Abstract
Background and Purpose
The presence of dysphagia and aspiration in stroke patients is associated with increased mortality and morbidity. Early recognition and management of these two conditions via reliable, minimally invasive bedside procedures before complications arise remains challenging in everyday clinical practice. This study reviews the available bedside screening tools for detecting swallowing status and aspiration risk in acute stroke by qualitatively observing reference population study design, clinical flexibility, reliability and applicability to acute-care settings.
Methods
The primary search was conducted using the PubMed, Embase, and Cochrane Library databases. The search was limited to papers on humans written in English and published from 1991 to 2016. Eligibility criteria included the consecutive enrollment of acute-stroke inpatients and the development of a protocol for screening aspiration risk during oral feeding in this population.
Results
Of the 652 sources identified, 75 articles were reviewed in full however, only 12 fulfilled the selection criteria. Notable deficiencies in most of the bedside screening protocols included poor methodological designs and inadequate predictive values for aspiration risk which render clinicians to be more conservative in making dietary recommendations.
Conclusions
The literature is dense with screening methods for assessing the presence of dysphagia but with low predictive value for aspiration risk after acute stroke. A standard, practical, and cost-effective screening tool that can be applied at the bedside and interpreted by a wide range of hospital personnel remains to be developed. This need is highlighted in settings where neither trained personnel in evaluating dysphagia nor clinical instrumentation procedures are available.
Keywords: acute stroke, aspiration risk, oropharyngeal dysphagia, screening, swallowing
INTRODUCTION
Stroke ranks as the third leading cause of death1,2 and has a staggering human and economic toll.3 There are 1.8 million new cases of stroke in the European Union annually.4,5 The high prevalence of stroke might be at least partially attributable to continued record levels of cigarette smoking6 and to the lack of unified prevention and treatment planning policies.
Dysphagia is commonly observed after a cerebrovascular accident (CVA),7 and if not recognized early, it may lead to aspiration of saliva, food, and/or fluid entering the airway below the level of the vocal cords and into the trachea.8 The incidence of oropharyngeal dysphagia exceeds 50% within the first 72 hours after stroke.9 Dysphagia is transient and resolves spontaneously for many acute-stroke survivors,10,11 while about 50% of patients with persisting dysphagia will show slower recovery and will typically resort to alternative methods of nutrition for some time following stroke.12
Despite the propensity for recovering after acute stroke, the presence of dysphagia carries a 12-times greater risk of pulmonary complications such as aspiration pneumonia,13 an infection that increases the catabolic condition of the patient14 and is associated with the highest attributable mortality rate of all medical complications following a CVA.2,15,16 Several studies have further revealed significant relationships between dysphagia, malnutrition, and dehydration, especially in patients who receive thickened liquids or modified diets.17,18 The limited ability of patients to achieve a safe and adequate oral intake is linked to longer hospital stays19 and an increased likelihood of being discharged to long-term care facilities, which further adversely affects patient outcome.20
Managing dysphagia early in the stroke care pathway is of international concern, and so worldwide clinical guidelines mandate systematic bedside swallowing screening within the first few hours of admission to the hospital and prior to offering any oral food, fluid, or medication.21,22,23 The current lack of a universally acceptable and validated screening protocol has meant that various bedside methodologies are applied in clinical settings. When available, the use of diagnostic imaging methods for evaluating dysphagia and aspiration after stroke provides additional evidence for treatment planning. However, significant patient-specific limitations apply to instrumental diagnostic tests, such as the inability to comply with instructions due to poor medical or cognitive-communicative state, which render them inappropriate to use for all patients in the acute phase of stroke.24 Moreover, many nursing homes and other facilities do not have access to diagnostic imaging for dysphagia. In several acute-care settings across the world, there are notable organizational and procedural limitations to applying these diagnostic tests, since clinical expertise is required to perform and interpret these tests and specialized equipment might not be readily available at all locations.24,25 These conditions may delay assessments for days, having clear implications for patients associated with an increased likelihood of malnutrition and susceptibility to nosocomial infection and medical complications. Thus, a clinical bedside screening tool is needed for identifying the swallowing status and aspiration risk of the acute-stroke patient.
There remains a need to find simple, valid, and clinically useful bedside screening procedures that will correctly estimate the presence of aspiration risk associated with post stroke dysphagia.26 This is especially true in countries where stroke care is still evolving, instrumentation by a qualified speech language pathologist (SLP), otorhinolaryngologist (ENT), or radiology team is lacking, and judgements are made by clinicians without sufficient training to assess the swallowing status of patients and determine appropriate nutritional plans. A standard bedside assessment of aspiration risk may lead to more clinically focused treatment, improved patient outcomes, and a reduction in unnecessary incurring costs.27
In this study we endeavored to review all bedside methodologies designed for clinical use by a range of health-care professionals and to critically evaluate already established practices for assessing aspiration risk following acute stroke.
