Abstract
Background and Purpose
The risk of vitamin D deficiency varies with the season. The frequency of vitamin D deficiency in migraine patients and its association with migraine are unclear.
Methods
We retrospectively evaluated first-visit migraine patients between January 2016 and May 2017, and investigated the demographics, season, migraine subtypes, frequency, severity, and impact of migraine, psychological and sleep variables, climate factors, and vitamin D levels. The nonfasting serum 25-hydroxyvitamin D concentration was measured to determine the vitamin D level, with deficiency of vitamin D defined as a concentration of <20 ng/mL.
Results
In total, 157 patients with migraine aged 37.0±8.6 years (mean±standard deviation) were analyzed. Their serum level of vitamin D was 15.9±7.4 ng/mL. Vitamin D deficiency was present in 77.1% of the patients, and occurred more frequently in spring and winter than in summer and autumn (89.1%, 85.7%, 72.4%, and 61.7%, respectively; p=0.008). In multivariate Poisson regression analysis, monthly headache was 1.203 times (95% confidence interval=1.046–1.383, p=0.009) more frequent in patients with vitamin D deficiency than in those without deficiency after adjusting for demographics, season, migraine subtype, depression, anxiety, and sleep quality. These associations were consistently noted in subgroup analysis of episodic migraine (odds ratio=1.266, p=0.033) and chronic migraine (odds ratio=1.390, p=0.041).
Conclusions
Our study found that a larger number of monthly days with headache was related to vitamin D deficiency among migraineurs. Future studies should attempt to confirm the causal relationship between vitamin D deficiency and migraine.
Keywords: migraine, vitamin D, seasonal variation, summer, winter
INTRODUCTION
Vitamin D deficiency is associated with chronic pain disorder, depression, and several neurological disorders.1,2,3,4,5,6 The brain has an abundance of vitamin D receptors and there is evidence of a nonskeletal role of vitamin D in inflammation, immunity, and the metabolism of neurotransmitters.6,7,8 In addition, vitamin D has been associated with severe headache and is considered a potential prophylactic agent or adjuvant in the management of migraine.9,10,11
The serum level of vitamin D is related to sun exposure (as determined by latitude, outdoor physical activity, and sun-seeking or avoidance behavior), dietary intake, and genetic components.6,12 Migraineurs tend to avoid sunlight due to photophobia during an attack, and low physical activity and long working hours reportedly increase the risk of headache or migraine.13,14 The risks of both vitamin D deficiency and migraine have been shown to vary with latitude, suggesting the need to investigate the association of vitamin D deficiency with migraine frequency.15
Previous studies investigating vitamin D deficiency in migraine had limitations such as lack of adjustment for seasonal variation, climate, or other variables, and the absence of appropriate headache parameters.16,17,18 A cross-sectional study suggested that the vitamin D level is lower in migraine patients than in nonheadache controls among nonsmokers, but it did not evaluate the association of vitamin D levels with the severity of headache.19 Therefore, the association of vitamin D and migraine needs to be investigated using comprehensive headache parameters, seasonal consideration, and psychological comorbidities. The present study investigated the frequency of vitamin D deficiency in migraine patients and the relationship between vitamin D deficiency and headache parameters.
METHODS
Subjects
This study was conducted in the Department of Neurology at the University Hospital of Hwaseong, Korea, in an urban setting at latitude 37° N. The study performed a retrospective review of prospective headache registry records of patients who presented for the first time with complaints of headache between January 2016 and May 2017. The inclusion criteria were as follows: 1) migraine with aura, migraine without aura, chronic migraine, and probable migraine diagnosed according to the third edition of the International Classification of Headache Disorders (ICHD-3),20 2) aged 19–65 years, 3) detailed information available about the headache and psychological status obtained a self-administered questionnaire, and 4) measurement of the vitamin D level. The exclusion criteria were 1) the presence of secondary headaches due to causes other than medication overuse, 2) incomplete questionnaire data, 3) use of preventive medication for migraine, or 4) duration of more than 1 month between the vitamin D measurement and assessment of the migraine status.
The study protocol was reviewed and approved by the Dongtan Sacred Heart Hospital Institutional Review Board/Ethic Committee (IRB No. 2016-10-439) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants were kept fully informed during the clinical procedures. The Institutional Review Board waived the need to obtain informed consent due to this being a post-hoc analysis of collected clinical data and the guarantee of anonymity of personal data.
