, Fowler S, Beck M. Buffered 4% Articaine as a Primary Buccal Infiltration of the Mandibular First Molar: A Prospective, Randomized, Double-blind Study. J Endod. 2015;41:1403–1407. doi: 10.1016/j.joen.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Ridenour S, Reader A, Beck M, Weaver J. Anesthetic efficacy of a combination of hyaluronidase and lidocaine with epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2001;48:9–15. [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan PA, Morley MR, Langdon JD. A study of the effectiveness of dental 2% lignocaine local anaesthetic solution at different pH values. Br Dent J. 1987;163:158–159. doi: 10.1038/sj.bdj.4806226. [DOI] [PubMed] [Google Scholar]
- 22.Syverud SA, Jenkins JM, Schwab RA, Lynch MT, Knoop K, Trott A. A comparative study of the percutaneous versus intraoral technique for mental nerve block. Acad Emerg Med. 1994;1:509–513. doi: 10.1111/j.1553-2712.1994.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 23.Saatchi M, Farhad AR, Shenasa N, Haghighi SK. Effect of Sodium Bicarbonate Buccal Infiltration on the Success of Inferior Alveolar Nerve Block in Mandibular First Molars with Symptomatic Irreversible Pulpitis: A Prospective, Randomized Double-blind Study. J Endod. 2016;42:1458–1461. doi: 10.1016/j.joen.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. The cochrane collaboration; 2011. [Google Scholar]
- 25.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Lem LC. Efficacy and pain of inferior alveolar nerve block with alkalinized lidocaine. J Oral Maxillofac Surg. 1991;49:86. [Google Scholar]
- 29.Reader A, Nusstein J, Drum M. Successful local anesthesia for restorative dentistry and endodontics. Quintessence Publishing; 2017. Clinical Factors Related to Local Anesthesia; pp. 1–40. [Google Scholar]
- 30.Reader A, Nusstein J, Drum M. Successful local anesthesia for restorative dentistry and endodontics. Quintessence Publishing; 2017. Mandibular Anesthesia; pp. 41–91. [Google Scholar]
- 31.Parirokh M. Buffered Lidocaine With Sodium Bicarbonate did not Increase Inferior Alveolar Nerve Block Success Rate in Patients Having Symptomatic Irreversible Pulpitis. J Evid Based Dent Pract. 2016;16:59–61. doi: 10.1016/j.jebdp.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Aulestia-Viera PV, Braga MM, Borsatti MA. The effect of adjusting the pH of local anaesthetics in dentistry: a systematic review and meta-analysis. Int Endod J. 2018 Jan 29; doi: 10.1111/iej.12899. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor D, Green S, Higgins J. Cochrane handbook for systematic reviews of interventions. 2008. Defining the review question and developing criteria for including studies; pp. 81–94. Cochrane book series. [Google Scholar]