METHODS
An electronic search was performed of the Medline (PubMed), Embase, and Cochrane databases from 1991 to 2016 for studies on humans reported in English for which the full text was available. The following search terms were applied to the medical subject headings: (stroke OR cerebrovascular accident) AND (dysphagia OR swallowing OR aspiration) AND (screening OR assessment). Other potentially relevant papers were identified for full-text review from the reference lists of selected articles and from online searches of the tables of contents during the same period. Two of the authors independently completed the full article reviews to verify their inclusion, with disagreements resolved by consensus-based discussion among the review authors.
Findings of the literature search
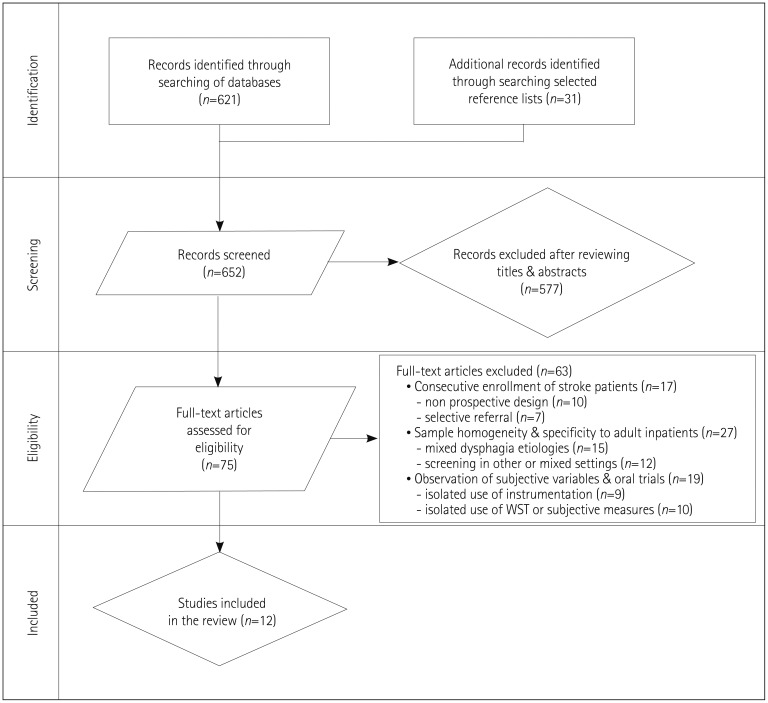
Of the 652 articles retrieved, 75 original articles pertained to screening oropharyngeal swallowing impairments and aspiration risk following an acute ischemic CVA (Fig. 1). The inclusion criteria were fulfilled by only 12 articles. Information on sample size, length of training, and overall administrative burden on the clinician were noted but not required for inclusion.
Fig. 1. Flow chart of study inclusion. WST: water swallowing test.
Selection criteria
Eligibility criteria included originally validated studies involving 1) the consecutive enrollment of acute-stroke patients with or without suspected dysphagia, 2) the specificity of the bedside tool for adult stroke survivors in an inpatient care unit, and 3) the combined use of subjective non-swallowing variables with subsequent food or liquid trials for estimating a patient's swallowing risk. Accordingly, papers were eliminated from full review if 1) the study did not have a prospective design, 2) the described assessment or screening protocols were administered to a heterogeneous sample of adults with confirmed or suspected dysphagia with different neurogenic etiologies, and 3) the bedside screening procedures did not entail some form of direct swallowing stimuli for determining swallowing integrity and predicting whether the patients had a high or low risk of aspiration during food and/or liquid intake.
The following exclusion criteria were applied: 1) failure to explicitly state an appraisal of swallowing status or aspiration risk after the bedside screening protocol, 2) main outcome via the bedside screening test other than the early detection of a aspiration and/or dysphagia, 3) sole use of oral trials for determining aspiration risk without the use of other clinical identifiers, 4) sole use of subjective clinical indicators without subsequent bolus trials, 5) sole use of instrumental methods for detecting dysphagia and aspiration, 6) highly heterogeneous patient samples with other kinds of neurogenic etiologies besides confirmed stroke, and 7) samples drawn exclusively from a rehabilitation setting, nursing home setting, or mixed inpatient and outpatient setting, since these were not considered patients with an acute risk of aspiration.
Articles meeting the inclusion criteria were evaluated for methodological rigor (as presented in Table 1) using diagnostic study appraisal criteria from the Centre for Evidence-Based Medicine (http://www.cebm.net/critical-appraisal/) and the Quality Assessment for Diagnostic Accuracy of Studies-2 (QUADAS-2) tool, which are recommended by Cochrane.28 These criteria were modified for consistency with the present study focusing on patients with stroke using clinical features associated with swallowing risk and a reference test. The criteria were rated as either ‘yes’ or ‘no’ if sufficient information was provided, or ‘unclear’ if the information was insufficient.