Demographics data and questionnaire
Migraine was diagnosed by an investigator based on clinical history, a neurological examination, and laboratory or imaging data, as appropriate. Migraine with aura, migraine without aura, and probable migraine were classified into episodic migraine. Demographics data including age and sex, monthly frequency of attacks, headache duration in hours, monthly days of taking abortive medication use, and the severity of pain [assessed using a Visual Analog Scale (VAS) ranging from 0 to 10] were collected and assessed. The applied questionnaires included Headache Impact Test-6 (HIT-6) to measure headache-related impact, Patient Health Questionnare-9 (PHQ-9) to assess depression, Generalized Anxiety Disorder-7 (GAD-7) to assess anxiety, and the Pittsburgh Sleep Quality Index (PSQI) to measure the quality of sleep.21,22,23,24
Climate factors
Detailed climate data for the catchment area of our study population were acquired from the Korea Meteorological Administration (www.kma.go.kr). In brief, monthly temperatures were defined as the air temperatures at 1.5 m above ground level in Korea. The monthly relative humidity refers to the ratio of the amount of water vapor in the air to the amount of water vapor at saturation at that temperature during the corresponding month. Precipitation was defined by the depth of water in the measuring equipment installed at the Korea Meteorological Administration. Total sunshine hours refer to sunlight shining on the ground without being obscured by clouds or fog.
Measurement and analysis of vitamin D
The serum 25-hydroxyvitamin D [25(OH)D] concentration is used to assess the vitamin D status. Nonfasting levels of serum 25(OH)D were measured using a chemiluminescence immunoassay (ARCHITECT i4000SR, Abbott Diagnostics, Abbott Park, IL, USA). The intra- and interassay coefficients of variation were 1.7–2.8% and 2.7–4.1%, respectively. The assay was standardized against NIST Standard Reference Material 2972 (NIST, Gaithersburg, MD, USA) and certified by the Centers for Diseases Control and Prevention Vitamin D Standardization Program.25 Vitamin D deficiency, insufficiency, and sufficiency were defined as <20, ≥20 and <30, and ≥30 ng/mL 25(OH)D, respectively.6,26 The month and year were recorded for when the vitamin D test was performed.
Statistical analysis
A descriptive analysis of vitamin D deficiency and insufficiency was carried out in absolute terms and according to season. The independent-samples t test was applied to continuous variables, and the chi-square test was used for categorical variables. The association of vitamin D deficiency with monthly headache frequency was analyzed using Poisson regression analysis after adjusting for demographics, migraine subtype, depression, anxiety, and sleep quality. A p value of <0.05 was considered statistically significant. All analyses were performed using the Statistical Package for the Social Sciences (Windows version 22.0, IBM Corp., Armonk, NY, USA).
RESULTS
Patients and clinical demographics
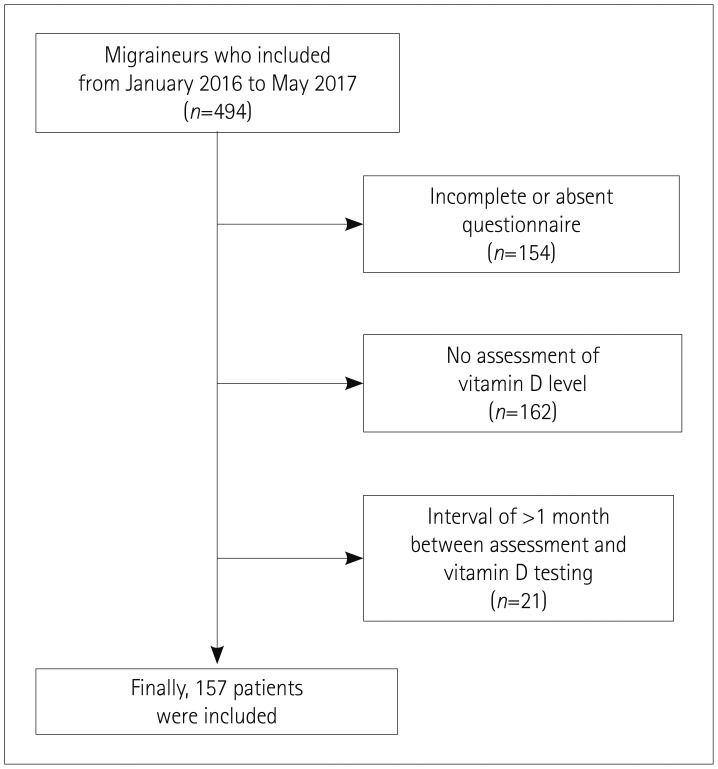
There were 494 adult patients with migraine who were registered in the headache records between January 2016 to May 2017, of which 154 had an incomplete response or absent questionnaire, 162 had no assessment of the vitamin D level, and 21 had an interval between the assessment and vitamin D testing of longer than 1 month, and so were excluded, resulting in 157 migraineurs being included in this study (Fig. 1). The sex distribution, number of days of migraine attacks per month, VAS score, and season distribution did not differ between the included and excluded patients, but the frequency of chronic migraine was higher and that of probable migraine was lower in the included patients (Supplementary Table 1 in the online-only Data Supplement).
Fig. 1. Flowchart outlining patient inclusion.
The 157 included patients were aged 37.0±8.6 years (mean±standard deviation), and 75.2% (118/157) of them were female. They were classified into migraine without aura (n=114), migraine with aura (n=8), chronic migraine (n=31), and probable migraine (n=4) according to the ICHD-3 classification. The vitamin D level was 15.9±7.4 ng/mL. The frequency of vitamin D deficiency was observed in 121 migraineurs (77.0%), and was more common in spring and winter than in summer and autumn (89.1%, 85.7%, 72.4%, and 61.7%, respectively; p=0.008). Based on a <30 ng/mL cutoff for serum vitamin D, 149 (94.9%) migraineurs were considered to have vitamin D insufficiency.
Comparison of demographics and clinical factors according to presence of vitamin D deficiency
The demographics, headache characteristics, and accompanying problems (HIT-6 score, PHQ-9 score, GAD-7 score, and PSQI) did not differ significantly between migraineurs with and without vitamin D deficiency, with the exception of the seasonal distribution. The number of monthly days with headache tended to be higher in patients with vitamin D deficiency than in those without deficiency (p=0.073) (Table 1). The temperature, relative humidity, and total sunshine hours of the months in which the vitamin D concentration was measured were lower in those with vitamin D deficiency than in those without deficiency (Table 1). When dichotomizing seasons into summer/autumn and winter/spring, the serum vitamin D level was negatively correlated with the HIT-6 score in summer/autumn (ρ=−0.239, p=0.037) but not in winter/spring (ρ=0.094, p=0.405).
Table 1. Comparison of demographics and clinical factors based on the presence or absence of vitamin D deficiency.
| Characteristic | Total (n=157) | Migraineurs with vitamin D deficiency (n=121)* | Migraineurs without vitamin D deficiency (n=36) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 36.9±8.6 | 36.8±0.1 | 37.6±7.5 | 0.593 |
| Females | 118 (75.2) | 94 (79.7) | 24 (66.7) | 0.179 |
| Season | 0.008 | |||
| Spring | 46 (29.3) | 41 (33.9) | 5 (13.9) | |
| Summer | 29 (18.5) | 21 (17.4) | 8 (22.2) | |
| Autumn | 47 (29.9) | 29 (24.0) | 18 (50.0) | |
| Winter | 35 (22.3) | 30 (24.8) | 5 (13.9) | |
| ICHD-3 classification | 0.406 | |||
| Migraine without aura | 114 (72.6) | 89 (73.6) | 25 (69.4) | |
| Migraine with aura | 8 (5.1) | 5 (4.1) | 3 (8.3) | |
| Chronic migraine | 31 (19.7) | 25 (20.7) | 6 (16.7) | |
| Probable migraine | 4 (2.5) | 2 (1.7) | 2 (5.6) | |
| Headache characteristics | ||||
| Monthly days with headache | 9.0±7.4 | 9.5±7.8 | 7.3±5.9 | 0.073 |
| Attack duration, hours | 39.5±49.3 | 40.9±53.1 | 34.6±33.3 | 0.753 |
| Medication, days/month | 4.6±6.3 | 5.0±6.9 | 3.5±3.3 | 0.712 |
| VAS score | 7.0±1.7 | 7.1±1.8 | 6.8±1.7 | 0.242 |
| Accompanying problems | ||||
| HIT-6 score | 61.2±7.3 | 61.5±7.6 | 60.6±6.8 | 0.554 |
| PHQ-9 score | 7.2±5.1 | 7.4±5.2 | 6.7±4.7 | 0.552 |
| GAD-7 score | 6.1±5.1 | 6.4±5.3 | 5.1±4.6 | 0.27 |
| PSQI | 8.7±3.8 | 9.0±3.8 | 8.1±4.3 | 0.224 |
| Climate factors, monthly | ||||
| Temperature, ℃ | 13.6±9.7 | 12.4±9.6 | 17.6±9.2 | 0.006 |
| Relative humidity, % | 67.8±6.9 | 66.7±6.6 | 71.6±6.3 | 0.001 |
| Precipitation, mm | 78.0±76.1 | 72.7±72.6 | 95.7±85.7 | 0.113 |
| Total sunshine hours | 189.8±36.4 | 187.0±35.1 | 199.3±39.6 | 0.075 |
Data are mean±standard deviation or n (%) values.