Table 1. Quality assessment measures in the studies reviewed.
| Study | Was selection bias minimized? | Did the study avoid inappropriate exclusions? | Was an index clinical test used? | Did all patients complete a reference standard test? | Was an appropriate protocol used? | Was there an acceptable interval between the index and reference tests? | Were the reference test results interpreted while blinded to those of the clinical test? | Were psychometric analysis data adequately reported? | Were accuracy measures adequately interpreted? | Was the description of the study protocol sufficient to allow replication? | Number of criteria met |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leigh et al.41 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 6 |
| Edmiaston et al.33 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Antonios et al.36 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Turner-Lawrence et al.30 | U | 0 | 1 | 0 | U | 1 | 1 | 0 | 0 | 0 | 3 |
| Trapl et al.32 | 1 | U | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ramsey et al.29 | 0 | 0 | 1 | 1 | 1 | U | 1 | 1 | 0 | 1 | 6 |
| Nishiwaki et al.40 | 0 | 1 | 1 | 1 | 1 | 1 | U | 1 | 0 | 1 | 7 |
| Leder & Espinosa35 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Lim et al.34 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Smith et al.31 | 1 | 0 | 1 | 1 | U | 0 | 1 | 1 | 1 | 0 | 6 |
| Daniels et al.38 | 1 | 1 | 1 | 1 | 1 | U | 1 | 1 | 0 | 1 | 8 |
| Smithard et al.37 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 8 |
1 indicates criteria met, 0 indicates criterion not met, U indicates unclear whether the criterion was met (assigned a final score of 0).
RESULTS
The results from the methodological appraisal of the included studies are presented in Table 1. None of the 12 articles were consistent with all ten quality-analysis measures. The need for informed consent and the ability to cooperate with bedside or instrumental assessment procedures resulted in a high rate of exclusion in some studies29,30,31 and a bias toward patients with mild-to-moderate strokes. Five of the studies32,33,34,35,36 conformed with nine of the methodological-rigor measures, while a study involving the hyper-acute stroke phase and emergency-room physicians30 conformed to only three of them. The procedures in two studies30,31 were not described in sufficient detail or with sufficient clarity to allow their replication.
Bedside evaluation tools included different non-swallowing stimuli, such as medical history information, subjective variables,33,35,36,37 oral motor measures,29,35,36,37,38,39,40 oxygen desaturation recordings,30,31,34 and scores on neurological scales such as the National Institutes of Health Stroke Severity Scale or the Glasgow Coma Scale.33 For the purposes of this review, all of the bedside tools that were investigated in detail utilized some form of direct oral trial such as either a water swallowing test (WST) and/or a bolus swallowing test (BST) with multiple or alternative oral (per os) intake consistencies administered in varied volumes. Almost half of the included studies (42%)32,36,37,40,41 incorporated a preliminary assessment of patient's dry (saliva) swallowing ability prior to administering swallowing trials involving boluses with other textures or specifically measured for swallowing reflex ability. One study used small aliquots of diluted radiopaque contrast agent and looked for signs of aspirated contrast in chest radiography.29
Four of the reviewed studies involved drinking water in gradually increasing volumes ranging from 3 mL to a 90-mL sequential drinking task based on the patient's initial tolerance to smaller volumes.32,37,39,41 Each subtest was typically terminated if a patient exhibited any overt sign of swallowing difficulty, aspiration, or voice-quality compromise. Seven studies (58%) utilized a single volume of water in combination with other subjective clinical data in order to determine the swallowing integrity, aspiration, or dysphagia risk of each patient30,31,33,34,36,40,41 and their eligibility to receive oral nutrition at the time of assessment. One study35 incorporated single sips of water boluses administered via a straw, and two studies32,38 added swallowing of different bolus types: semisolids, solids, and liquids.