*Vitamin D deficiency: serum total 25-hydroxyvitamin D <20 ng/mL.
GAD-7: Generalized Anxiety Disorder-7, HIT-6: Headache Impact Test-6, ICHD-3: third edition of the International Classification of Headache Disorders, PHQ-9: Patient Health Questionnaire-9, PSQI: Pittsburgh Sleep Quality Index, VAS: Visual Analog Scale.
Factors associated with monthly headache frequency
In multivariate Poisson regression analysis, monthly headache was 1.203 times [95% confidence interval (CI)=1.046–1.383, p=0.009] more frequent in patients with vitamin D deficiency than in those without deficiency after adjusting for age, sex, and variables with p<0.1 in the univariate analysis [season (winter/spring vs. summer/autumn), migraine subtype (chronic vs. episodic), depression (PHQ-9 score), anxiety (GAD-7 score), and sleep quality (PSQI)]. Chronic migraine was more strongly associated than episodic migraine with the number of monthly days with headache [odds ratio (OR)=2.562, 95% CI=2.292–2.864, p<0.001] (Table 2).
Table 2. Analysis of contributing factors related to the monthly frequency of headache in patients with migraine.
| Factor | Exponential (B) (95% CI) | p* |
|---|---|---|
| Age | 1.001 (0.995–1.007) | 0.774 |
| Sex, female | 0.960 (0.851–1.084) | 0.511 |
| Season, winter/spring† | 1.078 (0.966–1.204) | 0.181 |
| Chronic migraine‡ | 2.562 (2.292–2.864) | <0.001 |
| PHQ-9 score | 1.005 (0.988–1.023) | 0.561 |
| GAD-7 score | 1.001 (0.986–1.017) | 0.886 |
| PSQI | 1.011 (0.993–1.029) | 0.236 |
| Vitamin D <20 ng/mL | 1.203 (1.046–1.383) | 0.009 |
The goodness of fit of our model in the Poisson regression analysis was confirmed by a p value of 0.001 in the Omnibus test.
*Results of multivariate Poisson regression analysis of monthly headache frequency as the dependent variable, †Summer/autumn as reference, ‡Episodic migraine as reference.
GAD-7: Generalized Anxiety Disorder-7, PHQ-9: Patient Health Questionnaire-9, PSQI: Pittsburgh Sleep Quality Index.
Comparison of demographics and clinical data according to sex and chronicity
Vitamin D deficiency was observed more frequently in females (79.7%) than in males (69.2%), although the difference was not statistically significant (p=0.179). In subgroup analysis, males with vitamin D deficiency tended to have higher VAS and PSQI scores than those without deficiency (Table 3). In subgroup multivariate analysis after adjusting for age, season (winter/spring vs. summer/autumn), and migraine subtype (chronic vs. episodic), vitamin D deficiency was independently associated with more monthly days with headache in females (OR=1.220, 95% CI=1.026–1.451, p=0.024) but not in males (OR=1.123, 95% CI=0.866–1.456, p=0.383).
Table 3. Comparison of demographics and clinical factors according to vitamin D deficiency in males and females.
| Characteristic | Males (n=39) | Females (n=118) | ||
|---|---|---|---|---|
| Migraineurs with vitamin D deficiency (n=27) | Migraineurs without vitamin D deficiency (n=12) | Migraineurs with vitamin D deficiency (n=94) | Migraineurs without vitamin D deficiency (n=24) | |
| Demographics | ||||
| Age, years | 37.4±8.7 | 35.0±7.7 | 36.6±9.0 | 38.9±7.1 |
| Season | ||||
| Spring | 12 (44.4) | 4 (33.3) | 29 (30.9) | 1 (25.4)* |
| Summer | 4 (14.8) | 3 (25.0) | 17 (18.1) | 5 (20.8)* |
| Autumn | 6 (22.2) | 5 (41.7) | 23 (24.5) | 13 (54.2)* |
| Winter | 5 (18.5) | 0 (0.0) | 25 (26.6) | 5 (20.8)* |
| ICHD-3 classification | ||||
| Migraine without aura | 21 (77.8) | 7 (58.3) | 68 (72.3) | 18 (75.0) |
| Migraine with aura | 0 (0.0) | 1 (8.3) | 5 (5.3) | 2 (8.3) |
| Chronic migraine | 6 (22.2) | 3 (25.0) | 19 (20.2) | 3 (12.5) |
| Probable migraine | 0 (0.0) | 1 (8.3) | 2 (2.1) | 1 (4.2) |
| Headache characteristics | ||||
| Monthly days with headache | 10.1±8.1 | 8.2±5.6 | 9.3±7.7 | 6.8±6.1 |
| Attack duration, hours | 30.5±24.2 | 34.3±25.5 | 43.9±58.6 | 34.8±37.1 |
| Medication, days/month | 6.7±8.8 | 4.1±4.1 | 4.4±6.2 | 3.2±2.9 |
| VAS score | 7.4±1.7 | 6.3±1.4† | 7.0±1.8 | 6.9±1.7 |
| Accompanying problems | ||||
| HIT-6 score | 62.1±7.4 | 61.3±6.5 | 61.3±7.6 | 60.2±6.9 |
| PHQ-9 score | 7.3±6.0 | 6.6±4.2 | 7.3±5.0 | 6.7±5.1 |
| GAD-7 score | 6.6±5.9 | 4.6±3.7 | 6.3±5.1 | 5.3±4.9 |
| PSQI | 9.8±3.4 | 7.4±3.7† | 8.7±3.8 | 8.5±4.5 |
| Climate factors, monthly | 14.1±8.9 | 19.3±7.5† | 12.0±9.8 | 16.7±10.0* |
| Temperature, ℃ | ||||
| Relative humidity, % | 66.4±6.3 | 69.3±5.7 | 66.8±6.7 | 72.8±6.4* |
| Precipitation, mm | 73.5±63.0 | 78.3±44.2 | 72.5±75.4 | 104.4±100.0 |
| Total sunshine hours | 205.8±47.9 | 223.6±41.4† | 181.6±28.5 | 181.6±24.2 |
Data are mean±standard deviation or n (%) values.
*p<0.05, †p<0.1.
GAD-7: Generalized Anxiety Disorder-7, HIT-6: Headache Impact Test-6, ICHD-3: third edition of the International Classification of Headache Disorders, PHQ-9: Patient Health Questionnaire-9, PSQI: Pittsburgh Sleep Quality Index, VAS: Visual Analog Scale.
In chronic migraine patients, the number of monthly days with headache was higher in migraineurs with vitamin D deficiency than in those without deficiency (19.3±7.0 days vs. 13.3±4.0 days, p=0.058). None of the other variables differed significantly with vitamin D deficiency in either episodic migraineurs or chronic migraineurs (Table 4). In subgroup multivariate analysis after adjusting for age, sex, and season (winter/spring vs. summer/autumn), vitamin D deficiency was independently associated with more monthly days with headache in both episodic migraine patients (OR=1.266, 95% CI=1.019–1.573, p=0.033) and chronic migraine patients (OR=1.390, 95% CI= 1.013–1.905, p=0.041).
Table 4. Comparison of demographics and clinical factors according to vitamin D deficiency in episodic migraine and chronic migraine.