The clinical bedside screening test was completed before the diagnostic reference test in all studies. More than half of the studies (58%) used videofluoroscopy (VFSS) as the reference diagnostic test,29,31,33,37,38,40,41 while 25%32,34,35 used videoendoscopy [fiberoptic endoscopic evaluation of swallowing (FEES)]. In the study conducted by Antonios and colleagues,36 subsequent validation was conducted by an SLP who performed the Mann Assessment of Swallowing Ability42 while the results from the Emergency Physician Screening tool30 were compared with those from an unspecified standardized dysphagia assessment performed by an expert SLP. Blinding of clinical results from the health-care professional completing the diagnostic reference test was reported for all studies except for that conducted by Leigh and colleagues.41 The time frame between the two assessments was reported for all of the studies, but four studies used instrumental techniques with a delay of >1 day between the bedside screening and the diagnostic test (ranging from a few days to several weeks poststroke).31,38,40,41
Across all of the included studies (as listed in Table 2), the sensitivities of the procedures described for detecting dysphagia ranged from as low as 54.6%41 to as high as 100%,32 while their specificities exhibited less variability, ranging from 66%33 to 86.3%.36 The sensitivities of the tests for identifying aspiration risk ranged from 65.2%41 to 100%,32 and their specificities ranged from 30%35 to 84.4%.38 Eight studies used aspiration or the risk of aspiration as the outcome measure,29,31,32,34,35,37,38,40 one study solely used dysphagia as the outcome measure,30 and the remaining studies used both aspiration and dysphagia measures.33,36,41
Table 2. Validity measures in the included studies.
| Research study and protocol name (index test) | Descriptive measures and test components | Criterion standard | Main outcome | Psychometric analysis data |
|---|---|---|---|---|
| Leigh et al.41 | Check mental status and ability to open the mouth | VFSS | Dysphagia | Sensitivity=54.6% |
| Specificity=80.9% | ||||
| PPV=75.7% | ||||
| NPV=62.1% | ||||
| +LR (95% CI)=2.86% | ||||
| Bedside swallowing screening test | Dry and wet swallowing tests (20-mL WST) | Aspiration risk | Sensitivity=65.2% | |
| Descriptive three-point scale classifying the aspiration risk | Specificity=71.4% | |||
| PPV=42.9% | ||||
| NPV=86.2% | ||||
| +LR (95% CI)=2.28% | ||||
| Edmiaston et al.33 | Four screening items: mental status (Glasgow Coma Scale score <13), and presence of facial, tongue, or palatal asymmetry or weakness | VFSS | Dysphagia | Sensitivity=94% |
| Specificity=66% | ||||
| PPV=71% | ||||
| NPV=93% | ||||
| Barnes Jewish Hospital Stroke Dysphagia Screen | Subjective signs of aspiration on 90-mL WST | Aspiration | Sensitivity=95% | |
| Specificity=50% | ||||
| PPV=41% | ||||
| NPV=96% | ||||
| Antonios et al.36 | Physician-weighted screening of 12 items: alertness, cooperation, respiration, expressive dysphasia, auditory comprehension, dysarthria, saliva, tongue movement, tongue strength, gag reflex, voluntary cough, and palate movement (maximum score=100) | Evaluation of dysphagia by SLPs using the MASA42 | Dysphagia | Sensitivity=92.6% |
| Specificity=86.3% | ||||
| PPV=79% | ||||
| NPV=95% | ||||
| Modified MASA | Aspiration | Sensitivity=93% | ||
| Specificity=53% | ||||
| Turner-Lawrence et al.30 | Two-tier bedside assessment: | Formal swallowing evaluation by SLP | Dysphagia | Sensitivity=96% |
| Specificity=56% | ||||
| 1) voice quality, swallowing complaints, facial asymmetry, and aphasia; and | +LR=2.2% | |||
| Emergency Physician Swallowing Screen | 2) Signs of aspiration on WST and observation of pulse oximetry desaturation (≥2%) | |||
| Trapl et al.32 | Preliminary assessment/indirect swallowing test: vigilance, throat clearing, and SST | FEES (using the Penetration Aspiration Scale) | Aspiration risk (grouped according to the Penetration Aspiration Scale)48 | First group (n=19) |
| Sensitivity=100% | ||||
| Specificity=50% | ||||
| PPV=81% | ||||
| NPV=100% | ||||
| The Gugging Swallowing Screen | Subsequent direct swallowing trials with three bolus types: semisolids, liquids, and solids | Second group (n=30) | ||
| Sensitivity=100% | ||||
| Specificity=69% | ||||
| PPV=74% | ||||
| NPV=100% | ||||
| Ramsey et al.29 | Oral motor function examination | VFSS | Aspiration/unsafe swallowing | Failed MBSA±oxygen desaturation >2% |
| Observation after three 5-mL aliquots of diluted radiopaque contrast agent | Sensitivity=60% | |||
| Specificity=41% | ||||
| PPV=28% | ||||
| NPV=73% | ||||
| Modified bedside swallowing test | Simultaneous 10-min desaturation recordings | Failed MBSA±oxygen desaturation >5% | ||
| Sensitivity=53% | ||||
| Specificity=67% | ||||
| PPV=38% | ||||
| NPV=79% | ||||
| Nishiwaki et al.40 | Six oral motor items: lip closure, tongue movement, palatal elevation, gag reflex, voice quality, and motor speech function | VFSS | Aspiration | Sensitivity=72% |
| Modified screening tool | Two swallowing screening tests: SST and 30-mL WST | Specificity=67% (for cough/voice change in WST) | ||