| Characteristic | Episodic migraine (n=126) | Chronic migraine (n=31) | ||
|---|---|---|---|---|
| Migraineurs with vitamin D deficiency (n=96) | Migraineurs without vitamin D deficiency (n=30) | Migraineurs with vitamin D deficiency (n=25) | Migraineurs without vitamin D deficiency (n=6) | |
| Demographics | ||||
| Age, years | 35.9±8.6 | 36.8±6.2 | 40.2±9.4 | 41.3±12.0 |
| Season | ||||
| Spring | 34 (35.4) | 4 (13.3)* | 7 (28.0) | 1 (16.7) |
| Summer | 17 (17.7) | 6 (20.0)* | 4 (16.0) | 2 (33.3) |
| Autumn | 24 (25.0) | 16 (53.3)* | 5 (20.0) | 2 (33.3) |
| Winter | 21 (21.9) | 4 (13.3)* | 9 (36.0) | 1 (16.7) |
| Headache characteristics | ||||
| Monthly days with headache | 7.0±5.7 | 6.1±5.5 | 19.3±7.0 | 13.3±4.0† |
| Attack duration, hours | 37.7±34.6 | 33.5±29.6 | 53.2±95.8 | 40.5±51.3 |
| Medication, days/month | 3.9±5.0 | 2.5±2.1 | 8.6±10.9 | 8.3±4.2 |
| VAS score | 7.1±1.6 | 6.8±1.6 | 6.9±2.2 | 6.3±1.9 |
| Accompanying problems | ||||
| HIT-6 score | 61.4±7.5 | 60.8±5.9 | 61.6±7.8 | 59.5±10.6 |
| PHQ-9 score | 7.0±5.0 | 6.7±4.5 | 8.6±5.9 | 6.5±6.2 |
| GAD-7 score | 6.2±5.0 | 4.7±3.6 | 7.2±6.1 | 6.8±8.1 |
| PSQI | 8.7±3.6 | 7.8±4.1 | 9.7±4.0 | 9.5±5.0 |
| Climate factors, monthly | ||||
| Temperature, ℃ | 13.0±9.4 | 17.9±8.9* | 10.4±10.5 | 16.0±11.7 |
| Relative humidity, % | 67.1±6.8 | 71.8±6.0* | 65.4±6.1 | 71.0±8.6† |
| Precipitation, mm | 77.1±74.1 | 95.3±81.6 | 55.9±65.2 | 97.6±113.0 |
| Total sunshine hours | 186.8±35.4 | 198.1±41.2 | 187.8±34.3 | 205.2±32.4 |
Data are mean±standard deviation or n (%) values.
*p<0.05, †p<0.1.
GAD-7: Generalized Anxiety Disorder-7, HIT-6: Headache Impact Test-6, ICHD-3: third edition of the International Classification of Headache Disorders, PHQ-9: Patient Health Questionnaire-9, PSQI: Pittsburgh Sleep Quality Index, VAS: Visual Analog Scale.
DISCUSSION
We found that proportion of migraineurs with vitamin D deficiency varied from 61.7% in autumn to 89.1% in spring. Vitamin D deficiency was independently associated with an increased frequency of monthly headache among migraineurs even after adjusting for season, depression, anxiety, and sleep quality.
The reported prevalence rates of vitamin D insufficiency and deficiency in patients with migraine have varied from 40% to 68% and from 13% to 80%, respectively.16,17,19,27,28 These differences are presumably due to differences in race, research design, and residence area.15 The incidence rates of vitamin D deficiency or insufficiency in our study were similar to previous data from Korea, with the prevalence of vitamin D deficiency reportedly being 68.5% in males and 83.1% in females.29 Although previous studies have found vitamin D deficiency to be more common in nonmigraine headaches or chronic tension-type headaches, the relative prevalence rates of vitamin deficiency in migraineurs versus nonheadache controls have not been established.19,30
Our study found that the number of monthly days with headache was associated with serum vitamin D deficiency. Despite of the limitation of small samples in the subgroup analysis, this association was consistently noted among females, episodic migraine patients, and chronic migraine patients. Previous studies found that the frequency of headache attack tended to increase in winter and decrease in summer, which is consistent with the seasonal variation of serum vitamin D levels.15 Combination therapy with simvastatin and vitamin D was found to exert migraine-preventive effects in a previous randomized clinical trial,10 but vitamin D had been prescribed for its anti-inflammatory effect against cytokines or the alleviation of muscle pain.11 Furthermore, there are few reports on the association of vitamin D and headache frequency in females and chronic migraine patients, and so our study provides new information on this research topic.
The severity of migraine was found to not be related to vitamin D level among migraineurs in middle East Asian countries.16 Studies have also shown no significant relationship between serum vitamin D and migraine severity.17 Our results are in line with these previous findings because the VAS scores did not differ with vitamin D deficiency. However, considering the effect of season, our study demonstrated that the HIT-6 score was negatively correlated with vitamin D level during summer/autumn only. It is possible that the effect of HIT-6 was not clear due to low individual differences in normal vitamin D concentrations during spring and winter.
The mechanisms underlying the associations of migraine and migraine frequency with vitamin D remain to be elucidated. However, a few hypotheses are as follows: First, the vitamin D receptors 1-hydroxylase (the enzyme responsible for the formation of the active form of vitamin D) and vitamin-D-binding protein are located in the brain. Vitamin D facilitates the differentiation of brain cells, regulates axonal growth, and regulates calcium signaling directly in the brain, modulates the production of brain-derived reactive oxygen species, and stimulates the production of neurotrophic factors. These roles of vitamin D elicit peripheral and central sensitization of neurons in the periosteum during bone swelling, resulting in headache.7,17 Second, although this was not evaluated in our study, magnesium is the second-most-plentiful intracellular cation and an important component of bone mineralization that plays a crucial role in the synthesis and metabolism of vitamin D. Lower levels of vitamin D are generally associated with lower serum magnesium levels.31 A previous study found that the serum levels of magnesium were lower during migraine attacks than in healthy individuals.32 Third, neurological diseases such as depression and fibromyalgia, which are associated with vitamin D, are also closely related to migraine itself. It is therefore possible that our findings are attributed to accompanying health conditions.7,33
Our study had several limitations. First, a retrospective analysis was performed based on headache clinic data and a relatively small sample. Moreover, although the clinical features were similar in the included and excluded patients, the frequency of vitamin D deficiency in this study was limited to the entire population because of differences in the rates of chronic migraine. However, the purpose of this study was to determine the relationship between vitamin D deficiency and headache variables. Furthermore, the relationship between vitamin D deficiency and monthly days with headache was statistically significant in both episodic and chronic migraineurs. Second, the number of monthly days with headache was based on recall rather than being assessed using a headache diary. Third, vitamin D replacement by diet, medication, or fortification was not assessed.
Notwithstanding these limitations, our study has the following strengths. First, it was conducted in Asia at relatively high latitudes without prominent sun-seeking behaviors. The geographic and cultural characteristics provide novel insights into the association of vitamin D with migraine in an area with a high risk of vitamin D deficiency. Second, our study systematically considered depression, anxiety, and sleep quality, which are closely related to the characteristics of migraine. Third, our study made adjustments for seasonal variation and climate factors that influence serum vitamin D levels.
In conclusion, our study found that a larger number of monthly days with headache is related to vitamin D deficiency among migraineurs. The vitamin D level may also be associated with the occurrence or impact of headache among migraineurs. Future studies should attempt to confirm the causal relationship between vitamin D deficiency and migraine.
Acknowledgements
This study was supported by Hallym University Research Fund (HURF-2016-54). This study was supported by a grant from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01057934). No other financial relationships relevant to this publication were disclosed.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2018.14.3.366.
Comparison of demographics and headache characteristics between included and excluded patients
References
- 1.Atherton K, Berry DJ, Parsons T, Macfarlane GJ, Power C, Hypponen E. Vitamin D and chronic widespread pain in a white middle-aged British population: evidence from a cross-sectional population survey. Ann Rheum Dis. 2009;68:817–822. doi: 10.1136/ard.2008.090456. [DOI] [PubMed] [Google Scholar]
- 2.Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56–61. doi: 10.1016/j.jad.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 3.Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, et al. The association between low vitamin D and depressive disorders. Mol Psychiatry. 2014;19:444–451. doi: 10.1038/mp.2013.36. [DOI] [PubMed] [Google Scholar]
- 4.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger KL, Hongell K, Åivo J, Soilu-Hänninen M, Surcel HM, Ascherio A. 25-hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology. 2017;89:1578–1583. doi: 10.1212/WNL.0000000000004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 7.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Garcion E, Nataf S, Berod A, Darcy F, Brachet P. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45:255–267. doi: 10.1016/s0169-328x(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 9.Thys-Jacobs S. Vitamin D and calcium in menstrual migraine. Headache. 1994;34:544–546. doi: 10.1111/j.1526-4610.1994.hed3409544.x. [DOI] [PubMed] [Google Scholar]