| Leder & Espinosa35 | Bedside evaluation with six clinical identifiers: dysphonia, dysarthria, abnormal gag reflex, abnormal volitional cough, and voice change after swallowing | FEES | Aspiration risk | Sensitivity=86% |
| Specificity=30% | ||||
| PPV=50% | ||||
| Clinical bedside examination | Per-os trials of single sips of water boluses via straw | NPV=73% | ||
| Lim et al.34 | 50-mL WST (in 10-mL aliquots) and pulse oximetry recordings before and after each 10-mL WST (≥2% desaturation was clinically significant) | FEES | Aspiration | WST and oxygen desaturation test combined: |
| Sensitivity=100% | ||||
| Specificity=70.8% | ||||
| PPV=78.8% | ||||
| NPV=100% | ||||
| Bedside aspiration test | Monitoring for evidence of aspiration pneumonia | Aspiration pneumonia risk | RR if evidence of aspiration on FEES=1.24 (1.03<RR<1.49) | |
| Smith et al.31 | Subjective evaluation of swallowing physiology at rest and on swallowing various quantities and consistencies (not clearly outlined) | VFSS | Aspiration risk | Bedside examination and oxygen desaturation ≥2% |
| Sensitivity=73% | ||||
| Specificity=76% | ||||
| PPV=55% | ||||
| Clinical bedside examination | NPV=88% | |||
| Daniels et al.38 | Oral motor examination 70-mL WST in small ordinal aliquots and clinical swallowing trial with semisolids and solids | VFSS | Aspiration risk | Stepwise logistic regression highlighted two of six predictor variables: abnormal volitional cough and cough with swallowing combined |
| Sensitivity=69.6% | ||||
| Bedside swallowing examination | Specificity=84.4% | |||
| Smithard et al.37 | Medical bedside assessment: consciousness level, head and trunk control, breathing pattern, lip closure, palate movement, laryngeal function, gag reflex, and voluntary cough | VFSS | Aspiration | Multiple logistic regression analysis revealed two independent predictors of aspiration: impairment of consciousness level and weak voluntary cough |
| Sensitivity=75% | ||||
| Specificity=72% | ||||
| Signs of aspiration during WST (three 5-mL aliquots followed by 60-mL challenge if passed) | PPV=41% | |||
| NPV=91% | ||||
| Clinical judgement by SLP |
CI: confidence interval, FEES: fiberoptic endoscopic evaluation of swallowing, MASA: Mann Assessment of Swallowing Ability, MBSA: modified bedside swallowing assessment, NPV: negative predictive value, PPV: positive predictive value, RR: relative risk, SLP: speech language pathologist, SST: saliva swallowing test, VFSS, videofluoroscopy, WST, water swallowing test, +LR: positive likelihood ratio.
DISCUSSION
Dysphagia with or without aspiration is highly prevalent after stroke and is associated with increased nutritional deficits and pneumonia risk. Worldwide stroke guidelines advise the early recognition and management of dysphagia in hospitalized patients in order to reduce its devastating sequelae. Initial bedside swallowing screening remains the cornerstone of routine clinical practice, but the heterogeneity of the existing screening protocols reflects that a consensus has yet to be established.
The quality of the articles included in the methodological analysis varied. Although all quality measures are important for developing a highly valid and reliable tool that can detect the swallowing status and aspiration risk following acute stroke, the exact relevance of specific quality criteria such as a short interval between assessments and the ability to replicate administration protocols may vary according to the needs of the facilities developing swallowing screening tests. Aspiration or aspiration risk was the primary outcome in the vast majority of the studies. However, a patient may present with significant dysphagia without aspiration43 that may also lead to a reduced nutritional status and lower quality of life.44 One study34 also followed up on the hospital stay of the patients for evidence of aspiration pneumonia, which was not performed in any of the other studies. It appears that a screening tool needs to be able to detect both the swallowing status and aspiration risk with high sensitivity and specificity, and be correlated with clinical outcomes such as pneumonia. In addition, the tool should be sufficiently simple to allow it to be implemented by the hospital personnel assigned to assess and care for stroke patients.
Significant variability also existed in the components used to screen for swallowing and aspiration risk across the studies. Non-swallowing measures included medical history items such as the presence of pneumonia, assessment of mental status, speech and language deficits, and oral motor categorical items such as unilateral jaw weakness, tongue strength, gag reflex, secretion management, and volitional cough. However, not all of these items have been demonstrated to be strong predictors of aspiration. McCullough et al.39 examined the utility of non-swallowing bedside indicators and trial swallowing measures in detecting aspiration. Sound methodology and lengthy statistical recording for each measure were noted, but they reported low sensitivities for each measure individually, leading a clinician to the conclusion that the presence of two measures is much more meaningful than their absence. Regression analysis demonstrated that the best factors in the model for detecting aspiration overall were the failure of the 90-mL WST and dysphonia.