- 10.Buettner C, Nir RR, Bertisch SM, Bernstein C, Schain A, Mittleman MA, et al. Simvastatin and vitamin D for migraine prevention: a randomized, controlled trial. Ann Neurol. 2015;78:970–981. doi: 10.1002/ana.24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buettner C, Burstein R. Association of statin use and risk for severe headache or migraine by serum vitamin D status: a cross-sectional population-based study. Cephalalgia. 2015;35:757–766. doi: 10.1177/0333102414559733. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato K, Hayashino Y, Yamazaki S, Takegami M, Ono R, Otani K, et al. Headache prevalence and long working hours: the role of physical inactivity. Public Health. 2012;126:587–593. doi: 10.1016/j.puhe.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Varkey E, Hagen K, Zwart JA, Linde M. Physical activity and headache: results from the Nord-Trondelag Health Study (HUNT) Cephalalgia. 2008;28:1292–1297. doi: 10.1111/j.1468-2982.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- 15.Prakash S, Mehta NC, Dabhi AS, Lakhani O, Khilari M, Shah ND. The prevalence of headache may be related with the latitude: a possible role of Vitamin D insufficiency? J Headache Pain. 2010;11:301–307. doi: 10.1007/s10194-010-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zandifar A, Masjedi SS, Banihashemi M, Asgari F, Manouchehri N, Ebrahimi H, et al. Vitamin D status in migraine patients: a case-control study. Biomed Res Int. 2014;2014:514782. doi: 10.1155/2014/514782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottaghi T, Khorvash F, Askari G, Maracy MR, Ghiasvand R, Maghsoudi Z, et al. The relationship between serum levels of vitamin D and migraine. J Res Med Sci. 2013;18(Suppl 1):S66–S70. [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G, Cervellin G, Mattiuzzi C. No evidence for an association of vitamin D deficiency and migraine: a systematic review of the literature. Biomed Res Int. 2014;2014:827635. doi: 10.1155/2014/827635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjaergaard M, Eggen AE, Mathiesen EB, Jorde R. Association between headache and serum 25-hydroxyvitamin D: the Tromsø Study: Tromsø 6. Headache. 2012;52:1499–1505. doi: 10.1111/j.1526-4610.2012.02250.x. [DOI] [PubMed] [Google Scholar]
- 20.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. No authors listed. [DOI] [PubMed] [Google Scholar]
- 21.Chu MK, Im HJ, Ju YS, Yu KH, Ma HI, Lee BC. [Validity and reliability assessment of Korean Headache Impact Test-6 (HIT-6)] J Korean Neurol Assoc. 2009;27:1–6. [Google Scholar]
- 22.Seo JG, Park SP. Validation of the Patient Health Questionnaire-9 (PHQ-9) and PHQ-2 in patients with migraine. J Headache Pain. 2015;16:65. doi: 10.1186/s10194-015-0552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo JG, Park SP. Validation of the Generalized Anxiety Disorder-7 (GAD-7) and GAD-2 in patients with migraine. J Headache Pain. 2015;16:97. doi: 10.1186/s10194-015-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (US) CDC Vitamin DStandardization-Certification Program (VDSCP)-total 25 hydroxy vitaminD certified procedures [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2017. [cited 2017 Dec 8]. Available from: https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures.pdf. [Google Scholar]
- 26.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler SD. Vitamin D deficiency in chronic migraine. Headache. 2008;48(S1):S52–S53. [Google Scholar]
- 28.Khorvash F, Mottaghi T, Askari G, Maracy MR, Ghiasvand R, Maghsoudi Z, et al. The association between serum vitamin D levels with general and abdominal obesity among patients with migraine. Int J Prev Med. 2013;4(Suppl 2):S313–S317. [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong H, Hong S, Heo Y, Chun H, Kim D, Park J, et al. Vitamin D status and associated occupational factors in Korean wage workers: data from the 5th Korea National Health and Nutrition Examination Survey (KNHANES 2010–2012) Ann Occup Environ Med. 2014;26:28. doi: 10.1186/s40557-014-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakash S, Rathore C, Makwana P, Dave A, Joshi H, Parekh H. Deficiency in patients with chronic tension-type headache: a case-control study. Headache. 2017;57:1096–1108. doi: 10.1111/head.13096. [DOI] [PubMed] [Google Scholar]
- 31.Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, et al. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013;11:187. doi: 10.1186/1741-7015-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assarzadegan F, Asgarzadeh S, Hatamabadi HR, Shahrami A, Tabatabaey A, Asgarzadeh M. Serum concentration of magnesium as an independent risk factor in migraine attacks: a matched case-control study and review of the literature. Int Clin Psychopharmacol. 2016;31:287–292. doi: 10.1097/YIC.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 33.Makrani AH, Afshari M, Ghajar M, Forooghi Z, Moosazadeh M. Vitamin D and fibromyalgia: a meta-analysis. Korean J Pain. 2017;30:250–257. doi: 10.3344/kjp.2017.30.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of demographics and headache characteristics between included and excluded patients