The ability to sustain adequate alertness level for a short period of time appears to be a prerequisite for direct oral trials. Furthermore, testing that a patient can actually swallow should be included in any screening tool, but the optimal method of assessment still requires investigation. Most of the studies included a WST, but the volumes administered varied over a very wide range (from 3 mL to 90 mL), and the number of trials also varied in some studies. Research has shown that silent aspiration is volume-dependent,45 and thus one concern with the bedside administration of small bolus volumes for determining aspiration risk is that the absence of overt behavioral signs such as a reflexive cough can lead to high false-negative rates. Meanwhile, there is a need to determine the optimal trade-off between assessing the swallowing ability of patients and their swallowing safety. The safety of requiring an acute-stroke patient to self-administer 90 mL of water without stopping is questionable when research has shown poor awareness of swallowing deficits in the acute phases of stroke, with most patients consuming larger volumes of water more rapidly.46
Several of the investigators used pulse oximetry in conjunction with bedside swallowing trials to determine the presence of an aspiration risk25,30,31,34 but there are conflicting data of its usefulness.29,47 Lim and colleagues34 measured oxygen desaturation 10 minutes after applying a modified WST that involved small equal aliquots. A bedside procedure that simultaneously applies a sequential drinking task and pulse oximetry measurements may provide more meaningful results while maintaining the test-retest reliability. Equally controversial findings from small subject groups were found by Smith and colleagues31 Although they used a combination of bedside screening and oxygen desaturation testing, they did not clearly report on their bedside assessment procedure, making it almost impossible to utilize their research in clinical practice.
Most of the studies used an instrumental reference test (VFSS or FEES) to objectively confirm the presence of dysphagia or aspiration. There is support in the literature for the need to routinely assess the swallowing function of patient in the acute phase of stroke using a diagnostic test (when this is readily available) and when the patient is able to sit up and cooperate with the procedure. However, few studies applied the reference standard test and the clinical index screening test within a few days of each other, which represents an unacceptable delay in this patient population given that the spontaneous recovery typically occurs rapidly.
The necessity for identifying post-stroke dysphagia and aspiration early in the care pathway of a patient indicates that frontline medical professionals who are the first to make contact with the patient after a CVA need to apply swallowing screening. Several studies have highlighted interdisciplinary dysphagia screening,30,32,33,34 but this had limitations associated with the poorly defined screening procedures making it difficult to integrate them into clinical practice. It is clear from the existing literature that the reported statistical data can be influenced by whether patients are selectively referred due to probable dysphagia or whether they are consecutively recruited into a research study. The selection of different swallowing and non-swallowing features in the evaluation process and their perceived importance in identifying dysphagia and aspiration as well as the significant variability in the volumes and consistencies of boluses applied as direct swallowing stimuli at the bedside can further lead to discrepant assumptions.
Consistent empirical evidence is required to achieve best practice for swallowing screens. The absence of a consensus on the best screening methodology should not be interpreted as “no screening should be performed” or that it is a “one fits all” process. Broader patient-specific and facility-specific factors should be taken into account before making any recommendation regarding oral nourishment.
CONCLUSION
Stroke is the leading cause of neurogenic dysphagia and medical professionals in the acute situation rely on a bedside screening test to determine the swallowing status. How instrumental examinations are organized may present logistics problems at locations worldwide where their routine use is not possible. Screening for dysphagia and aspiration is important to reduce negative outcomes, decrease hospital re-admissions for pneumonia, and to expedite the safe nutritional management of patients in the acute phase of recovery. Although numerous screening tools have been developed, no present screening protocol provides high specificity and sensitivity for predicting the risk of aspiration. It appears that a cluster of swallowing and non-swallowing features may achieve both high sensitivity and specificity at the bedside.
Acknowledgements
The authors would like to express their deep gratitude to Dr. Thomas Murry, PhD, CCC-SLP for his thoughtful comments on the content of this manuscript.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136:784–789. doi: 10.1001/archoto.2010.129. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong JR, Mosher BD. Aspiration pneumonia after stroke: intervention and prevention. Neurohospitalist. 2011;1:85–93. doi: 10.1177/1941875210395775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Lobban T, Knight E, Wait S, Harding E. The Route Map for Change & the European Atlas on the Prevention of AF-Related Stroke. London: The Health Policy Partnership; 2014. [Google Scholar]
- 5.OECD/EU. Health at a Glance: Europe 2010. Paris: OECD Publishing; 2010. [Google Scholar]
- 6.Health and Social Care Information Centre (GB) Statistics on Smoking, England: 2016. London: Health and Social Care Information Centre; 2016. [Google Scholar]
- 7.Flowers HL, Silver FL, Fang J, Rochon E, Martino R. The incidence, co-occurrence, and predictors of dysphagia, dysarthria, and aphasia after first-ever acute ischemic stroke. J Commun Disord. 2013;46:238–248. doi: 10.1016/j.jcomdis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Wieseke A, Bantz D, Siktberg L, Dillard N. Assessment and early diagnosis of dysphagia. Geriatr Nurs. 2008;29:376–383. doi: 10.1016/j.gerinurse.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Ickenstein GW, Höhlig C, Prosiegel M, Koch H, Dziewas R, Bodechtel U, et al. Prediction of outcome in neurogenic oropharyngeal dysphagia within 72 hours of acute stroke. J Stroke Cerebrovasc Dis. 2012;21:569–576. doi: 10.1016/j.jstrokecerebrovasdis.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Smithard DG, O'Neill PA, England RE, Park CL, Wyatt R, Martin DF, et al. The natural history of dysphagia following a stroke. Dysphagia. 1997;12:188–193. doi: 10.1007/PL00009535. [DOI] [PubMed] [Google Scholar]
- 11.Okubo PC, Fábio SR, Domenis DR, Takayanagui OM. Using the National Institute of Health Stroke Scale to predict dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2012;33:501–507. doi: 10.1159/000336240. [DOI] [PubMed] [Google Scholar]
- 12.Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- 13.Wirth R, Smoliner C, Jäger M, Warnecke T, Leischker AH, Dziewas R DGEM Steering Committee. Guideline clinical nutrition in patients with stroke. Exp Transl Stroke Med. 2013;5:14. doi: 10.1186/2040-7378-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaker R, Geenen JE. Management of dysphagia in stroke patients. Gastroenterol Hepatol (N Y) 2011;7:308–332. [PMC free article] [PubMed] [Google Scholar]
- 15.Gosney MA, Martin MV, Wright AE, Gallagher M. Enterobacter sakazakii in the mouths of stroke patients and its association with aspiration pneumonia. Eur J Intern Med. 2006;17:185–188. doi: 10.1016/j.ejim.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G Canadian Stroke Network; Stroke Outcome Research Canada (SORCan) Working Group. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 17.Finestone HM, Greene-Finestone LS, Wilson ES, Teasell RW. Malnutrition in stroke patients on the rehabilitation service and at follow-up: prevalence and predictor. Arch Phys Med Rehabil. 1995;76:310–316. doi: 10.1016/s0003-9993(95)80655-5. [DOI] [PubMed] [Google Scholar]
- 18.Foley NC, Salter KL, Robertson J, Teasell RW, Woodbury MG. Which reported estimate of the prevalence of malnutrition after stroke is valid? Stroke. 2009;40:e66–e74. doi: 10.1161/STROKEAHA.108.518910. [DOI] [PubMed] [Google Scholar]
- 19.Yoo SH, Kim JS, Kwon SU, Yun SC, Koh JY, Kang DW. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. 2008;65:39–43. doi: 10.1001/archneurol.2007.12. [DOI] [PubMed] [Google Scholar]
- 20.Kammersgaard LP, Jørgensen HS, Rungby JA, Reith J, Nakayama H, Weber UJ, et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke. 2002;33:1759–1762. doi: 10.1161/01.str.0000019910.90280.f1. [DOI] [PubMed] [Google Scholar]
- 21.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence (GB) Stroke and transient ischaemic attack in over 16s: diagnosis and initial management [Internet] London: National Institute for Health and Care Excellence; 2008. [cited 2017 Aug 26]. Available from: http://nice.org.uk/guidance/cg68. [Google Scholar]
- 23.Intercollegiate Stroke Working Party (GB) National Clinical Guideline for Stroke. 4th ed. London: Royal College of Physicians; 2012. [Google Scholar]
- 24.Boaden E, Doran D, Burnell J, Clegg A, Dey P, Hurley M, et al. Screening for aspiration risk associated with dysphagia in acute stroke (diagnostic test accuracy protocol) Cochrane Database Syst Rev. 2017:CD012679. doi: 10.1002/14651858.CD012679.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey DJ, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34:1252–1257. doi: 10.1161/01.STR.0000066309.06490.B8. [DOI] [PubMed] [Google Scholar]
- 26.Schultheiss C, Nusser-Müller-Busch R, Seidl RO. The semisolid bolus swallow test for clinical diagnosis of oropharyngeal dysphagia: a prospective randomised study. Eur Arch Otorhinolaryngol. 2011;268:1837–1844. doi: 10.1007/s00405-011-1628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan NJ, Daniels SK, Edmiaston J, Weinhardt J, Summers D, Mitchell PH American Heart Association Council on Cardiovascular Nursing and Stroke Council. Dysphagia screening: state of the art: invitational conference proceeding from the State-of-the-Art Nursing Symposium, International Stroke Conference 2012. Stroke. 2013;44:e24–e31. doi: 10.1161/STR.0b013e3182877f57. [DOI] [PubMed] [Google Scholar]
- 28.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey DJ, Smithard DG, Kalra L. Can pulse oximetry or a bedside swallowing assessment be used to detect aspiration after stroke? Stroke. 2006;37:2984–2988. doi: 10.1161/01.STR.0000248758.32627.3b. [DOI] [PubMed] [Google Scholar]
- 30.Turner-Lawrence DE, Peebles M, Price MF, Singh SJ, Asimos AW. A feasibility study of the sensitivity of emergency physician Dysphagia screening in acute stroke patients. Ann Emerg Med. 2009;54:344–348. doi: 10.1016/j.annemergmed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Smith HA, Lee SH, O'Neill PA, Connolly MJ. The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age Ageing. 2000;29:495–499. doi: 10.1093/ageing/29.6.495. [DOI] [PubMed] [Google Scholar]
- 32.Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38:2948–2952. doi: 10.1161/STROKEAHA.107.483933. [DOI] [PubMed] [Google Scholar]
- 33.Edmiaston J, Connor LT, Steger-May K, Ford AL. A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23:712–716. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim SH, Lieu PK, Phua SY, Seshadri R, Venketasubramanian SH, et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16:1–6. doi: 10.1007/s004550000038. [DOI] [PubMed] [Google Scholar]
- 35.Leder SB, Espinosa JF. Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17:214–218. doi: 10.1007/s00455-002-0054-7. [DOI] [PubMed] [Google Scholar]
- 36.Antonios N, Carnaby-Mann G, Crary M, Miller L, Hubbard H, Hood K, et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the modified Mann Assessment of Swallowing Ability. J Stroke Cerebrovasc Dis. 2010;19:49–57. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Smithard DG, O'Neill PA, Park C, England R, Renwick DS, Wyatt R, et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998;27:99–106. doi: 10.1093/ageing/27.2.99. [DOI] [PubMed] [Google Scholar]
- 38.Daniels SK, Brailey K, Priestly DH, Herrington LR, Weisberg LA, Foundas AL. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79:14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- 39.McCullough GH, Rosenbek JC, Wertz RT, McCoy S, Mann G, McCullough K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. J Speech Lang Hear Res. 2005;48:1280–1293. doi: 10.1044/1092-4388(2005/089). [DOI] [PubMed] [Google Scholar]
- 40.Nishiwaki K, Tsuji T, Liu M, Hase K, Tanaka N, Fujiwara T. Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37:247–251. doi: 10.1080/16501970510026999. [DOI] [PubMed] [Google Scholar]
- 41.Leigh JH, Lim JY, Han MK, Bae HJ, Kim WS, Paik NJ. A prospective comparison between bedside swallowing screening test and videofluoroscopic swallowing study in post-stroke dysphagia. Brain Neurorehabil. 2016;9:e7 [Google Scholar]
- 42.Mann G. MASA: the Mann Assessment of Swallowing Ability. Clifton Park: Singular/Thomson Learning; 2002. [Google Scholar]
- 43.Martino R, Pron G, Diamant N. Screening for oropharyngeal dysphagia in stroke: insufficient evidence for guidelines. Dysphagia. 2000;15:19–30. doi: 10.1007/s004559910006. [DOI] [PubMed] [Google Scholar]
- 44.Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S Stroke Practice Improvement Network Investigators. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36:1972–1976. doi: 10.1161/01.STR.0000177529.86868.8d. [DOI] [PubMed] [Google Scholar]
- 45.Leder SB, Suiter DM, Green BG. Silent aspiration risk is volume-dependent. Dysphagia. 2011;26:304–309. doi: 10.1007/s00455-010-9312-2. [DOI] [PubMed] [Google Scholar]
- 46.Parker C, Power M, Hamdy S, Bowen A, Tyrrell P, Thompson DG. Awareness of dysphagia by patients following stroke predicts swallowing performance. Dysphagia. 2004;19:28–35. doi: 10.1007/s00455-003-0032-8. [DOI] [PubMed] [Google Scholar]
- 47.Leder SB. Use of arterial oxygen saturation, heart rate, and blood pressure as indirect objective physiologic markers to predict aspiration. Dysphagia. 2000;15:201–205. doi: 10.1007/s004550000028. [DOI] [PubMed] [Google Scholar]
- 48.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Woods JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]